Introduction
Esophageal cancer (EC) is one of the most common
tumors of the gastrointestinal system. The prognosis of EC patients
is relatively poor, since the majority of patients are diagnosed at
an advanced stage. The most common metastatic sites include the
lungs, liver, and bones (1). The
incidence of brain oligometastasis (oligo-BM) is extremely rare
(1–3% incidence in clinical series) (2,3). The
majority of patients with BM are diagnosed at an advanced clinical
stage, and the majority of BMs tend to occur together with other
organ metastases (2,4,5).
Since brain imaging as part of a metastasis work-up
is not routinely performed, detection of solitary BM at diagnosis
is difficult. However, with the increased use of positron emission
tomography (PET) at initial staging, along with advances in
neuroimaging, the incidence of BM has gradually increased, as
reported in the literature (6,7). Due to
the rarity of BM in patients with EC, there is no standardized
treatment for these patients. The survival time of patients with EC
and metastasis to the brain ranges from 2 weeks to 25 months,
depending on the extent of disease and the treatment modalities
employed (7,8). Previously, it was demonstrated that
patients with one to three BMs had improved survival rates with
advanced treatment modalities (9).
Therefore, an aggressive treatment approach may be effective for
only a limited number of patients with good performance status and
solitary metastasis. However, it is important to make balanced
clinical decisions for treatment planning; specifically, how to
identify patients with oligometastasis who would benefit from
localized therapy. How long localized therapy may be able to extend
life expectancy has yet to be determined for EC patients with BM.
In the present study, the long-term survival of seven EC patients
with isolated synchronous BM treated with definitive
chemoradiotherapy (CRT) of the primary site, and localized
treatments such as surgery, radiotherapy (RT) or radiosurgery of
the BM, is reported.
Patients and methods
Patient population
A retrospective study of the clinical records from
125 patients with EC who had been treated at Baskent University
Faculty of Medicine Department of Radiation Oncology, Adana,
Turkey, between October 2007 and December 2015 was designed. Of
these 125 patients, 10 patients (8%) had BM. Seven of the 10
patients (6%) had solitary BM diagnosed prior to or during
treatment, while three patients (2%) had multiple BMs (two patients
had multiple BMs only, and one patient had BM and lung metastasis).
The seven patients with a solitary BM were chosen for analysis, and
their characteristics are summarized in Table I. In these patients, BM was diagnosed
via PET, or a combination with computed tomography (CT) and
