Introduction
Lung cancer is currently the leading cause of
cancer-related mortality worldwide. Although certain combinations
of chemotherapeutic agents (cisplatin/irinotecan,
cisplatin/vinorelbin, cisplatin/gemcitabine and
carboplatin/paclitaxel) as first-line therapy of advanced NSCLC
demonstrated similar efficacy (1),
the response rate (RR) to chemotherapy is low, with <40% of
patients exhibiting significant tumor reduction. The 5-year
survival remains unacceptably low, in the order of 4–17% (2).
Bevacizumab is a recombinant humanized monoclonal
antibody against vascular endothelial growth factor (VEGF)
(3). A number of clinical studies on
bevacizumab have been performed, and some demonstrated that the
addition of bevacizumab to chemotherapy in the first-line setting
significantly increased the progression-free survival (PFS),
overall survival (OS) and RR of patients diagnosed with advanced
lung cancer (4–10). However, there have been few studies
on their feasibility or efficacy of bevacizumab in nonSq-NSCLC
patients who were previously treated with a platinum-based
regimen.
Vinorelbine, one of the vinca alkaloid agents, was
selected to be used with bevacizumab in this regimen for several
reasons as follows: Vinorelbine was suggested to possess
antiangiogenic properties by preclinical data (11,12). The
toxicity profile of vinorelbine is similar to that of bevacizumab,
as neither typically causes severe thrombocytopenia or requires
corticosteroid premedication. In addition, the present trial was
conducted with a lower burden on the patients, as these agents may
be administered by injection; therefore, vinorelbine was
administered within a few minutes from the time of bevacizumab
administration. In addition, two previous phase III trials of
single-agent vinorelbine as first-line treatment for elderly
patients with advanced NSCLC, revealed that vinorelbine was
well-tolerated by elderly patients (13,14).
Therefore, a prospective study was conducted to investigate the
combination of vinorelbine and bevacizumab in nonSq-NSCLC patients
who were previously treated with a platinum regimen.
Patient and methods
Study design
The present feasibility study was conducted to
evaluate the efficacy and safety of vinorelbine and bevacizumab
combination therapy in patients with previously treated
nonSq-NSCLC. The primary endpoint was feasibility and the secondary
endpoints were RR, the incidence of adverse events, OS and PFS.
Patient eligibility
The Ethics Review Board of Toho University Omori
Medical Center approved the present study. The eligibility criteria
were as follows: Histological or cytological evidence of NSCLC;
prior administration of first-line platinum-based chemotherapy; an
interval of at least 4 weeks after the last administration of
agents from the prior chemotherapy regimen; no prior administration
of vinorelbine or bevacizumab; stage IIIB/IV or recurrent disease
after surgery; measurable or assessable disease; Eastern
Cooperative Oncology Group performance status (PS) of 0–2; age
>20 years; a white blood cell count of >3×103/µl,
hemoglobin (Hb) >9.5g/dl, platelet count
>75×103/µl, bilirubin <1.5 mg/dl, aspartate
aminotransferase and alanine aminotransferase level <twice the
upper limit of normal, creatinine <1.5 mg/dl and PaO2
>70 Torr; anticipated survival ≥3 months; and provision of
written informed consent. The exclusion criteria were as follows:
Serious concomitant systemic disorders, including severe heart
failure, uncontrollable angina, hypertension, diabetes mellitus,
interstitial pneumonia, active infection, ulcer, or another primary
malignancy; history of severe hypersensitivity; and pregnancy. All
the patients provided written informed consent to participation
prior to study enrollment.
