Introduction
Liver dysfunction, which may affect drug metabolism,
is a major concern in patients receiving chemotherapy for
colorectal cancer. Thus, the assessment of the degree of any liver
dysfunction, such as hepatic fibrosis and inflammation, is crucial
for predicting the effects or adverse events of chemotherapy.
Currently, the gold standard method for the assessment of liver
dysfunction is liver biopsy; however, this is a costly and invasive
method, with a high risk of complications. In addition, the typical
sample is small and may result in sampling error (1,2). Certain
benefits of non-invasive clinical scoring systems, constructed from
routine clinical and laboratory variables, have been reported for
several liver conditions, including non-alcoholic fatty liver
disease (NAFLD) (3). Among those,
the NAFLD fibrosis score (NFS) has been recommended even for
Japanese populations, in which the incidence of obesity is
relatively low (4,5).
Irinotecan (CPT-11) is a key drug used to treat
unresectable/recurrent colorectal cancer. Polymorphisms in the
uridine diphosphate-glucuronosyltransferase (UGT)1A1 gene, which is
involved in the metabolism of CPT-11 in the liver, may cause severe
adverse events, such as neutropenia (6). However, testing for such polymorphisms
is time-consuming and costly. In addition, the number of UGT1A1*28
homozygous patients who experience severe adverse events due to
CPT-11 is quite small in the Japanese population. Thus, it is
important to identify a more appropriate surrogate marker
reflecting liver dysfunction in chemotherapy with CPT-11. The aim
of this study was to evaluate whether NFS is useful for predicting
the effects and adverse events of chemotherapy including CPT-11 for
colorectal cancer.
Materials and methods
Patients and clinicopathological
characteristics
Between January, 2007 and May, 2013, 118 patients
were diagnosed with unresectable/recurrent colorectal cancer at
Keio University Hospital (Tokyo, Japan), of whom 87 patients who
underwent first-line chemotherapy including CPT-11 were enrolled in
this study.
The tumor location, from the vermiform appendix (VA)
to the rectum, was classified according to the 7th edition of the
TNM Classification of Malignant Tumours (http://www.uicc.org/sites/main/files/private/TNM_Classification_of_Malignant_Tumours_Website_15%20MAy2011.pdf).
The colon was defined as spanning from the cecum to the sigmoid
colon, while ‘other’ was defined as the rectum; thus, tumors of the
VA were excluded. In terms of histological types, well- and
moderately differentiated tubular adenocarcinomas were defined as
highly differentiated carcinomas, whereas poorly differentiated,
mucinous adenocarcinomas and signet-ring cell carcinomas were
defined as poorly differentiated carcinomas.
Demographic variables were retrospectively collected
from medical records and included age, gender, tumor location,
histological type, number of metastatic sites (single or multiple),
temporal association of metastasis (synchronous or metachronous),
cycles of chemotherapy, use of bevacizumab, pretreatment total
bilirubin serum levels and pretreatment NFS. NFS was calculated as
follows: NFS=1.675 + 0.037x age (years) + 0.094x BMI
(kg/m2) + 1.13x impaired fasting glucose/diabetes
(yes=1, no=0) + 0.99x AST/ALT ratio-0.013x platelet
(x109/l)-0.66x albumin (g/dl), where BMI, body mass
index; AST, aspartate aminotransferase; and ALT, alanine
aminotransferase.
Treatment and evaluation
Our regimen including CPT-11 was FOLFIRI or
uracil/ftorafur/leucovorin combined with CPT-11 (TEGAFIRI); thus,
the participants received FOLFIRI (CPT-11 150 mg/m2 on
day 1, leucovorin 200 mg/m2 on day 1, 5-fluorouracil 400
mg/m2 on day 1, 5-fluorouracil 2,400 mg/m2 as
a 46-h infusion, every 2 weeks) or TEGAFIRI (uracil/ftorafur 300
mg/m2/day on days 1–21, leucovorin 75 mg/day on days
1–21, CPT-11 150 mg/m2/day on days 1 and 15, every 4
weeks). The National Cancer Institute Common Terminology Criteria
for Adverse Events (CTCAE), version 4.0, were used to assess
toxicity (7).