magnetic resonance imaging (MRI). In addition, three patients
received histological confirmation of the condition following
surgical resection of the brain lesion. Written informed consent
was obtained from each patient prior to inclusion of their data in
the present study.
 | Table I.Characteristics of EC patients with
solitary BM. |
Table I.
Characteristics of EC patients with
solitary BM.
| Patient no. | Age, sex | Diagnosis | Stage | Location | Treatment
(esophagus) | Treatment (BM) | Systemic
treatment |
|---|
| 1 | 50, M | Adeno Ca | T4N1 | Distal 1/3 | CRT (54 Gy +
capecitabine) + capecitabine | WBRT | 6 × cisplatin |
| 2 | 48, M | Adeno Ca | T3N1 | Distal 1/3 | CRT (54 Gy +
5-FU) | Surgery + WBRT | 6 × CFF |
| 3 | 48, M | Adeno Ca | T3N1 | Distal 1/3 | CRT (60 Gy+
5-FU) | GK + WBRT | 6 × ECF |
| 4 | 76, M | Adeno Ca | T4N0 | Distal 1/3 | CRT (50.4 Gy +
5-FU) | Surgery + WBRT | 6 × FU-FA |
| 5 | 59, M | SCC | T4N1 | Mid 1/3 | CRT (60 Gy +
cisplatin) | Surgery + WBRT | 6 × CFF |
| 6 | 77, M | Adeno Ca | T3N1 | Distal 1/3 | CRT (54 Gy +
capecitabine) | WBRT | 8 × capecitabine |
| 7 | 69, M | SCC | T3N0 | Proximal 1/3 | RT (60 Gy) | WBRT | 6 × CFF |
Treatment of primary tumors
Aggressive treatment of the primary tumors was
performed in three stages. First, seven patients with solitary BM
were treated with neoadjuvant chemotherapy for treatment of the
primary tumors (Table I). One
patient was diagnosed with BM following the second cycle of
chemotherapy. Three patients received six cycles of cisplatin and
5-fluorouracil (5-FU); one patient received six cycles of
epirubicin, cisplatin, and 5-FU; one patient received six cycles of
cisplatin and capecitabine; one patient received six cycles of 5-FU
and folinic acid; and one patient received eight cycles of
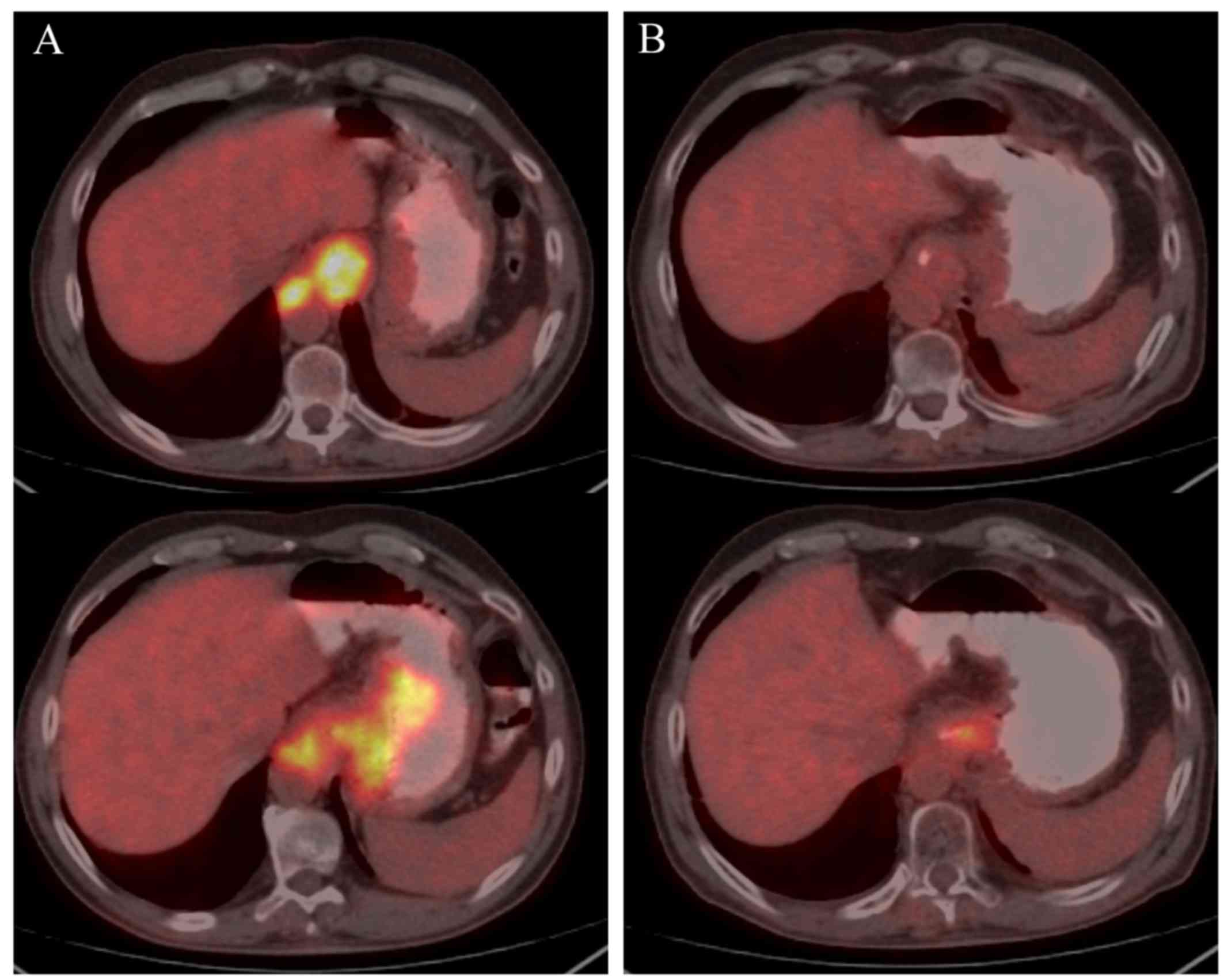
capecitabine. Following completion of the neoadjuvant chemotherapy,
PET-CT was used for restaging and assessing the treatment response
(Fig. 1). The primary tumor was
treated in cases with no progression of the disease following
systemic chemotherapy. In the second course of treatment, all the
patients received definitive CRT with a median RT dose of 50.4 Gy
(range, 50.4–60 Gy) using conventional fractionation with either a
3-dimensional conformal (two patients) or intensity-modulated (five
patients) RT technique. One patient refused additional treatment,
and received palliative care only. Finally, six of the seven
patients underwent a second round of chemotherapy: Three patients
received 5-FU, two patients received capecitabine, and one received
cisplatin concurrently with RT. All the patients tolerated the
treatment well; no serious side-effects were observed during or
after completion of the treatment.
