Introduction
Breast cancer (BC) is the most common malignancy in
women around the world, with ~1.7 million new cases reported per
100,000 women every year (1).
Although the prognosis of BC continues to improve due to advances
in systemic therapies, there are disparities between countries
according to their development index and between ethnic groups
(2,3). Approximately 140,000 Latin American
women develop BC each year, representing a high burden of disease,
with a higher incidence in young women compared with that observed
in other regions of the world (1,4).
The seminal work of Perou, Sorlie et al
(5–7)
demonstrated that BC is a heterogeneous disease with three main
driver mutations, involving the estrogen receptor 1 (ESR1),
progesterone receptor (PGR) and HER2 genes, leading to a
classification of the breast tumors into four main subtypes: The
luminal A, luminal B, basal, and HER2-enriched subtypes. Molecular
subtyping is based upon the evaluation of mRNA profiling with
microarrays or an analysis of 50 genes by reverse
transcription-polymerase chain reaction, a procedure that is not
suitable for routine use. Later studies identified surrogate
subtypes, based on the immunohistochemical (IHC) assessment of the
estrogen receptor (ER), PgR and HER2 genes (8). Further studies demonstrated that the
cellular marker, Ki-67, is useful to distinguish between luminal
subtypes (9,10). The majority of the luminal B tumors
may lack the expression (or amplification) of HER2, leading to a
misclassification of a luminal A subtype. For this reason, Ki-67 is
an important marker to identify appropriately surrogates of the BC
subtypes (9).
There are different distribution patterns of the
subtypes across the ethnic groups. Several studies have
demonstrated a higher incidence of the triple-negative subtype in
Hispanic women and women of African descent compared with Caucasian
women (11–13). Although several previous reports have
described the frequencies of BC phenotypes in certain Latin
American countries, the majority of them did not include the use of
Ki-67 in the IHC panel to discriminate luminal B tumors from
luminal A ones. The aim of the present study was to determine the
effect of Ki-67 on the distribution of BC phenotypes defined in a
prospective cohort of Latin American women.
Patients and methods
Study design
The present study is a prospective evaluation in
patients diagnosed with BC between 2012 and 2013 in three hospitals
from Peru and one from Uruguay [the Hospital Nacional Edgardo
Rebagliatti Martins, the Hospital Nacional Guillermo Almenara
Irigoyen, the Hospital Nacional Alberto Sabogal (all located in
Lima) and the Instituto Nacional del Cáncer in Montevideo].
Patient selection and sample size
Patients diagnosed with BC between 2012 and 2013
were enrolled. Exclusion criteria included a lack of pathological
material for IHC analysis, or the patient refusing at any time to
participate in the present study.
Biomarker evaluation
Evaluation of all the biomarkers was performed in
tumors that has been fixed in 10% neutral formalin and were
paraffin-embedded. Samples taken from biopsies (n=248, 42.8%),
lumpectomies (n=177, 30.5%), and mastectomies (n=155, 26.7%) were
evaluated. A set of 5-µm tissue slides were cut and loaded on to
electrically charged Superfrost Plus™ (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) glass slides for IHC or silver in
situ hybridization (SISH) staining.
IHC
The following primary antibodies were employed in
the present study: Anti-ER (alpha; clone 1D5), anti-progesterone
receptor (PR) (PgR636), anti-Ki-67 (MIB-1), and PATHWAY®
anti-HER2 (clone 4B5). Anti-ER, anti-PR, and anti-Ki-67 antibodies
were obtained from Agilent Technologies, Inc. (Dako Products;
Agilent Technologies, Inc., Santa Clara, CA, USA) and
PATHWAY® anti-HER2 was obtained from Roche (Roche
Diagnostics GmbH, Mannheim, Germany). Evaluation of the level of ER
and PR was performed according to American Society of Clinical
Oncology (ASCO)/College of American Pathologists (CAP) ER and PgR
guideline recommendations (14).
HER2 evaluation was performed according to the ASCO/CAP guidelines,
and scored in four categories (HER2 0+, HER2
1+, HER2 2+ and HER2 3+) (15). Antigen retrieval and IHC processing
was performed according to manufacturer's protocols, and all IHC
procedures were performed in local laboratories. The IHC evaluation
for HER2 was performed using the Ventana BenchMark Classic
Automated system (Ventana Medical Systems, Oro Valley, AZ, USA). If
any sample was identified as being HER2 equivocal (HER2
2+), a SISH evaluation was subsequently performed.