Treatment was administered until disease
progression, development of unacceptable toxicity, or the patient's
wish to withdraw. The doses of CPT-11 were lowered by 20% if severe
toxic effects (CTCAE grade ≥3) occurred. Patients were evaluated
for progression every four cycles by computed tomography. During
the follow-up period, any continuing adverse effects were closely
monitored.
The primary outcome was the association between
pretreatment NFS and adverse events, such as hematological and
non-hematological toxicity, of chemotherapy including CPT-11.
This study was approved by the Ethics Review Board
of our institute (no. 20140459).
Statistical methods
Logistic regression analysis was used to identify
univariate and multivariate risk factors for hematological and
non-hematological toxicity (CTCAE grade ≥3). The coefficient (coef)
was used to estimate the impact on hematological and
non-hematological toxicity. All predictors with P<0.05 in
univariate analysis and clinically important factors were retained
in the multivariate models.
A receiver operating characteristic (ROC) curve
analysis was performed to determine a cut-off point for NFS as a
continuous variable. Youden's index was calculated and the maximum
value was used as the optimum cut-off point. Using this cut-off
value, the impact on adverse events was also assessed. Each risk
factor was assessed using the χ2 test, Mann-Whitney U
test, Fisher's exact test and logistic regression analysis. All
statistical analyses were two-sided and statistical significance
was set at P<0.05. Statistical analyses were performed using the
STATA software, version 11; (StataCorp LP, College Station, TX,
USA).
Results
Patient background
The clinicopathological demographics of the 87
patients are summarized in Table I.
The median age was 67 years (range, 36–82 years) and 59 of the
participants (67.8%) were men. The tumors were located in the colon
in 59 patients (67.8%). The median pretreatment total bilirubin
serum levels were 0.6 mg/dl (range, 0.3–1.5 mg/dl) and the median
NFS was 1.302 (range, 5.158–2.620). Grade ≥3 hematological and
non-hematological toxicities occurred in 6 (6.9%) and 13 (14.9%)