Patients with multiple BMs were treated with
palliative RT delivered to the primary tumor site after whole-brain
RT (WBRT), followed by systemic chemotherapy.
Treatment of BMs
Six of seven patients had solitary BM diagnosed
prior to initiation of any treatment, whereas in one patient, BM
was identified at the end of the second cycle of chemotherapy. Four
patients reported headache and nausea/vomiting at the first
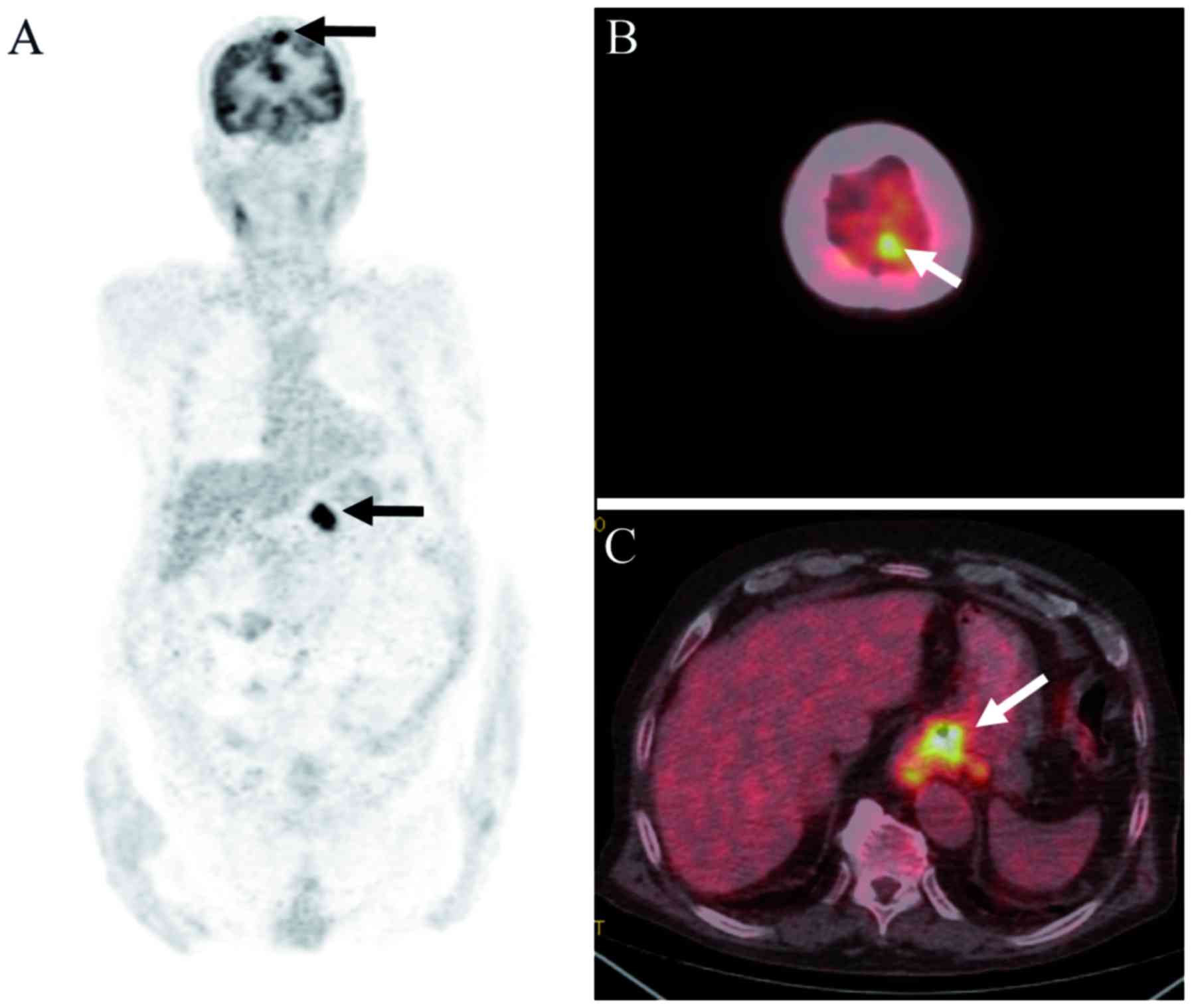
diagnosis, and BM was confirmed using MRI. In three patients, no
neurological symptoms were observed, and BM was identified
incidentally by initial PET-CT (Fig.
2). Although the treatment regimens of EC patients with BM were
determined by the treating oncologists, all the patients underwent
WBRT. Three patients underwent surgical resection followed by WBRT,
and one patient also had Gamma Knife radiosurgery prior to WBRT.
WBRT was administered via a 6 MV linear accelerator in daily
fractions of 3 Gy for a total dose of 30 Gy delivered in 10
fractions. The Gamma Knife radiosurgery dose was 18 Gy, delivered
to the solitary metastasis. All the patients tolerated the
treatment well; only corticosteroids were administered during the
RT, if required.
Results
Patient outcomes for patients with
solitary BM
The median age for the seven patients at diagnosis
was 59 years (range, 48–77 years). All the patients were male, and
six succumbed to mortality resulting from their cancer (Table II). The median survival time of the
patients was 18.9 months (range, 10.0–27.2 months). The median time