Silver in situ hybridization
(SISH)
HER2 determination by SISH was performed by using
the Ventana HER2 dual-color ISH assay automated staining system,
performed in the Ventana BenchMark Classic Automated system
(Ventana Medical Systems). Slides were pretreated with Cell
Conditioning Solution (CC2; pH 6.0, Ventana Medical Systems),
followed by protein digestion with ISH protease (for 12 min) and
subsequent incubation with the INFORM HER2 Dual ISH DNA PROBE
cocktail (Vendana Medical Systems) for 6 h. Detection was performed
using an ultraView SISH DNP Detection kit, according to the
manufacturer's protocol. Silver precipitation was deposited in the
nuclei, and single copies of the HER2 gene were visualized as
single black dots and single copies of chromosome 17 [the
chromosome enumeration probe 17 (CEP)] as red dots on the same
slide. The slides were then counterstained using haematoxylin II
and a bluing reagent. The SISH staining was carried out using the
Ventana BenchMark Classic Automated system (Ventana Medical
Systems). The numbers of chromosome 17 CEP and HER2 signals were
counted in 20 non-overlapping nuclei per core. Samples with HER2/17
CEP <1.8 were considered negative, whereas those cases with
HER2/17 CEP >2.2 were considered to be positive, and cases with
HER2/17 CEP between 1.8–2.2 were considered to be equivocal.
Definition of BC phenotypes
Luminal tumors were defined by the expression of any
of the hormonal receptors. Among this group, luminal A tumors were
classified as negative for HER2 where Ki-67 expression was low
(with <14% tumor cells being positive), whereas luminal B tumors
had high Ki-67 expression (≥14% tumor cell positivity). The HER2
phenotype was positive for HER2, and negative for hormonal
receptors. Tumors with negative status for HER2 and hormonal
receptors were classified as being triple-negative.
Statistical analysis
As the present work did not include hypothesis
testing, sample size calculations were not performed. Descriptive
data are presented, and associations between categorical data were
evaluated using the Chi-square or Fisher's test. Associations
between categorical data and quantitative variables were evaluated
with the Student's t-test or an analysis of variance (one-way
ANOVA) test. P<0.05 was considered to indicate a statistically
significant difference.
Ethical considerations
The present study was approved by the local
institutional review boards (IRBs) and the Ministry of Health
(MoH). Patients were enrolled in this study after having signed a
form to give their informed consent.
Results
Patient characteristics
In total, 580 out of 599 patients screened for the
present study met the eligibility criteria. A total of 19 patients
refused to participate or had important data missing in the
clinical record, and were excluded. Accrual of patients across the
centers was as follows: 398 patients (68.6%) from the Hospital
Nacional Edgardo Rebagliatti Martins (Lima, Peru); 93 (16%) from
the Hospital Nacional Guillermo Almenara Irigoyen (Lima, Peru); 70
(12.1%) from the Hospital Nacional Alberto Sabogal Sologuren (Lima,
Peru); and 19 (3.3%) from the Instituto Nacional del Cancer
(Montevideo, Uruguay).
The median age was 58 years (range: 27–90 years).
With regard to the menopausal status, 18.1% were premenopausal
(n=100); 11.1% were postmenopausal (n=61), and 70.8% (n=552) were
postmenopausal. In 28 cases, the menopausal status was unknown
(Table I).
 | Table I.Hormonal receptors and HER2 and their
association with clinicopathological parameters. |
Table I.
Hormonal receptors and HER2 and their
association with clinicopathological parameters.