Treatment schedule
All the patients received combination therapy with
vinorelbine (25 mg/kg on days 1 and 8) and bevacizumab (15 mg/kg on
day 1). Treatment was performed in 3-week cycles with vinorelbine
and bevacizumab administered on days 1 and 8, and on day 1,
respectively. Vinorelbine was scheduled to be administered on day 1
as a 5-min intravenous (i.v.) infusion of 25 mg/kg, followed by a
90-min i.v. infusion of 15 mg/kg bevacizumab. If no incident
occurred, the second dose was administered over 60 min and
subsequent doses over 30 min. On day 8 of each cycle, only a 5-min
i.v. infusion of 25 mg/kg vinorelbine was administered. The
treatment plan was repeated until the development of progressive
disease (PD), unacceptable adverse events, or patient withdrawal of
consent. Colony-stimulating factors were used at the doctor's
discretion and 5-HT3 antagonists were used as antiemetics.
Treatment schedule amendment
Halfway through the study the treatment schedule was
modified to prevent the development of phlebitis. Betamethasone
(4.0 mg i.v.) was administered immediately prior to vinorelbine
infusion.
Dose adjustment
The criteria for discontinuation of the study
included grade 3/4 drug-related toxicity requiring treatment delay
of ≥3 weeks, progressive disease, withdrawal of consent, and any
changes in the patient's condition that made further treatment
inappropriate. The criteria for dose reduction included grade 4
hematological and grade 3 non-hematological toxicity, in which case
the vinorelbine dose was reduced by 20%.
Statistical analysis
Tumor response was evaluated using the Response
Evaluation Criteria in Solid Tumors, version1.1 (15). All statistical analyses were
performed using SPSS statistical software, version 11.0 (SPSS Inc.,
Chicago, IL, USA). Survival curves were drawn using the
Kaplan-Meier method and statistical analysis was performed using
the log-rank test. A P-value of <5% was considered to indicate
statistically significant differences.
This single-center study was conducted at the Toho
University Omori Medical Center (Tokyo, Japan) and was approved by
its Human Genome/Gene Analysis Research Ethics Committee
(authorization number, 22–86).
Results
Patient characteristics
Between June, 2011 and January, 2013, a total of 15
patients with nonSq-NSCLC were enrolled in this study. All the
patients were evaluable for toxicity and response assessment. The
patient characteristics are presented in Table I. Of the 15 patients, 12 (80%) had
received at ≤3 prior courses of chemotherapy and all the patients
had a PS of 0–2; 5 patients harbored a mutation of the epidermal
growth factor receptor gene conferring sensitivity to tyrosine
kinase inhibitors.
 | Table I.Patient characteristics (n=15). |
Table I.
Patient characteristics (n=15).
| Characteristics | No. |
|---|
| Age (years) | 68 |
| Median (range) | 57–82 |
| Gender |
|
| Male | 7 |
|
Female | 8 |
| PS |
|
| 0 | 2 |
| 1 | 11 |
| 2 | 2 |
| Histology |
|
| Ad | 15 |
| NOS | 0 |
| Clinical stage |
|
| IV | 7 |
| Rec | 8 |
| Smoking history |
|
|
Current | 6 |
|
Former | 9 |
|
Never | 0 |
| EGFR
mutation |
|
|
Positive | 5 |
|
Negative | 10 |
| Prior
chemotherapy |
|
| 1
regimen | 1 |
| 2
regimens | 2 |
| 3
regimens | 2 |
| 4
regimens | 4 |
| ≥5
regimens | 6 |
| Platinum
doublet (+/−) | 14/1 |
| EGFR-TKI
(+/−) | 5/10 |
| Single
agent (+/−) |
|
|
PEM (+/−) | 9/6 |
|
DTX
(+/−) | 2/13 |
|
GEM
(+/−) | 3/12 |
|
CPT-11
(+/−) | 1/14 |
Treatment administration
Overall, a total of 68 cycles were administered
(median, 4; range, 1–12) and 8 patients required a dose reduction
due to toxicity. The reasons for treatment discontinuation included
disease progression (n=11), toxicity (n=3) and the patient's wishes
(n=1).