to progression after completion of all the treatments was 8 months
(range, 3–9 months). Two patients had tumor progression at the
primary site, and one patient had BM progression. Two patients with
localized progression of the primary tumor also had intra-abdominal
lymph node metastasis, which was observed 3 and 9 months,
respectively, following completion of the treatment. These patients
received systemic chemotherapy: One patient succumbed to the cancer
as a consequence of disease progression, whereas the other patient
remained alive, although with the disease (Table II). The patient who had progression
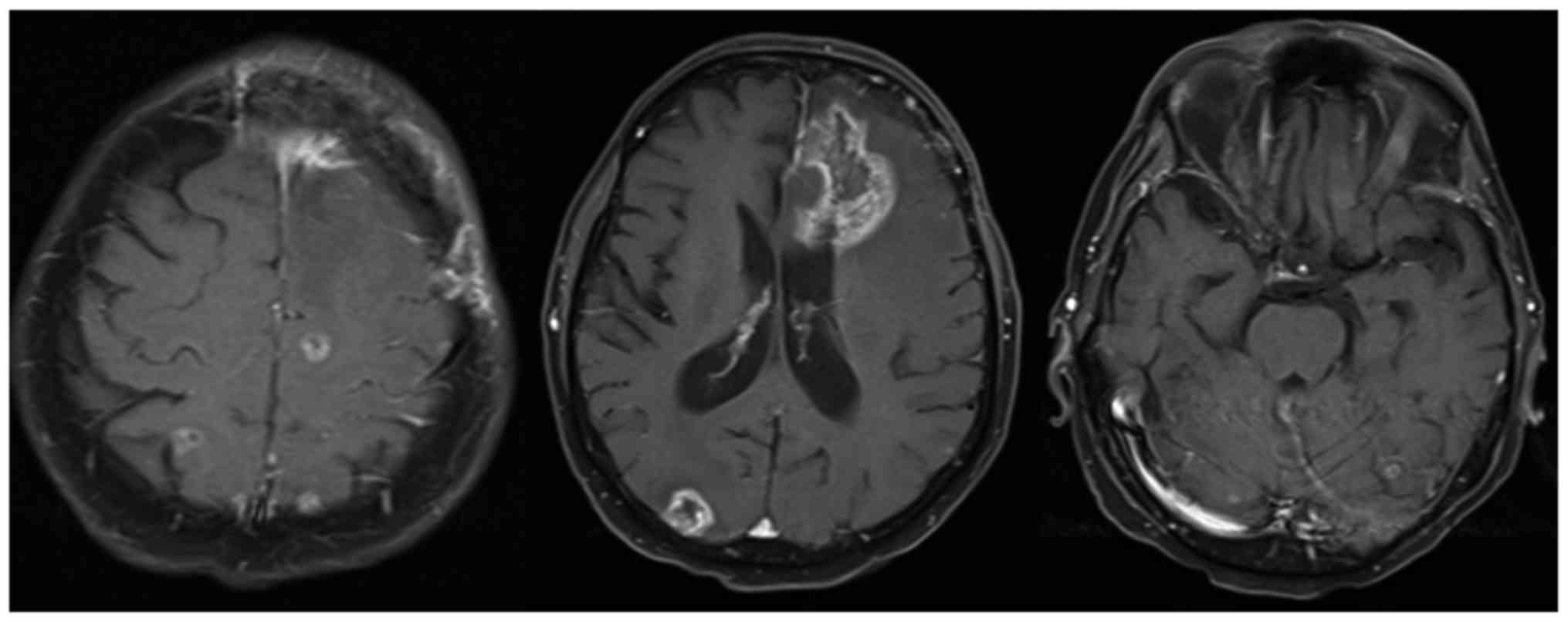
of the BM was treated with excision of the BM, followed by WBRT.
Eight months following completion of the treatment, new metastatic
foci in the brain and lung were identified (Fig. 3). Evidence of systemic progression
was observed at multiple sites, although mostly in the liver and
lungs. Two patients with adenocarcinoma had liver metastasis only
at 5 and 9 months, respectively, after the completion of treatment.
One patient had liver metastasis, together with lung metastasis and
BM 9 months after completion of the treatment. One patient refused
systemic chemotherapy, and succumbed from the disease under
supportive care.
 | Table II.Treatment outcomes for EC patients
with BM. |
Table II.
Treatment outcomes for EC patients
with BM.
| Patient no. | Progression site | Status | Time to progression
(months) | Overall survival time
(months) |
|---|
| 1 | Esophagus,