|
| ER status | PR status | Her2 status |
|---|
|
|
|
|
|
|---|
|
| Neg | Pos | P-value | Neg | Pos | P-value | Neg | Pos | P-value |
|---|
| Age at diagnosis
(mean ± SD) | 56.7±12.4 | 58.9±13.3 | 0.066 | 58.9±12.2 | 57.4±13.8 | 0.176 | 58.8 | 55.8 | 0.017 |
| Age group
(years) |
|
| 0.280 |
|
| 0.004 |
|
| 0.056 |
|
<40 | 19 (9.4%) | 24 (6.4%) |
| 17 (5.9%) | 26 (9.0%) |
| 31 (6.8%) | 11 (9.6%) |
|
|
40–49 | 40 (19.7%) | 82 (21.8%) |
| 46 (15.9%) | 76 (26.2%) |
| 100 (21.8%) | 21 (18.3%) |
|
|
50–59 | 61 (30.0%) | 92 (24.4%) |
| 89 (30.7%) | 64 (22.1%) |
| 109 (23.7%) | 42 (36.5%) |
|
|
60–69 | 46 (22.7%) | 92 (24.4%) |
| 77 (26.6%) | 61 (21.0%) |
| 109 (23.7%) | 28 (24.3%) |
|
| ±70 | 37 (18.2%) | 87 (23.1%) |
| 61 (21:0%) | 63 (21.7%) |
| 110 (24.0%) | 13 (11.3%) |
|
| Menopausal
status |
|
| 0.961 |
|
| 0.011 |
|
| 0.703 |
|
Premenopausal | 36 (18.8%) | 64 (17.8%) |
| 38 (13.8%) | 62 (22.5%) |
| 79 (18.2%) | 19 (17.1%) |
|
|
Perimenopausal | 21 (19.9%) | 40 (11.1%) |
| 27 (76.4%) | 34 (12.3%) |
| 50 (11.5%) | 10 (9%) |
|
|
Postmenopausal | 135 (70.3%) | 256 (71.1%) |
| 211 (76.4%) | 180 (65.2%) |
| 306 (70.3%) | 82 (73.9%) |
|
|
Unknown | 11 | 17 |
| 14 | 14 |
| 24 | 4 |
|
| Nuclear grade |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
| Grade
1 | 0 | 18 (4.8%) |
| 6 (2.1%) | 12 (2.1%) |
| 18 (3.9%) | 0 |
|
| Grade
2 | 61 (30%) | 279 (74%) |
| 129 (44.5%) | 211 (72,8%) |
| 287 (62.5%) | 48 (41.7%) |
|
| Grade
3 | 142 (70%) | 80 (21.2%) |
| 155 (53.4%) | 67 (23,1%) |
| 154 (33.6%) | 67 (58.3%) |
|
| Histological
grade |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
Well-differentiated | 20 (9.9%) | 97 (25.8%) |
| 45 (15.6%) | 72 (24.9%) |
| 108 (23.6%) | 6 (5.3%) |
|
|
Moderately differentiated | 79 (39.1%) | 231 (61.4%) |
| 131 (45.3%) | 179 (61.9) |
| 250 (54.6%) | 58 (50.9%) |
|
| Poorly
differentiated | 103 (51%) | 48 (12.8%) |
| 113 (45.3%) | 38 (13.1%) |
| 100 (21.8%) | 50 (43.9%) |
|
|
Unknown | 1 | 1 |
| 1 | 1 |
| 1 | 1 |
|
| Lymphovascular
invation |
|
| 0.03 |
|
| 0.025 |
|
| 0.129 |
|
Present | 141 (69.5%) | 226 (60.1%) |
| 197 (67.9%) | 170 (58.8%) |
| 299 (65.3%) | 66 (57.4%) |
|
|
Absent | 150 (30.5%) | 62 (39.9%) |
| 93 (32.1%) | 119 (41.2%) |
| 159 (34.7%) | 49 (42.6%) |
|
|
Unknown | 0 | 1 |
| 0 | 1 |
| 1 | 0 |
|
| Ki-67 value |
|
| <0.001 |
|
| 0.045 |
|
| <0.001 |
|
Low | 65 (32%) | 194 (51.5%) |
| 117 (40.3%) | 142 (49%) |
| 224 (48.8%) | 32 (27.8%) |
|
|
High | 138 (68%) | 183 (48.5%) |
| 173 (59.7%) | 148 (51%) |
| 235 (51.2%) | 83 (72.2%) |
|
Histopathological features. With regard to the
histological subtypes, the carcinoma ductal NOS was present in
78.3% of cases (n=454), followed by lobular carcinoma, 10.9%
(n=63); mucinous carcinoma, 3.6% (n=21); mixed carcinoma, 1.2%
(n=7); metaplastic carcinoma, 1.2%; and others, 4.8%. Histological
grade 1 was present in 20.2% (n=117); grade 2 was present in 53.6%
(n=310) and grade 3 in 26.1% (n=151). Two cases were of unknown
histological grade. With regard to the nuclear grade, 3.1% (n=18)
had grade 1; 58.6% (n=340) had grade 2 and 38.3% (222) had grade 3.
Vascular or lymphatic invasion was present in 63.3% of cases
(n=367) (Table II). Information
concerning the nodal status and metastases was not included due to
the large number of patients who were referred to other centers
following the primary diagnosis or surgery (note that the Peruvian
Hospitals involved in the present study belong to the Social
Security System, EsSalud Perú).
 | Table II.Clinicopathological characteristics
of the phenotypes of breast cancer. |
Table II.