patients, respectively.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variables | All (n=87) |
|---|
| Gender
(male/female) | 59/28 |
| Age, years | 67
(36–82)a |
| Tumor location
(C/A/T/D/S/R) | 5/15/9/6/24/28 |
| Histological type
(well/poorly differentiated) | 77/10 |
| Single/multiple
metastasis | 49/38 |
|
Synchronous/metachronous metastasis | 37/50 |
| Number of cycles | 13
(1–126)a |
| Use of bevacizumab
(yes/no) | 45/42 |
| Total serum
bilirubinb, mg/dl | 0.6
(0.3–1.5)a |
| NFSb | −1.302 (−5.158 to
2.62)a |
| Hematological
toxicityc | 6 |
| Decreased
platelet count | 1 |
| Decreased
neutrophil count | 5 |
| Non-hematological
toxicityc | 13 |
|
Vomiting | 5 |
|
Diarrhea | 4 |
| Nervous
system disorders | 1 |
| Increased
serum bilirubin | 1 |
|
Cough | 2 |
Association between NFS and adverse
events
In the univariate analysis, pretreatment NFS was
significantly associated with hematological toxicity (Table II). Furthermore, pretreatment NFS
was an independent risk factor for hematological toxicity in a
multivariate analysis (coef=0.932, 95% CI: 0.083–1.781; P=0.031),
adjusted for gender, age, temporal association of metastasis, cycle
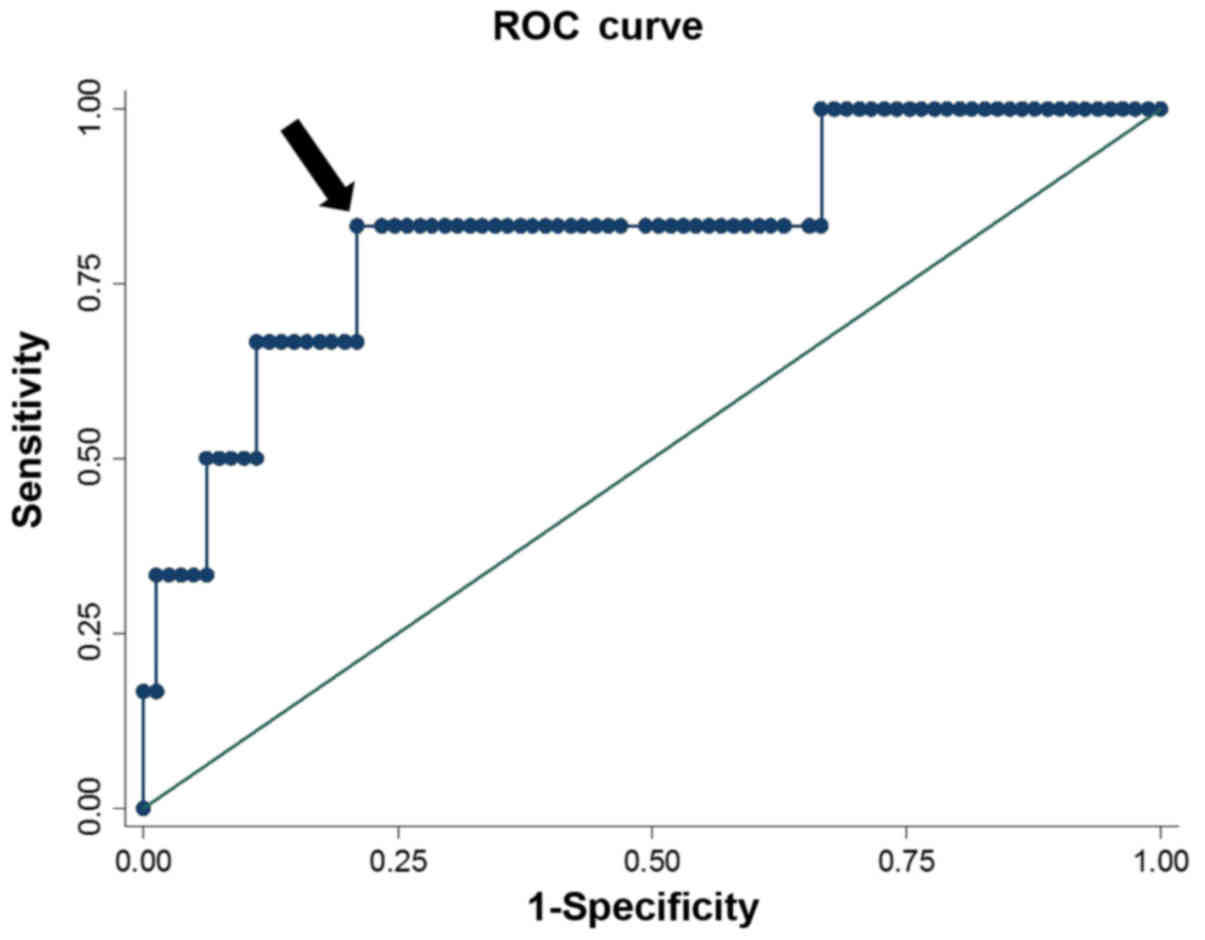
number and pretreatment total bilirubin serum levels. ROC analysis,
including Youden's index of NFS, identified 0.347 as the optimal
cut-off value associated with hematological toxicity (Fig. 1). Using this cut-off, 21 (24.1%)
patients were classified as high NFS, while 66 (75.9%) patients had
a low NFS (Table III). High NFS
was found to be a significant risk factor for hematological
toxicity (coef=2.019, 95% CI: 0.239–3.798; P=0.026) but not for
non-hematological toxicity (P=0.546). This model exhibited a
sensitivity of 83.3%, a specificity of 79.3%, a likelihood ratio
for a positive finding of 3.97, and a likelihood ratio for a
negative finding of 0.21. However, pretreatment NFS was not
identified as a risk factor for non-hematological toxicity in any
univariate or multivariate analysis (Table IV). The results were similar even
when the investigation was limited to diarrhea among
non-hematological toxicities (coef=0.214, 95% CI: −0.514 to 0.942;
P=0.564).
 | Table II.Uni- and multivariate analysis for
hematological toxicity. |
Table II.