Treatment efficacy
All the patients were evaluable. There was no
complete response and 4 patients exhibited a partial response,
accounting for an overall RR of 26.7% (95% CI: 1.3–52.3). Another 7
patients exhibited stable disease as their best response, resulting
in an overall disease control rate (DCR) of 73.3% (95% CI:
47.98–98.68; Table II). The
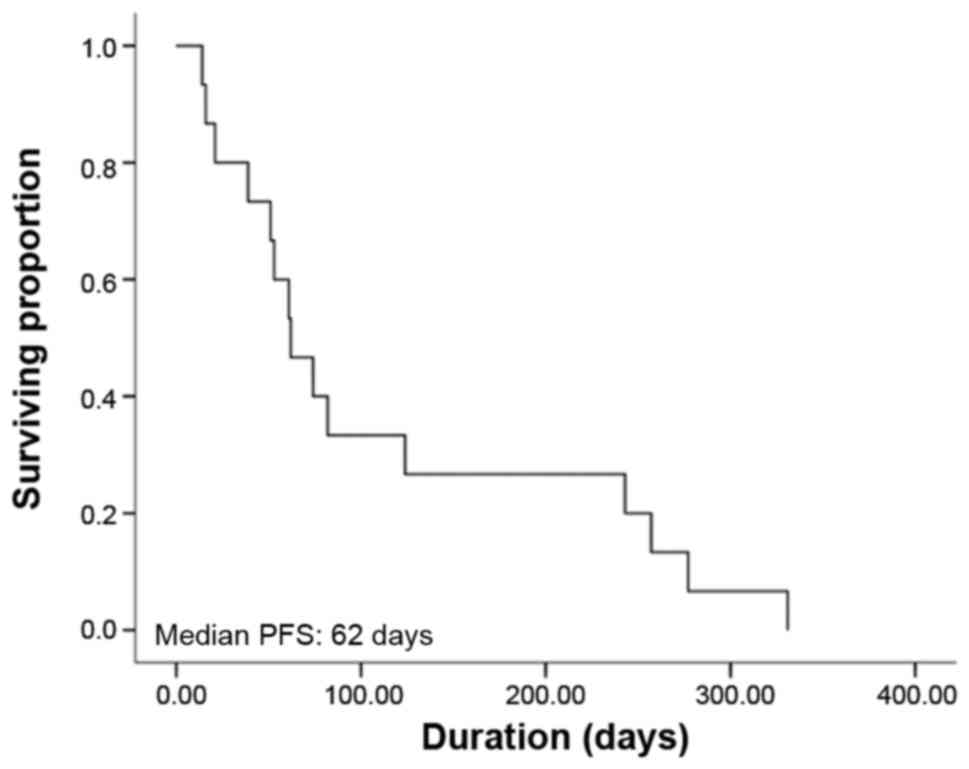
Kaplan-Meier curve for PFS is shown in Fig. 1; the estimated median PFS was 2.1
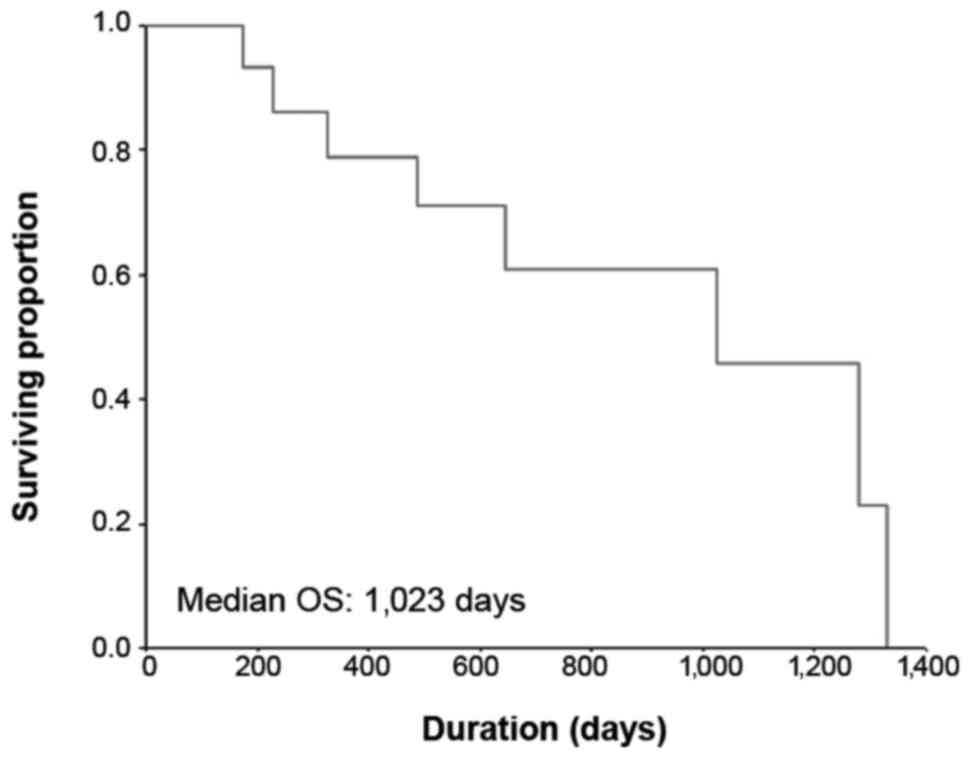
months (95% CI: 1.2–4.6 months). The Kaplan-Meier curve for OS is
shown in Fig. 2; the median OS was
34.1 months (95% CI: 15.6–52.6 months) and the 1-year OS rate was
78.6%.
 | Table II.Adverse events (n=15). |
Table II.
Adverse events (n=15).
|
| NCI CTC |
|---|
|
|
|
|---|
| Toxicities | Grade 1/2 (%) | Grade 3/4 (%) |
|---|
| Hematological |
|
|
|
Leukopenia | 20.0 | 33.3 |
|
Neutropenia | 13.3 | 53.3 |
|
Anemia | 40.0 | 6.7 |
|
Thrombocytopenia | 20.0 | 0.0 |
|
Non-hematological |
|
|
|
Nausea/vomiting | 13.3 | 6.7 |
|
Anorexia | 6.7 | 13.3 |
|
Diarrhea | 0.0 | 0.0 |
|
Phlebitis | 26.6 | 20.0 |
| Rash | 33.3 | 0.0 |
|
Hemorrhage | 0.0 | 0.0 |
| Febrile
neutropenia | 0.0 | 6.7 |
|
Hypertension | 33.3 |
0.0 |
Treatment toxicity
All the patients ware evaluable for toxicity. The
adverse events of this study are summarized in Table II. The incidence of grade 3–4
neutropenia, anemia and febrile neutropenia was 53.3, 6.7 and 6.7%,
respectively. The incidence of grade 3–4 nausea/vomiting and
anorexia was 6.7 and 13.3%, respectively. Grade 3–4 phlebitis
occurred in 3 patients (20%); phlebitis improved by placement of a
central venous catheter in 1 patient and by administration of
corticosteroid treatment in 2 other patients. There were no
reported grade 3/4 bevacizumab-related adverse events, such as
thrombosis, hemorrhage, bowel perforation, hypertension,
proteinuria, or hemorrhagic events.
Discussion
The tolerability and efficacy of bevacizumab
combined with vinorelbine were evaluated in patients receiving
second-line therapy for recurrent NSCLC. The study demonstrated an
overall RR of 26.7% and an overall DCR of 73.3%. The median PFS was
2.1 months and the median OS was 34.1 months.