Clinicopathological characteristics
of the phenotypes of breast cancer.
| Characteristic | All patients | Luminal A | Luminal B | HER2 | Triple
Negative | P-value |
|---|
| Number (n) | 574a | 183 (31.9%) | 201 (35.0%) | 70 (12.1%) | 120 (20.7%) |
|
| Age at diagnosis
(mean ± SD) | 58.1±13 | 58.8±58.8 | 56.4±11.9 | 55.4±11.5 | 58.4±12.7 | 0.175 |
| Age group
(years) |
|
|
|
|
| 0.003 |
|
<40 | 42 (7.3%) | 6 (3.3%) | 21 (10.4%) | 7 (10%) | 8 (6.7%) |
|
|
40–49 | 121 (21.1%) | 40 (21.9%) | 44 (21.9%) | 13 (18.6%) | 24 (20%) |
|
|
50–59 | 151 (26.3%) | 35 (19.1%) | 58 (28.9%) | 27 (38.6%) | 31 (25.8%) |
|
|
60–69 | 137 (23.9%) | 46 (25.1%) | 47 (23.4%) | 14 (20%) | 30 (25%) |
|
|
±70 | 123 (21.4%) | 56 (30.6) | 31 (15.4%) | 9 (12.9%) | 27 (22.5%) |
|
| Menopausal
status |
|
|
|
|
| 0.074 |
|
Premenopausal | 98 (17.1%) | 21 (12%) | 46 (24.1%) | 13 (19.1%) | 18 (16.1%) |
|
|
Perimenopausal | 60 (10.5%) | 25 (14.3%) | 16 (8.4%) | 8 (11.8%) | 11 (9.8%) |
|
|
Postmenopausal | 388 (67.6%) | 129 (73.7%) | 129 (67.5%) | 47 (69.1%) | 83 (74.1%) |
|
|
Unknown | 28 | 8 | 10 | 2 | 8 |
|
| Nuclear grade |
|
|
|
|
| <0.001 |
| Grade
1 | 18 (3.1%) | 10 (5.5%) | 8 (4.0%) | 0 | 0 |
|
| Grade
2 | 335 (58.46%) | 148 (80.9%) | 131 (65.2%) | 18 (25.7%) | 38 (31.7%) |
|
| Grade
3 | 221 (38.5%) | 25 (13.7%) | 62 (30.8%) | 52 (74.3%) | 82 (68.3%) |
|
| Histological
grade |
|
|
|
|
| <0.001 |
|
Well-differentiated | 114 (19.9%) | 66 (36.3%) | 30 (14.9%) | 3 (4.3%) | 15 (12.5%) |
|
|
Moderately differentiated | 308 (53.8%) | 104 (57.1%) | 132 (65.7%) | 27 (39.1%) | 45 (37.5%) |
|
| Poorly
differentiated | 150 (26.2%) | 12 (6.6%) | 39 (19.4%) | 39 (56.5%) | 60 (50.0%) |
|
|
Unknown | 2 | 1 | 0 | 1 | 0 |
|
| Lymphovascular
invation |
|
|
|
|
| 0.001 |
|
Present | 365 (63.7%) | 122 (67.0%) | 114 (56.7%) | 38 (54.3%) | 91 (75.8%) |
|
|
Absent | 208 (36.3%) | 60 (33.0%) | 87 (43.3%) | 32 (45.7%) | 29 (24.2%) |
|
|
Unknown | 1 | 1 | 0 | 0 | 0 |
|
| ER status |
|
|
|
|
| <0.001 |
|
Positive | 372 (64.8%) | 179 (97.8%) | 193 (96%) | 0 | 0 |
|
|
Negative | 202 (35.2%) | 4 (2.2%) | 8 (4%) | 70 (100%) | 120 (100%) |
|
| PR status |
|
|
|
|
| <0.001 |
|
Positive | 286 (49.8%) | 132 (72.1%) | 154 (76.6%) | 0 | 0 |
|
|
Negative | 288 (50.2%) | 51 (27.9%) | 47 (23.4%) | 70 (100%) | 120 (100%) |
|
| HER2 status |
|
|
|
|
| <0.001 |
|
Positive | 115 (20.0%) | 0 | 45 (22.4%) | 70 (100%) | 0 |
|
|
Negative | 459 (80%) | 183 (100%) | 156 (77.6%) | 0 | 120 (100%) |
|
IHC biomarker status and HER2 SISH evaluation. The
ER was positive in 65% (n=377) of cases, while the PR was positive
in 50% (n=203) of them. With regard to HER2 status, 59.1% (n=343)
of the cases were scored 0; 16.7% (n=97) were scored 1+; 6.7%
(n=39) were scored 2+ and 17.4% (n=101) were scored as
3+. A total of 38 out 39 cases scored as 2+ were evaluated using
SISH, and the results were negative in 50% (n=19) of cases,
positive in 36.8% (n=14) and equivocal in 13.2% (n=5) of them. In
total, 79.1% (n=459) were HER2-negative, 19.8% (n=115) were
HER2-positive and 1% (n=6) were equivocal for HER2. The median
index for Ki-67 expression in tumor cells was 27% in total, and
55.3% (n=321) and 44.7% (n=259) had low Ki-67 and high Ki-67
expression, respectively.