intra-abdominal ln | DwD | 3 | 10.0 |
| 2 | Lung, brain | DwD | 8 | 20.3 |
| 3 | Liver | DwD | 5 | 20.6 |
| 4 | Liver | DwD | 9 | 27.2 |
| 5 | Lung | DwD | 5 | 13.9 |
| 6 | Esophagus,
intra-abdominal ln | AwD | 9 | 14.5 |
| 7 | Lung, liver,
bones | DwD | 9 | 18.9 |
Neurological symptoms at diagnosis in four patients
were resolved completely after the conclusion of WBRT. Mild to
moderate headache, which was resolved following corticosteroid
administration, was observed during RT. The neurological status of
three patients with BM that had been identified incidentally during
staging work-up did not deteriorate after WBRT.
Outcomes for patients with multiple
BMs
Two patients with multiple BMs, who were provided
palliative treatment, only survived 2.1 and 3.1 months after the
diagnosis. One of these patients only had BM, whereas the other
patient had liver metastasis together with BM.
Six patients were diagnosed with multiple BMs at a
median of 2.5 months (range 0.8–16.2 months) following completion
of the primary tumor treatment. Three of six patients had BM only,
while one patient had local recurrence, one patient had liver
metastasis, and one patient had lung and liver metastasis, together
with multiple BMs. The median survival of patients with BM observed
after completion of the curative treatment of EC was 5.0 months
(range, 1.0–9.6 months).
Discussion
In the present patient series, the efficacy of
treatment of both BM and primary tumor in EC patients with
synchronous solitary BM was investigated, and longer survival times
were observed.