Several studies on doublet chemotherapy including
bevacizumab as second-line chemotherapy for NSCLC have been
reported (16–19). Herbst et al (20) conducted a randomized second-line
phase II study that evaluated the efficacy of bevacizumab in
combination with standard second-line chemotherapies that included
pemetrexed, docetaxel or erlotinib, and it demonstrated a RR of
12.5%, a median PFS of 4.8 months and an OS of 12.6 months in the
bevacizumab plus chemotherapy arm. Our study results included an RR
of 26.7%, a median PFS of 2.1 months and a median OS of 34.1
months. These values were better compared with the abovementioned
historical data for second-line chemotherapy. A limitation of the
present study was that it was conducted on a highly selected
patient group and our patient sample was insufficient for accurate
evaluation. The aim of this study was to investigate the
feasibility and effectiveness of third-generation chemotherapy with
bevacizumab. Vinorelbine was selected as the other third-generation
chemotherapeutic agent, as it is a vinca alkaloid with barely any
reported adverse events in previous second-line studies (21).
In terms of adverse events, there was a high rate of
severe phlebitis. Several studies have reported that bevacizumab
has enhanced the toxicity and increased the activity of another
agent in a combination regimen (22,23).
Bevacizumab targets VEGF and alters tumor vessel physiology,
thereby increasing intratumoral drug uptake (24,25).
Seto et al (7) reported that
the addition of bevacizumab may increase the toxicity to a certain
degree (hypertension, proteinuria and haemorrhagic events).
Adverse events were carefully monitored,
particularly phlebitis; therefore, halfway through the study,
steroids were administered as a preventive measure against the
development of phlebitis, however, 2 of 7 patients who received
steroid treatment as a precaution developed phlebitis; thus, the
preventive effect of steroids was not confirmed. In our study, the
combination therapy enhanced the typical adverse events associated
with cytotoxic anticancer drugs, although not those particularly
associated with bevacizumab, such as hypertention, proteinuria and
bleeding. These results are similar to previous findings on the
adverse events of bevacizumab combined with other agents (4–10). The
bevacizumab and vinorelbine combination therapy was considered as
high-risk in terms of phlebitis or vascular events; thus, further
phase II studies on bevacizumab combined with vinorelbine are
required.
The aim of the AvaALL study, which is an open-label,
randomized, multicenter phase III study, is to evaluate the
efficacy and safety of bevacizumab in combination with standard of
care treatment in patients with nonSq-NSCLC (26). Patients will be randomly assigned to
one of two treatment arms to receive either bevacizumab plus
standard chemotherapy or standard chemotherapy alone, from the
first- through to the third-line setting. The result of this study
may indicate that new treatment strategies should be established
for previously treated nonSq-NSCLC patients.
In conclusion, combination treatment with
vinorelbine and bevacizumab may prove to be effective and feasible
for patients with previously treated nonSq-NSCLC. However, this
regimen should be managed carefully due to the associated adverse
events, particularly the increased risk of phlebitis.
Glossary
Abbreviations
Abbreviations:
|
nonSq-NSCLC
|
non-squamous non-small-cell lung
cancer
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
RR
|
response rate
|
|
DCR
|
disease control rate
|
|
VEGF
|
vascular endothelial growth factor
|
|
PD
|
progressive disease
|
|
DLT
|
dose-limiting toxicity
|
References
|
1
|
Ohe Y, Ohashi Y, Kubota K, Tamura T,
Nakagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y and Fukuoka
M: Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm Cooperative Study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr and Wu YL: Paz-Ares: Lung cancer: current
therapies and new targeted treatments. 389:299–311. 2017.
|
|
3
|
Giaccone G: The potential of
antiangiogenic therapy in non-small cell lung cancer. Clin Cancer
Res. 13:1961–1970. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S,
Feng J, He J, Han B, Wang J, et al: BEYOND: A randomized,
double-blind, placebo-controlled, multicenter, phase III study of
First-Line carboplatin/paclitaxel plus bevacizumab or placebo in
chinese patients with advanced or recurrent nonsquamous
non-small-cell lung cancer. J Clin Oncol. 33:2197–2204. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galetta D, Cinieri S, Pisconti S, Gebbia
V, Morabito A, Borsellino N, Maiello E, Febbraro A, Catino A, Rizzo
P, et al: Cisplatin/Pemetrexed Followed by Maintenance Pemetrexed
Versus Carboplatin/Paclitaxel/Bevacizumab Followed by Maintenance
Bevacizumab in Advanced Nonsquamous Lung Cancer: The GOIM (Gruppo
Oncologico Italia Meridionale) ERACLE Phase III Randomized Trial.