Distribution of BC phenotypes. A total of 574 cases
were evaluable for phenotype determination (note that the phenotype
could not be determined in 6 cases due to the HER2 equivocal
status). When Ki-67 was included in the classification,
distribution of subtypes was: Luminal A, 31.9% (n=183); luminal B,
35% (n=201); HER2, 12.1% (n=70) and triple-negative, 20.9% (n=120)
(Table II). In the luminal B
phenotype, 148 cases were HER2-positive and 53 cases were
HER2-negative (Table I).
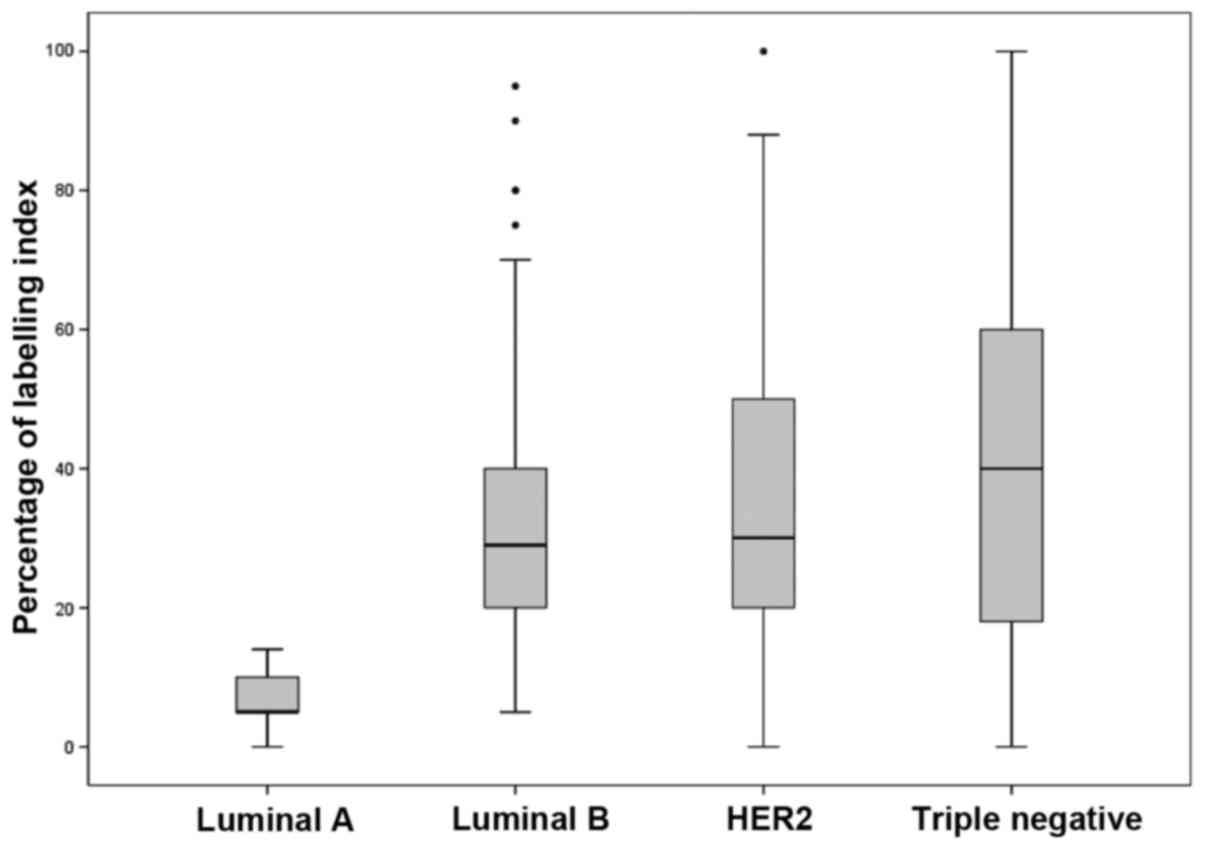
Effect of Ki-67 on the distribution of BC subtypes.