BM from EC is extremely rare, and only limited
information is available. In the literature, the incidence of BM
was 0–2% in clinical studies, and 0–5.1% in autopsy studies
(3,4). Kanemoto et al (10) reported that the crude and 3-year
cumulative incidences of BM in 391 patients with EC following CRT
were 3.1 and 6.6%, respectively. However, in the majority of
studies, the incidence of BM has probably been underestimated
since, in most series, routine brain imaging is not typically part
of the metastatic work-up. In the current study, the incidence of
BM was 6%, which was slightly higher compared with previously
published series (3–6). Although patients with BM were not
routinely evaluated with MRI in the present study, in four patients
with neurological symptoms, cranial CT or MRI was performed to rule
out BM. Furthermore, the majority of patients with EC were
evaluated with 18F-fluorodeoxyglucose (FDG)-PET/CT for
initial staging and RT planning. In three patients, the existence
of BM was demonstrated incidentally with FDG-PET/CT, although these
patients did not have any neurological symptoms. Since it was
reported that the sensitivity of PET-CT for detecting BM in other
cancer types was 70%, and the specificity was very high (11), the higher incidence of BM in the
current study may be connected with higher rates of PET-CT use
compared with previous studies.
There have been several efforts to assess whether
neuro staging is essential for patients with EC. Gabrielsen et
al (8) explored the usefulness
of preoperative cranial CT to investigate occult metastases in a
cohort of 240 patients who underwent esophagectomy. However, none
of the patients were found to have occult metastases by CT. The
authors suggested that a consistent, true incidence of BM from EC
in their material could not be determined exactly by CT, and that
the crude incidence of BM was 3.6% if MRI were to have been used
instead of CT. Studies detecting BM in routine staging are very
rare; the majority of studies involved lung cancer patients
(12,13). In a study by Seute et al
(12), the incidence of BM in 481
patients with small cell lung cancer was 10% with CT, but increased
up to 24% with MRI. Furthermore, in the CT group, all the patients
diagnosed with BM were symptomatic, whereas in the MRI group, 11%
of the patients were symptomatic. These authors concluded that the
estimated incidence of BM increased when MRI was used instead of
CT. Hjorthaug et al (13)
assessed whether PET-CT was suitable for selecting patients for MRI
on suspicion of BM in 596 patients with lung cancer. The
sensitivity, specificity, and positive predictive values were 72,
100, and 97%, respectively, and the authors concluded that PET-CT
may be suitable for selecting patients for MRI in diagnostic
centers that did not perform routine MRI in the pre-therapeutic
staging work-up. The results of the present study also supported
this finding: Higher rates of oligo-BM were detected at the time of
EC diagnosis, which could have been due to the routine use of
FDG-PET/CT for initial staging for the majority of the cases.
The stage of the primary tumor is the most important
risk factor for survival of patients with EC (2). Similarly, BM tended to occur in
patients with advanced clinical disease (4), which was supported by the present
series. In the current study, all the patients had clinical-stage
T3 or T4 disease, and five of seven patients also had clinical
lymph node metastasis. Another risk factor for predicting BM in
patients with EC is mean tumor length. It was previously reported
that primary tumor length in EC patients without BM was shorter
compared with EC patients with BM (4,8). In
addition, treatment modality influences the time to BM development.
Song et al (4) demonstrated
that the median time to BM development was longer in patients
treated with CRT compared with patients treated with chemotherapy
alone (15.2 vs. 7.6 months). These findings indicated that patients
with an extensive clinical stage have a high risk for developing
BM, and treatment of the primary tumor with definitive CRT may
extend the time prior to BM occurrence. In the current study,
another factor contributing towards longer survival time was
connected with successful local treatment with definitive CRT. Only
two patients had local recurrence, together with intra-abdominal
lymph node metastasis.
The prognosis is poor for EC patients with BM, and
only limited data are available concerning treatment approaches.