Uni- and multivariate analysis for
hematological toxicity.
| Variables | Unadjusted coef (95%
CI) | P-value | Adjusted coef (95%
CI) | P-value |
|---|
| Male gender | −1.558 (−3.321 to
0.205) | 0.083 | −1.247 (−3.480 to
0.985) | 0.274 |
| Age | 0.173 (−0.068 to
0.103) | 0.691 | 0.008 (−0.131 to
0.147) | 0.907 |
| Rectum | 0.056 (−1.704 to
1.816) | 0.950 |
|
|
| Multiple
metastasis | −0.470 (−2.223 to
1.283) | 0.599 |
|
|
| Synchronous
metastasis | −1.386 (−3.578 to
0.805) | 0.215 | −0.664 (−3.504 to
2.175) | 0.647 |
| Number of cycles | 0.016 (−0.016 to
0.049) | 0.320 | 0.022 (−0.015 to
0.058) | 0.243 |
| Use of
bevacizumab | −0.074 (−1.733 to
1.584) | 0.930 |
|
|
| Total serum
bilirubina | 1.248 (−1.619 to
4.116) | 0.394 | −0.434 (−4.516 to
3.648) | 0.835 |
| NFSa | 0.957 (0.257 to
1.658) | 0.007 | 0.932 (0.083 to
1.781) | 0.031 |
 | Table III.Patient characteristics by NFS. |
Table III.
Patient characteristics by NFS.
| Variables | High NFS (n=21) | Low NFS (n=66) | P-value |
|---|
| Gender
(male/female) | 13/8 | 46/20 | 0.506b |
| Age, years | 73
(47–82)a | 65
(36–80)a | 0.007c |
| Tumor location
(colon/rectum) | 16/5 | 43/23 | 0.346b |
| Histological type
(well/poorly differentiated) | 20/1 | 57/9 | 0.267b |
| Single/multiple
metastasis | 14/7 | 35/31 | 0.272b |
|
Synchronous/metachronous metastasis | 7/14 | 30/36 | 0.328b |
| Number of
cycles | 17
(3–87)a | 12
(1–126)a | 0.042c |
| Use of bevacizumab
(yes/no) | 12/9 | 33/33 | 0.568b |
| Total serum
bilirubine, mg/dl | 0.7
(0.5–1.3)a | 0.6
(0.3–1.5)a | 0.163c |
| NFSe | 0.823 | −1.692 |
<0.001c |
|
| (−0.268 to
2.62)a | (−5.158 to
−0.347)a |
|
| Hematological
toxicityf | 4 | 2 | 0.028d |
|
Decreased platelet count | 1 | 0 |
|
|
Decreased neutrophil
count | 3 | 2 |
|
| Non-hematological
toxicityf | 4 | 9 | 0.546d |
|
Vomiting | 1 | 4 |
|
|
Diarrhea | 1 | 3 |
|
| Nervous
system disorders | 0 | 1 |
|
|
Increased serum bilirubin | 1 | 0 |
|
|
Cough | 1 | 1 |
|
 | Table IV.Uni- and multivariate analysis for
non-hematological toxicity. |
Table IV.
Uni- and multivariate analysis for
non-hematological toxicity.