In the absence of Ki-67 in the BC subtype classification, the
frequency of luminal A was 41.1% (overestimated in 9.2% of cases)
and of luminal B, 25.8% (underestimated in 9.2% of cases). With
regard to Ki-67 expression, there were differences (according to
the ANOVA analysis) in the luminal A vs. luminal B (P<0.001),
luminal A vs. HER2 (P<0.001), luminal A vs. triple-negative
(P<0.001), and luminal B vs. triple-negative (P=0.001) groups
(Fig. 1).
Correlation among ER, PgR and HER2 status and
clinicopathological variables. A significant association was
identified between PgR status and the age group, and this
association was revealed by comparing the means of positive and
negative HER2 tumors (HER2-positive patients tended to be younger).
The same association was observed for the menopausal status.
ER-negative, PR-negative and HER2-positive cases presented higher
frequencies of tumors with nuclear grade 3 (P<0.001) and poorly
differentiated tumors (P<0.001). Lymphovascular invasion was
identified most commonly in ER-negative and PR-negative tumors
(P=0.03 and 0.025, respectively). High Ki-67 expression occurred
most frequently in ER-negative, PR-negative and HER2-positive
tumors (P<0.001, P=0.045 and <0.001, respectively) (Table I). The Ki-67 status was highly
correlated with the histological grade, where 30.8, 54.2, and 77.5%
of cases were Ki-67 positive for histological grades I, II and III,
respectively (P<0.001).
Correlation of BC phenotypes with
clinicopathological features. Significant associations were
identified between the age groups and phenotypes, where luminal A
patients were more likely to be older (P=0.003). With regard to the
nuclear grade, patients with triple-negative and HER2 tumors were
more likely to have grade 3 tumors (P<0.001) (Table II).
Discussion
BC is a malignancy with a high incidence in Latin
American women, and it presents with different epidemiological
characteristics in comparison with Caucasian, Asian or African
ethnic groups (1).
Although several reports have previously described
BC subtypes according to IHC classification, there is a lack of
information about the distribution of molecular BC subtypes.
Although the IHC subtype classification is important, the molecular
approach itself may be able to identify subtle levels of mRNA
expression of the HER2 or hormonal receptors in triple-negative
patients, providing opportunities for targeted therapy in this
group of patients (16).
The distribution of tumors positive for ER, PgR or
HER2 may change on the basis of ethnicity. Chu et al
(17), in a study from 2002 that
evaluated 123,732 patients, did not identify any variation in the
incidence of ER-positive or PgR-positive cases among different
ethnic groups, although differences in incidence were identified
according to the age group and clinical stage.
As shown in Table
III, it seems that ancestry has an important role in BC subtype
distribution. A report in 2014 by Carvalho et al (18) based on the Brazilian population
revealed a regional variation in BC distribution, predominantly in
luminal B tumors; an association of luminal B tumors with regions
of European ancestry (south and southeast) was also observed,
whereas the northern and central eastern regions presented high
frequencies of triple-negative tumors when the comparison was
made.
 | Table III.Distribution of BC phenotypes among
different Hispanic countries. |
Table III.
Distribution of BC phenotypes among
different Hispanic countries.
| Authors | Country | n | Markers | Luminal A (%) | Luminal B (%) | HER2 (%) | Triple-negative
(%) | Refs. |
|---|
| The present
study | Peru/Uruguay | 574 | ER, PR, HER2,
Ki-67 | 31.9 | 35.0 | 12.1 | 20.9 |
|
| Vallejos et
al | Peru | 1,198 | ER, PR, HER2 | 49.3 | 13.2 | 16.2 | 21.3 | (13) |
| Alarcon-Rosaz et
al | Peru | 142 | ER, PR, HER2 | 79.4 | 0 | 0 | 20.6 | (20) |
| Camejo et
al | Uruguay | 169 | ER, PR, HER2 | 58.0 | 8.0 | 18.0 | 16.0 | (21) |
| Abuchacra et
al | Argentina | 365 | ER, PR, HER2 | 76 | 6.0 | 3.0 | 15.0 | (22) |
| Carvalho et
al | Brazil | 5,665 | ER, PR, HER2,
Ki-67 | 27.7 | 47.6 | 8.9 | 15.8 | (18) |
| de Macêdo Andrade
et al | Brazil | 269 | ER, PR, HER2,
Ki-67 | 23.8 | 44.6 | 14.5 | 17.1 | (23) |
| Martinez et
al | Mexico | 416 | ER, PR, HER2 | 57.9 | 22.6 | 0 | 19.5 | (24) |
| Perez-Sanchez et
ala | Mexico | 478 | ER, PR, HER2 | 17.4 | 51.3 | 8.0 | 22.6 | (25) |
In the present study, the frequency of positive
cases for ER, PgR and HER2, and the association with
clinicopathological variables, was revealed to be similar to rates
previously described in the literature (8,13,17).