The median survival time after diagnosis of BM was only 3.8 months,
according to a report by Weinberg et al (5). However, it was reported in certain
series that surgical resection of isolated BM in EC patients, with
or without WBRT, resulted in a 3.8–26.2 month median survival time
(2,5,14). In
the largest series reported by Ogawa et al (2), the mean survival time was 1.8 months in
patients treated with WBRT alone, 3.8 months in patients who
underwent surgical resection of a solitary BM, and 9.6 months in
patients with EC treated with WBRT following surgical resection of
a solitary BM. However, 5 of 36 patients who underwent
postoperative WBRT, and who had no active extracranial disease,
survived >1 year. Weinberg et al (5) demonstrated in a study of 27 patients
that the longest survival time was observed in patients with single
BM who underwent surgery and WBRT (median survival, 9.6 months),
WBRT alone, surgery, surgery and WBRT, or stereotactic radiosurgery
(SRS) alone. Yoshida (14) evaluated
the treatment outcomes of patients with BM treated with WBRT alone,
surgery alone, and postoperative WBRT. The median survival time of
patients with single BM treated with SRS was 38.2 months, compared
with 16.4 months for patients with multiple BMs. The author
concluded that the best outcome was observed in patients treated
with surgical resection followed by RT. Song et al (4) reported a median survival time of 4.2
months for 26 patients with BM (12 with single BM, and 14 with
multiple BMs); the longest survival time was 7 months in patients
who underwent surgery and postoperative RT. In the present series,
although patients treated with postoperative WBRT or SRS and WBRT
survived slightly longer compared with patients treated with WBRT
alone, the progression of BM was observed only in one patient
treated with postoperative WBRT. However, patients with multiple
BMs at diagnosis who were provided palliative treatment only had
very low survival rates, which may have been due to disease
dissemination.
The kinetics of development of micrometastases, and
particularly of oligometastases, was explored after having made
certain assumptions. It is accepted that, beyond a threshold for
the initiation of metastatic spread, which varies widely among
different tumor types, the rate of primary tumor deposits with
metastatic potential increases exponentially (15). Therefore, the delivery of metastatic
clonogens from the primary tumor is accompanied by a similar
exponential growth of each micrometastasis that becomes newly
established at another site. The aim of treatment of
oligometastatic disease has the potential to prevent further
evolution of genetically unstable clones and metastatic spread,
which may potentially improve overall disease control (16,17).
Multiple trials are under way to determine whether localized
treatment of oligometastatic disease has been beneficial in various
types of cancers; however, no data on EC patients with BM are
available in the literature.
The present study had several limitations. First,
this was a retrospective study. Secondly, the patient number was
relatively limited, and therefore it was difficult to draw strong
conclusions. Thirdly, there may have been patient selection bias:
Although patients with single BM were analyzed who had an improved
performance status, in the current study, patients with multiple
BMs had a worse performance status and disseminated disease.
Therefore, it was not possible perform a head-to-head comparison.
Patients with multiple BMs who exhibited a good treatment response
should also be evaluated. Finally, patients who received different
treatment regimens were included in the analysis, which might have
affected treatment outcomes. In the present series, cases were
selected according to an approximately similar treatment strategy,
which was neoadjuvant chemotherapy with treatment of BM at
diagnosis, followed by definitive CRT or RT of the primary tumor
site, even in cases where a good treatment response was
observed.
Nevertheless, the present study has produced a
number of important findings. Improved survival times were observed
in selected patients treated with aggressive systemic and local
treatment modalities. In addition, patients with oligometastasis
are an important population, and the survival time of this
population may be extended with appropriate diagnostic and
treatment modalities.
In conclusion, the present study is, to the best of
our knowledge, the first to demonstrate the efficacy of aggressive
local and systemic treatment of the primary tumor site and BM of
patients with EC. Although the prognosis of EC with BM is
relatively poor, the findings in this study have revealed that the
long-term survival of EC patients with BM may be augmented by
locally ablative treatment of the primary tumor and the BM.
Therefore, in selected patients, a curative approach with effective
concurrent CRT, rather than palliative treatment, may result in
improved local control and prolonged survival rates. With novel
targeted therapeutics and effective adjuvant systemic therapies to
control systemic disease, longer survival rates may be observed in
selected EC patients with BM. Finally, physicians should be aware
of the metastatic status of such patients prior to their deciding
upon a treatment plan.