Clin Lung Cancer. 16:262–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seto T, Kato T, Nishio M, Goto K, Atagi S,
Hosomi Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, et al:
Erlotinib alone or with bevacizumab as first-line therapy in
patients with advanced non-squamous non-small-cell lung cancer
harbouring EGFR mutations (JO25567): An open-label, randomised,
multicentre, phase 2 study. Lancet Oncol. 15:1236–1244. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niho S, Kunitoh H, Nokihara H, Horai T,
Ichinose Y, Hida T, Yamamoto N, Kawahara M, Shinkai T, Nakagawa K,
et al JO19907 Study Group, : Randomized phase II study of
first-line carboplatin-paclitaxel with or without bevacizumab in
Japanese patients with advanced non-squamous non-small-cell lung
cancer. Lung Cancer. 76:362–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herbst RS, Ansari R, Bustin F, Flynn P,
Hart L, Otterson GA, Vlahovic G, Soh CH, O'Connor P and Hainsworth
J: Efficacy of bevacizumab plus erlotinib versus erlotinib alone in
advanced non-small-cell lung cancer after failure of standard
first-line chemotherapy (BeTa): A double-blind, placebo-controlled,
phase 3 trial. Lancet. 377:1846–1854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reck M, von Pawel J, Zatloukal P, Ramlau
R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, et
al: Phase III trial of cisplatin plus gemcitabine with either
placebo or bevacizumab as first-line therapy for nonsquamous
non-small-cell lung cancer: AVAil. J Clin Oncol. 27:1227–1234.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vacca A, Iurlaro M, Ribatti D, Minischetti
M, Nico B, Ria R, Pellegrino A and Dammacco F: Antiangiogenesis is
produced by nontoxic doses of vinblastine. Blood. 94:4143–4155.
1999.PubMed/NCBI
|
|
12
|
Kruczynski A, Poli M, Dossi R, Chazottes
E, Berrichon G, Ricome C, Giavazzi R, Hill BT and Taraboletti G:
Anti-angiogenic, vascular-disrupting and anti-metastatic activities
of vinflunine, the latest vinca alkaloid in clinical development.
Eur J Cancer. 42:2821–2832. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gridelli C, Perrone F, Gallo C, Cigolari
S, Rossi A, Piantedosi F, Barbera S, Ferraù F, Piazza E, Rosetti F,
et al MILES Investigators, : Chemotherapy for elderly patients with
advanced non-small-cell lung cancer: The Multicenter Italian Lung
Cancer in the Elderly Study (MILES) phase III randomized trial. J
Natl Cancer Inst. 95:362–372. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
No authors listed: Effects of vinorelbine
on quality of life and survival of elderly patientswith advanced
non-small-cell lung cancer. The Elderly Lung Cancer
VinorelbineItalian Study Group. J Natl Cancer Inst. 91:66–72. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC, et al: New guidelines to evaluate the
response to treatment in solid tumors. European Organization for
Research and Treatment of Cancer, National Cancer Institute of the
United States, National Cancer Institute of Canada. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
16
|
Weiss JM, Villaruz LC, Socinski MA,
Ivanova A, Grilley-Olson J, Dhruva N and Stinchcombe TE: A
single-arm phase II trial of pazopanib in patients with advanced
non-small cell lung cancer with non-squamons histology with disease
progression on bevacizumab containing therapy. Lung Cancer.