| Variables | Unadjusted coef
(95% CI) | P-value | Adjusted coef (95%
CI) | P-value |
|---|
| Male gender | −0.326 (−1.547 to
0.895) | 0.600 | −0.745 (−2.109 to
0.619) | 0.284 |
| Age | −0.007 (−0.064 to
0.049) | 0.799 | −0.044 (−0.116 to
0.027) | 0.227 |
| Rectum | 0.326 (−0.895 to
1.547) | 0.600 |
|
|
| Multiple
metastasis | 0.481 (−0.703 to
1.666) | 0.426 | 0.823 (−0.588 to
2.234) | 0.253 |
| Synchronous
metastasis | 0.537 (−0.648 to
1.722) | 0.374 | 1.146 (−0.426 to
2.719) | 0.153 |
| Number of
cycles | −0.094 (−0.186 to
−0.003) | 0.043 | −0.127 (−0.238 to
−0.017) | 0.024 |
| Use of
bevacizumab | −0.262 (−1.444 to
0.920) | 0.664 |
|
|
| Total serum
bilirubina | 0.508 (−1.538 to
2.554) | 0.627 | 1.377 (−1.298 to
4.053) | 0.313 |
| NFSa | 0.053 (−0.313 to
0.419) | 0.776 | 0.405 (−0.101 to
0.910) | 0.117 |
Discussion
Our findings indicate that NFS may be a promising
marker for predicting the hematological adverse events of
chemotherapy including CPT-11 for colorectal cancer. Liver
dysfunction, such as fibrosis, may cause severe CPT-11-related
hematological adverse events by hampering its metabolism. The
metabolism of CPT-11 occurs via a complex cascade, with hydrolysis
of the parent prodrug by hepatic carboxylesterases into
7-ethyl-10-hydroxycamptothecin (SN-38) as an active metabolite,
detoxification of SN-38 into an inactive glucuronidated form
(SN-38G) by UGT1A1, and excretion of SN-38G in the urine and bile
(8). A fraction of SN-38 is also
transported directly via ATP-binding cassette transporters into the
bile and released in the intestinal lumen (9). In the intestine, SN-38 may undergo
glucuronidation by intestinal UGT1A1 or be excreted unchanged in
the feces (10). Thus, reduced
SN-38G formation is closely associated with severe toxicities. As
UGT1A1 is considered to be primarily responsible for the formation
of SN-38G, genetic polymorphisms of UGT1A1, such as UGT 1A1*6 and
UGT 1A1*28, increase CPT-11-related neutropenia (6,11–13).
UGT1A1 is also responsible for the detoxification of bilirubin, and
a correlation between pretreatment bilirubin levels and UGT1A1
polymorphisms has been reported (14,15).
Thus, high levels of total bilirubin in the serum prior to
treatment have been reported to be correlated with neutropenia
(12,13,16,17).
However, this study did not determine the impact of pretreatment
total bilirubin on the adverse effects of chemotherapy including
CPT-11; instead, NFS was found to be a robust predictor of
hematological adverse events. As the enzyme activity of UGT1A1 was
reported to be largely unchanged by liver fibrosis in rats
(18), other mechanisms through
which liver dysfunction may cause CPT-11-related adverse effects
have been suggested. However, CYP3A, one of the most important P450
enzymes, was dysregulated in those rats (19). Considering that bilirubin was not a
significant predictor in our study, liver fibrosis may affect the
metabolism of CPT-11, through mechanisms other than via UGT1A1,
such as CYP3A4 or a bile duct transporter, and result in higher
concentrations of SN-38.
Regarding non-hematological toxicity, the inactive
metabolite SN-38G is secreted into the duodenum and deconjugated
into SN-38 by intestinal bacterial β-glucuronidase, which may cause
diarrhea (20,21). SN-38 is also converted into SN-38G,
catalyzed by the UGT enzyme in the intestine (10). Thus, polymorphisms of UGT1A1 have
been reported to increase not only neutropenia but also delayed
diarrhea (22). NFS was not
significantly associated with non-hematological toxicity in our
findings, even when the investigation was limited to diarrhea, as
liver fibrosis may not affect the activity of UGT or bacterial
β-glucuronidase, at least in the intestine.
Our study revealed that NFS was a significant
predictor of hematological toxicity in chemotherapy including
CPT-11 for CRC. However, while some studies have pointed out the
significance of chemotherapy-related adverse events, such as
hematological toxicity, for the prognosis of colorectal cancer
(23,24), our study observed no correlation
between NFS and prognosis (data not shown). This may explain why
the impact of NFS was limited and why CPT-11 chemotherapy alone
does not determine the prognosis of colorectal cancer patients, nor
do pretreatment serum bilirubin levels or UGT1A1 polymorphisms
(16,25). Further investigation is required.
This study has several limitations. Although the
majority of studies on CPT-11 have included information on UGT1A1
polymorphisms, this was lacking in our medical records, as it is
not routinely investigated at our institution. Another limitation
was weakness of the statistical power of our study to a certain
extent, as this was a retrospective study with a small sample size
and data from a single institution. Thus, a prospective study with
information on UGT1A1 polymorphisms should be conducted.