References
|
1
|
Quint LE, Hepburn LM, Francis IR, Whyte RI
and Orringer MB: Incidence and distribution of distant metastases
from newly diagnosed esophageal carcinoma. Cancer. 76:1120–1125.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ogawa K, Toita T, Sueyama H, Fuwa N,
Kakinohana Y, Kamata M, Adachi G, Saito A, Yoshii Y and Murayama S:
Brain metastases from esophageal carcinoma: natural history,
prognostic factors, and outcome. Cancer. 94:759–764. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Go PH, Klaassen Z, Meadows MC and
Chamberlain RS: Gastrointestinal cancer and brain metastasis: a
rare and ominous sign. Cancer. 117:3630–3640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song Z, Lin B, Shao L and Zhang Y: Brain
metastases from esophageal cancer: clinical review of 26 cases.
World Neurosurg. 81:131–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weinberg JS, Suki D, Hanbali F, Cohen ZR,
Lenzi R and Sawaya R: Metastasis of esophageal carcinoma to the
brain. Cancer. 98:1925–1933. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto T, Kuroda J, Takezaki T,
Shinojima N, Hide T, Makino K, Nakamura H, Yano S, Nishi T and
Kuratsu J: Characteristics of brain metastases from esophageal
carcinoma. Surg Neurol Int. 5:1372014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takeshima H, Kuratsu J, Nishi T, Soyama N,
Miura M, Masumitsu T and Ushio Y: Metastatic brain tumours from
oesophageal carcinoma: neuro-imaging and clinicopathological
characteristics in Japanese patients. Acta Neurochir (Wien).
143:31–35; discussion 35–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gabrielsen TO, Eldevik OP, Orringer MB and
Marshall BL: Esophageal carcinoma metastatic to the brain: clinical
value and cost-effectiveness of routine enhanced head CT before
esophagectomy. AJNR Am J Neuroradiol. 16:1915–1921. 1995.PubMed/NCBI
|
|
9
|
Lutterbach J, Cyron D, Henne K and
Ostertag CB: Radiosurgery followed by planned observation in
patients with one to three brain metastases. Neurosurgery.
52:1066–1073; discussion 35–36 discussion 1073–1074. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanemoto A, Hashimoto T, Harada H, Asakura
H, Ogawa H, Furutani K, Boku N, Nakasu Y and Nishimura T:
Occurrence and clinical features of brain metastasis after
chemoradiotherapy for esophageal carcinoma. J Radiat Res (Tokyo).
52:509–515. 2011. View Article : Google Scholar
|
|
11
|
Harders SW, Madsen HH, Hjorthaug K,
Arveschoug AK, Rasmussen TR, Meldgaard P, Hoejbjerg JA, Pilegaard
HK, Hager H, Rehling M and Rasmussen F: Mediastinal staging in
non-small-cell lung carcinoma: computed tomography versus
F-18-fluorodeoxyglucose positron-emission tomography and computed
tomography. Cancer Imaging. 14:232014.PubMed/NCBI
|
|
12
|
Seute T, Leffers P, ten Velde GP and
Twijnstra A: Detection of brain metastases from small cell lung
cancer: consequences of changing imaging techniques (CT versus
MRI). Cancer. 112:1827–1834. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hjorthaug K, Højbjerg JA, Knap MM, Tietze
A, Haraldsen A, Zacho HD, Kramer SM and Borghammer P: Accuracy of
18F-FDG PET-CT in triaging lung cancer patients with suspected
brain metastases for MRI. Nucl Med Commun. 36:1084–1090. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshida S: Brain metastasis in patients
with esophageal carcinoma. Surg Neurol. 67:288–290. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Withers HR and Lee SP: Modeling growth
kinetics and statistical distribution of oligometastases. Semin
Radiat Oncol. 16:111–119. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Corbin KS, Hellman S and Weichselbaum RR:
Extracranial oligometastases: a subset of metastases curable with
stereotactic radiotherapy. J Clin Oncol. 31:1384–1390. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rastogi S, Gulia S, Bajpai J, Ghosh J and
Gupta S: Oligometastatic breast cancer: a mini review. Indian J Med
Paediatr Oncol. 35:203–206. 2014. View Article : Google Scholar : PubMed/NCBI
|