86:288–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Powell SF, Beitinjaneh A, Tessema M, Bliss
RL, Kratzke RA, Leach J, Dudek AZ and Phase II: Phase II study of
topotecan and bevacizumab in advanced, refractory non-small-cell
lung cancer. Clin Lung Cancer. 14:495–501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kubota T, Okano Y, Sakai M, Takaoka M,
Tsukuda T, Anabuki K, Kawase S, Miyamoto S, Ohnishi H, Hatakeyama
N, et al: Carboplatin plus Weekly Paclitaxel with Bevacizumab for
First-line Treatment of Non-small Cell Lung Cancer. Anticancer Res.
36:307–312. 2016.PubMed/NCBI
|
|
19
|
Ohyanagi F, Yanagitani N, Kudo K, Kawano
Y, Sakatani T, Tanimoto A, Nishizawa H, Horiike A, Hagiwara S,
Horai T, et al: Phase II study of docetaxel-plus-bevacizumab
combination therapy in patients previously treated for advanced
non-squamous non-small cell lung cancer. Anticancer Res.
34:5153–5158. 2014.PubMed/NCBI
|
|
20
|
Herbst RS, O'Neill VJ, Fehrenbacher L,
Belani CP, Bonomi PD, Hart L, Melnyk O, Ramies D, Lin M and Sandler
A: Phase II study of efficacy and safety of bevacizumab in
combination with chemotherapy or erlotinib compared with
chemotherapy alone for treatment of recurrent or refractory non
small-cell lung cancer. J Clin Oncol. 25:4743–4750. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kosmas C, Tsavaris N, Panopoulos C,
Vadiaka M, Stavroyianni N, Kourelis T, Malamos N, Antonopoulos M
and Kalofonos HP: Gemcitabine and vinorelbine as second-line
therapy in non-small-cell lung cancer after prior treatment with
taxane+platinum-based regimens. Eur J Cancer. 37:972–978. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshida M, Muro K, Tsuji A, Hamamoto Y,
Yoshino T, Yoshida K, Shirao K, Miyata Y, Takahari D, Takahashi T,
et al: Combination chemotherapy with bevacizumab and S-1 for
elderly patients with metastatic colorectal cancer (BASIC trial).
Eur J Cancer. 51:935–941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pujol JL, Lavole A, Quoix E, Molinier O,
Souquet PJF, Le Caer H, Moro-Sibilot D, Fournel P, Oster JP, et al:
French Cooperative Thoracic Intergroup (IFCT); FrenchCooperative
Thoracic Intergroup IFCT. Randomized phase II–III study
ofbevacizumab in combination with chemotherapy in previously
untreated extensivesmall-cell lung cancer: Results from the
IFCT-0802 trial. Ann Oncol. 26:908–914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wildiers H, Guetens G, De Boeck G,
Verbeken E, Landuyt B, Landuyt W, de Bruijn EA and van Oosterom AT:
Effect of antivascular endothelial growth factor treatment on the
intratumoral uptake of CPT-11. Br J Cancer. 88:1979–1986. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dickson PV, Hamner JB, Sims TL, Fraga CH,
Ng CY, Rajasekeran S, Hagedorn NL, McCarville MB, Stewart CF and
Davidoff AM: Bevacizumab-induced transient remodeling of the
vasculature in neuroblastoma xenografts results in improved
delivery and efficacy of systemically administered chemotherapy.
Clin Cancer Res. 13:3942–3950. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gridelli C, Bennouna J, de Castro J,
Dingemans AM, Griesinger F, Grossi F, Rossi A, Thatcher N, Wong EK
and Langer C: Randomized phase IIIb trial evaluating the
continuation of bevacizumab beyond disease progression in patients
with advanced non-squamous non-small-cell lung cancer after
first-line treatment with bevacizumab plus platinum-based
chemotherapy: Treatment rationale and protocol dynamics of the
AvaALL (MO22097) trial. Clin Lung Cancer. 12:407–411. 2011.
View Article : Google Scholar : PubMed/NCBI
|