In conclusion, NFS is a significant predictor of
hematological adverse events in chemotherapy including CPT-11 for
colorectal cancer and its use may represent a non-invasive method
useful for determining regimens or doses of chemotherapy including
CPT-11.
References
|
1
|
Al Knawy B and Shiffman M: Percutaneous
liver biopsy in clinical practice. Liver Int. 27:1166–1173. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ratziu V, Charlotte F, Heurtier A, Gombert
S, Giral P, Bruckert E, Grimaldi A, Capron F and Poynard T; LIDO
Study Group, : Sampling variability of liver biopsy in nonalcoholic
fatty liver disease. Gastroenterology. 128:1898–1906. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McPherson S, Stewart SF, Henderson E, Burt
AD and Day CP: Simple non-invasive fibrosis scoring systems can
reliably exclude advanced fibrosis in patients with non-alcoholic
fatty liver disease. Gut. 59:1265–1269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Angulo P, Bugianesi E, Bjornsson ES,
Charatcharoenwitthaya P, Mills PR, Barrera F, Haflidadottir S, Day
CP and George J: Simple noninvasive systems predict long-term
outcomes of patients with nonalcoholic fatty liver disease.
Gastroenterology. 145:782–789.e4. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoneda M, Imajo K, Eguchi Y, Fujii H,
Sumida Y, Hyogo H, Ono M, Suzuki Y, Kawaguchi T, Aoki N, et al:
Noninvasive scoring systems in patients with nonalcoholic fatty
liver disease with normal alanine aminotransferase levels. J
Gastroenterol. 48:1051–1060. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minami H, Sai K, Saeki M, Saito Y, Ozawa
S, Suzuki K, Kaniwa N, Sawada J, Hamaguchi T, Yamamoto N, et al:
Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic
polymorphisms in Japanese: Roles of UGT1A1*6 and *28. Pharmacogenet
Genomics. 17:497–504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cancer Therapy Evaluation Program (CTEP),
. N.C.I. Adverse Events (CTCAE) Version 4.0. 2009 June 14, 2010
[cited 2009 May 28]. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40
|
|
8
|
Klein CE, Gupta E, Reid JM, Atherton PJ,
Sloan JA, Pitot HC, Ratain MJ and Kastrissios H: Population
pharmacokinetic model for irinotecan and two of its metabolites,
SN-38 and SN-38 glucuronide. Clin Pharmacol Ther. 72:638–647. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iyer L, King CD, Whitington PF, Green MD,
Roy SK, Tephly TR, Coffman BL and Ratain MJ: Genetic predisposition
to the metabolism of irinotecan (CPT-11). Role of uridine
diphosphate glucuronosyltransferase isoform 1A1 in the
glucuronidation of its active metabolite (SN-38) in human liver
microsomes. J Clin Invest. 101:847–854. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirose K, Kozu C, Yamashita K, Maruo E,
Kitamura M, Hasegawa J, Omoda K, Murakami T and Maeda Y:
Correlation between plasma concentration ratios of SN-38
glucuronide and SN-38 and neutropenia induction in patients with
colorectal cancer and wild-type UGT1A1 gene. Oncol Lett. 3:694–698.
2012.PubMed/NCBI
|
|
11
|
Innocenti F, Undevia SD, Iyer L, Chen PX,
Das S, Kocherginsky M, Karrison T, Janisch L, Ramírez J, Rudin CM,
et al: Genetic variants in the UDP-glucuronosyltransferase 1A1 gene
predict the risk of severe neutropenia of irinotecan. J Clin Oncol.
22:1382–1388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Onoue M, Terada T, Kobayashi M, Katsura T,
Matsumoto S, Yanagihara K, Nishimura T, Kanai M, Teramukai S,
Shimizu A, et al: UGT1A1*6 polymorphism is most predictive of
severe neutropenia induced by irinotecan in Japanese cancer
patients. Int J Clin Oncol. 14:136–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramchandani RP, Wang Y, Booth BP, Ibrahim
A, Johnson JR, Rahman A, Mehta M, Innocenti F, Ratain MJ and
Gobburu JV: The role of SN-38 exposure, UGT1A1*28 polymorphism, and
baseline bilirubin level in predicting severe irinotecan toxicity.