In the present study, it was possible to determine
the BC phenotype in 574 out of 580 patients, where 13.2% of
patients had an equivocal result for HER2 following SISH analysis,
a higher proportion compared with that reported previously (4.9%)
in studies involving SISH analysis (19).
A previous report by Vallejos et al (13) in 2010 described a frequency of 49.13%
of luminal A tumors; 13.2% of luminal B tumors; 16.2% of HER2
tumors; and 21.3% for triple-negative tumors; another study by
Alarcon-Rosas et al (20) in
2011 described an incidence of triple-negative BC of 20.6%. In the
present prospective analysis, in studies that reported cases of
Hispanic patients, it was observed that the proportion of
triple-negative BC was similar among different countries.
High rates of triple-negative BC in Latin American
women lead to a higher frequency of interval cancers (Table III) (18,21–25). It
adds a challenge for the cancer control programs that are based on
the use of mammograms for BC screening. The recent guidelines from
the American Cancer Society suggested that screening for BC should
be initiated for women at the age of 45 years; however, based on
the high frequency of triple-negative tumors, consideration should
be given to commencing screening for BC in Latin American women at
a younger age (26).
The distribution of luminal tumors has been revealed
to be variable among different reports in Hispanic cohorts: This
could be explained according to the different methodologies that
have been used to determine luminal A or B tumors, in comparison
with triple-negative or HER2 ones. In the cohort in the present
study, it was possible to recognize that 9.2% of luminal B tumors
were confusable with luminal A tumors if Ki-67 had not been
included in the analysis.
Cheang et al (9) demonstrated that 13.25% is the best
index cut-off point to distinguish luminal B from luminal A tumors.
Inclusion of Ki-67 in the panel of IHC biomarkers is important to
discriminate luminal B tumors that lack expression of HER2.
Distinction between these two groups is important due to their
different prognostic characteristics. The risk of recurrence is two
times greater for luminal B tumors compared with luminal A tumors
(10). In conclusion, a
misclassification of 9.2% of the cases of luminal tumors was
produced in the absence of Ki-67 in the IHC panel. These findings
have corroborated previously reported data on the distribution of
HER2- and triple-negative tumors in Latin American patients with
BC.
Acknowledgements
The present study was supported by an Academic Grant
of Roche, Peru. Note that R.M.C., M.E.D. and R.E.V. are employees
of Productos Roche del Perú, Lima, Peru.
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality
Worldwide: IARC CancerBase. (11)Lyon, France: http://globocan.iarc.frOctober 5–2014
|
|
2
|
Ooi SL, Martinez ME and Li CI: Disparities
in breast cancer characteristics and outcomes by race/ethnicity.
Breast Cancer Res Treat. 127:729–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Justo N, Wilking N, Jönsson B, Luciani S
and Cazap E: A review of breast cancer care and outcomes in Latin
America. Oncologist. 18:248–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Villarreal-Garza C, Aguila C,
Magallanes-Hoyos MC, Mohar A, Bargalló E, Meneses A, Cazap E, Gomez
H, López-Carrillo L, Chávarri-Guerra Y, et al: Breast cancer in
young women in Latin America: An unmet, growing burden. Oncologist.
18:1298–1306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carey LA, Perou CM, Livasy CA, Dressler
LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S,
et al: Race, breast cancer subtypes, and survival in the Carolina
Breast Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feeley LP, Mulligan AM, Pinnaduwage D,
Bull SB and Andrulis IL: Distinguishing luminal breast cancer
subtypes by Ki67, progesterone receptor or TP53 status provides
prognostic information. Mod Pathol. 27:554–561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang XR, Sherman ME, Rimm DL, Lissowska J,
Brinton LA, Peplonska B, Hewitt SM, Anderson WF,
Szeszenia-Dabrowska N, Bardin-Mikolajczak A, et al: Differences in
risk factors for breast cancer molecular subtypes in a
population-based study. Cancer Epidemiol Biomarkers Prev.
16:439–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spitale A, Mazzola P, Soldini D,
Mazzucchelli L and Bordoni A: Breast cancer classification
according to immunohistochemical markers: Clinicopathologic
features and short-term survival analysis in a population-based
study from the South of Switzerland. Ann Oncol. 20:628–635. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vallejos CS, Gómez HL, Cruz WR, Pinto JA,
Dyer RR, Velarde R, Suazo JF, Neciosup SP, León M, de la Cruz MA,
et al: Breast cancer classification according to
immunohistochemistry markers: Subtypes and association with
clinicopathologic variables in a peruvian hospital database. Clin
Breast Cancer. 10:294–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American Society of Clinical Oncology/College Of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, et al: American Society of Clinical Oncology/College of American
Pathologists: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for human epidermal
growth factor receptor 2 testing in breast cancer. Arch Pathol Lab
Med. 131:18–43. 2007.PubMed/NCBI
|
|
16
|
Bastien RR, Rodríguez-Lescure Á, Ebbert
MT, Prat A, Munárriz B, Rowe L, Miller P, Ruiz-Borrego M, Anderson
D, Lyons B, et al: PAM50 breast cancer subtyping by RT-qPCR and
concordance with standard clinical molecular markers. BMC Med
Genomics. 5:442012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chu KC and Anderson WF: Rates for breast
cancer characteristics by estrogen and progesterone receptor status
in the major racial/ethnic groups. Breast Cancer Res Treat.
74:199–211. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carvalho FM, Bacchi LM, Pincerato KM, Van
de Rijn M and Bacchi CE: Geographic differences in the distribution
of molecular subtypes of breast cancer in Brazil. BMC Womens
Health. 14:1022014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meijer SL, Wesseling J, Smit VT, Nederlof
PM, Hooijer GK, Ruijter H, Arends JW, Kliffen M, van Gorp JM, Sterk
L, et al: HER2 gene amplification in patients with breast cancer
with equivocal IHC results. J Clin Pathol. 64:1069–1072. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alarcon-Rozas AE, Cueva MR, Galarreta J,
Torres J, Gonzales E and Ramirez J: Features of recurrence of
triple-negative (TN), non-metastatic breast cancer (NMBC) patients:
A single institution study. J Clin Oncol. 29:abstr 1802011.
View Article : Google Scholar
|
|
21
|
Camejo N, Gonzalez V, Castillo C, Delgado
L, Ferrero L, Fresco R, Santander GK, Aguiar S, Heinzen S, Martinez
A, Meyer C, Sena G, Spera G, Ubillos L, Xavier F, Rodriguez R and
Sabini G: Survival analysis of breast cancer subtypes assessed by
hormone receptors and HER2 tumor expression in Uruguayan women with
operable breast cancer. J Clin Oncol. 29:e210642011. View Article : Google Scholar
|
|
22
|
Abuchacra LD, Alvarado GJ, Ferretti CN,
Gómez AL, Hernández PA, Sánchez N and Sidan MJ: Relación entre la
clasificación según tipos histológicos y subtipos moleculares más
frecuentes de carcinoma mamario entre los años 2007 y 2012 en San
Miguel de Tucumán, Argentina. CIMEL. 17:76–91. 2012.
|
|
23
|
de Macêdo Andrade AC, Ferreira J, únior
CA, Dantas G, uimarães B, Barros AW Pessoa, de Almeida G Sarmento
and Weller M: Molecular breast cancer subtypes and therapies in a
public hospital of northeastern Brazil. BMC Womens Health.
14:1102014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martinez ME, Wertheim BC, Natarajan L,
Schwab R, Bondy M, Daneri-Navarro A, Meza-Montenegro MM,
Gutierrez-Millan LE, Brewster A, Komenaka IK, et al: Reproductive
factors, heterogeneity, and breast tumor subtypes in women of
mexican descent. Cancer Epidemiol Biomarkers Prev. 22:1853–1861.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sánchez V Perez, Maldonado-Mtz HA, De
León, Trenado D, Bargalló Rocha E, Dragan A Stankov, Miranda A
Alvarado, Gaona A Ojeda and Medina F Lara: Breast Carcinoma in
Mexican Women, Molecular Subtypes Using Immunohistochemical
Surrogate Markers E. J Cancer. 14(S1)2012.Abstract 181.
|
|
26
|
Smith RA, Andrews K, Brooks D, DeSantis
CE, Fedewa SA, Lortet-Tieulent J, Manassaram-Baptiste D, Brawley OW
and Wender RC: Cancer screening in the United States, 2016 A review
of current American Cancer Society guidelines and current issues in
cancer screening. CA Cancer J Clin. 66:96–114. 2016. View Article : Google Scholar : PubMed/NCBI
|