J Clin Pharmacol. 47:78–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sai K, Saeki M, Saito Y, Ozawa S, Katori
N, Jinno H, Hasegawa R, Kaniwa N, Sawada J, Komamura K, et al:
UGT1A1 haplotypes associated with reduced glucuronidation and
increased serum bilirubin in irinotecan-administered Japanese
patients with cancer. Clin Pharmacol Ther. 75:501–515. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bosma PJ, Saeki M, Saito Y, Ozawa S,
Katori N, Jinno H, Hasegawa R, Kaniwa N, Sawada J and Komamura K:
Bilirubin UDP-glucuronosyltransferase 1 is the only relevant
bilirubin glucuronidating isoform in man. J Biol Chem.
269:17960–17964. 1994.PubMed/NCBI
|
|
16
|
Meyerhardt JA, Kwok A, Ratain MJ, McGovren
JP and Fuchs CS: Relationship of baseline serum bilirubin to
efficacy and toxicity of single-agent irinotecan in patients with
metastatic colorectal cancer. J Clin Oncol. 22:1439–1446. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raymond E, Boige V, Faivre S, Sanderink
GJ, Rixe O, Vernillet L, Jacques C, Gatineau M, Ducreux M and
Armand JP: Dosage adjustment and pharmacokinetic profile of
irinotecan in cancer patients with hepatic dysfunction. J Clin
Oncol. 20:4303–4312. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hao H, Zhang L, Jiang S, Sun S, Gong P,
Xie Y, Zhou X and Wang G: Thioacetamide intoxication triggers
transcriptional up-regulation but enzyme inactivation of
UDP-glucuronosyltransferases. Drug Metab Dispos. 39:1815–1822.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie Y, Wang G, Wang H, Yao X, Jiang S,
Kang A, Zhou F, Xie T and Hao H: Cytochrome P450 dysregulations in
thioacetamide-induced liver cirrhosis in rats and the counteracting
effects of hepatoprotective agents. Drug Metab Dispos. 40:796–802.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ikuno N, Soda H, Watanabe M and Oka M:
Irinotecan (CPT-11) and characteristic mucosal changes in the mouse
ileum and cecum. J Natl Cancer Inst. 87:1876–1883. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takasuna K, Hagiwara T, Hirohashi M, Kato
M, Nomura M, Nagai E, Yokoi T and Kamataki T: Involvement of
beta-glucuronidase in intestinal microflora in the intestinal
toxicity of the antitumor camptothecin derivative irinotecan
hydrochloride (CPT-11) in rats. Cancer Res. 56:3752–3757.
1996.PubMed/NCBI
|
|
22
|
Li M, Wang Z, Guo J, Liu J, Li C, Liu L,
Shi H, Liu L, Li H, Xie C, et al: Clinical significance of UGT1A1
gene polymorphisms on irinotecan-based regimens as the treatment in
metastatic colorectal cancer. Onco Targets Ther. 7:1653–1661.
2014.PubMed/NCBI
|
|
23
|
Rambach L, Bertaut A, Vincent J, Lorgis V,
Ladoire S and Ghiringhelli F: Prognostic value of
chemotherapy-induced hematological toxicity in metastatic
colorectal cancer patients. World J Gastroenterol. 20:1565–1573.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shitara K, Matsuo K, Takahari D, Yokota T,
Inaba Y, Yamaura H, Sato Y, Najima M, Ura T and Muro K:
Neutropaenia as a prognostic factor in metastatic colorectal cancer
patients undergoing chemotherapy with first-line FOLFOX. Eur J
Cancer. 45:1757–1763. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu CY, Chen PM, Chiou TJ, Liu JH, Lin JK,
Lin TC, Chen WS, Jiang JK, Wang HS and Wang WS: UGT1A1*28
polymorphism predicts irinotecan-induced severe toxicities without
affecting treatment outcome and survival in patients with
metastatic colorectal carcinoma. Cancer. 112:1932–1940. 2008.
View Article : Google Scholar : PubMed/NCBI
|