Introduction
Breast cancer is one of the most common malignant
tumors in women and the leading cause of death among middle-aged
women (1). Breast cancer may be
classified into several subtypes according to its different
etiology, clinicopathological presentation, molecular
characteristics and response to therapy. Molecular biological
methods are considered to be the gold standard for their accuracy
in diagnosis due to the nature of the specific biological
characteristic of the different breast cancer subtypes (2). In general, estrogen receptor (ER),
progesterone receptor (PR) and human epidermal growth factor
receptor 2 (HER2) are used as markers for invasive breast cancer
classification in order to predict the outcome and select the
optimal therapeutic strategies for the management of breast cancer
(3).
With the development of molecular biology technology
in recent years, an increasing number of molecular markers may be
selected to classify the subtypes of invasive breast cancer. Due to
the complexity, cost and lack of uniform standards of gene chip
technology, it is difficult to apply in clinical practice. In
clinical practice, immunohistochemistry for determining the ER, PR
and HER2 status has been used to classify breast cancer into four
subtypes, namely luminal A, luminal B, HER2-overexpressing and
triple-negative breast cancer (TNBC) (4).
TNBC is a subtype of breast cancer, in which the
expression of all three molecular markers is negative, and accounts
for 10–20% of all breast cancers. TNBC has a higher mortality and
risk of metastasis compared with other subtypes of breast cancer
(5,6). In addition, it is highly invasive and
is associated with a high local recurrence risk, and poor
cancer-specific and disease-free survival (7–9).
The reasons for the low survival rate of TNBC are
mainly as follows: First, compared with other subtypes of breast
cancer, TNBC is associated with a higher rate of chromosomal
mutations, high mitotic count, mutation of the p53 and BRCA1 genes,
and lymphatic dissemination (10,11).
Second, TNBC cannot be accurately diagnosed using conventional
imaging examinations and is often only detected at an advanced
stage (12). Third, therapies such
as hormone therapy, targeted therapy and chemotherapy have not been
shown to be effective (12).
Previous studies have demonstrated that there is a
strong association between TNBC and race. TNBC accounts for 12–17%
of all breast cancers in Western populations (13), whereas its incidence is higher (≤50%)
in African-American women. The incidence in Asian women is similar
to that in Caucasian women, and BRCA1 mutations are more common in
these patients (11,14). Previous findings have also
demonstrated that obese women are at a greater risk of developing
TNBC compared with non-obese women (15). The use of oral contraceptives (OCs)
has been identified as an important factor in the development of
breast cancer (16), but there are
currently no studies clearly indicating an association between OCs
and TNBC. Moreover, the established risk factors for breast cancer
as a whole may not apply to this unique subgroup of patients.
The aim of the present systematic review and
meta-analysis was to focus on case-control studies and elucidate
the strength of the association between OCs and TNBC, enabling a
better understanding of TNBC, helping clinicians integrate
biological and epidemiological characteristics, providing a
theoretical basis and more information for epidemiology researchers
focusing on the etiology, prevention and treatment of TNBC.
Materials and methods
Literature search and criteria for
eligible studies
The PubMed Central/PubMed and Web of Science
databases were systematically searched between January, 2005 and
March, 2016 using the search terms (triple-negative breast cancer
OR basal-like) AND (oral contraceptives). In addition, the
reference lists of the studies were manually searched for related
articles. Two authors (L.L. and Y.Z.) screened the research results
independently according to priorly established inclusion
criteria.
Based on the history of OC use, patients were
classified as non-OC users or OC users. If a patient had used OCs
for >1 year, she was classified as an OC user.
Case-control studies reporting the status of ER, PR
and HER2 as detected by immunohistochemistry or molecular biology
methods were included in the final analysis. Additional molecular
makers (cytokeratin 5/6, epidermal growth factor receptor) were
analyzed by certain studies, but were not used in the present
analysis. TNBCs, characterized by absence of expression of all
three markers, were assigned to the case arm. Patients who
expressed one or more of the abovementioned markers were assigned
to the control arm. When a healthy population was used in the
articles, it was assigned to another control arm. Therefore, the
control group included other subtypes of breast cancer patients or
healthy individuals for different groups of analysis.
The following inclusion criteria were established to
minimize bias or heterogeneity in the selection of studies: i)
Full-text articles published between January, 2005 and March, 2016;
ii) case-control studies; iii) data on the correlation between OC
use and TNBC; iv) original articles written in English; v)
diagnosis of TNBC implemented by immunohistochemistry stain or
molecular biology methods; vi) original data included in the
articles were sufficient to calculate the odds ratio (OR) and 95%
confidence interval (CI); vii) at least 20 patients included in the
case group; and viii) if there was an overlap in the cases
included, only the latest and most comprehensive data were
selected. Any disagreements were resolved through discussion.
Data extraction and quality
evaluation
The following information was independently
extracted from the original studies by two reviewers: Name of the
first author, year of publication, research location, histological
type of the tumors, sample size of the case and control groups, and
correction factor. In order to guarantee the methodological quality
of the included studies, the literatures were required to meet the
following basic criteria: The diagnosis of TNBC should be
definitive based on immunohistochemistry or molecular biological
methods and the major confounding factors should be specified and
analyzed. Two reviewers independently assessed the methodological
quality of the included studies according to the Newcastle-Ottawa
scale. Any disagreements were arbitrated by discussion and majority
voting of all authors.
Data synthesis and statistical
analysis
In order to analyze the association between OC use
and TNBC, the pooled ORs and 95% CIs were calculated by the
original data in the studies. The results indicated that OCs may
cause TNBC with a high probability if OR >1, compared with
non-use of OCs. Heterogeneity among studies was evaluated using the
Chi-squared test and I2 statistic. If the P-value of the
Chi-squared test was <0.1, the heterogeneity was considered to
be significant (17). The
I2 test may be quantified in the evaluation of study
heterogeneity (the greater the I2, the greater the
heterogeneity among studies). Under normal conditions, if the
I2 value was <50%, the heterogeneity was not
considered to be significant. Heterogeneity may be decreased by the
following methods: i) Subgroup analysis; and ii) sensitivity
analysis of the test results of potential bias. When the
Chi-squared test and the I2 statistic indicate that
heterogeneity is not significant, the pooled OR value may be
calculated using the Mantel-Haenszel fixed-effects model;
otherwise, the DerSimonian-Laird random-effects model is adopted
(14). A funnel plot was used to
evaluate the potential publication bias. Data synthesis and
statistical analysis were performed using RevMan v.5.3 software
(International Cochrane Collaboration Network, Copenhagen, Denmark)
and the CI was set to 95%.
Results
Study selection
A total of 392 articles were initially identified
from the database search, and 11 case-control studies met the
inclusion criteria and were included in the final meta-analysis.
Ultimately, 9 studies were included in the group using other
subtypes of breast cancer as the control arm, and 7 studies were
included in the quantitative analysis using the healthy population
as the control arm. A total of 15,427 patients participated and
3,279 were diagnosed with TNBC. The characteristics and original
data of the studies included in the case-control analysis are
summarized in Table I.
 | Table I.Characteristics of the studies
included in the meta-analysis. |
Table I.
Characteristics of the studies
included in the meta-analysis.
| Authors | Study year | Data source | Control types | Refs. |
|---|
| Beaber et
al | 2004–2010 | United States | H | (18) |
| Bethea et
al | 1993–2001, 1995–2015,
2003–2015 | BWHS, CBCS, WCHS | H | (19) |
| Dolle et
al | 1983–1990,
1990–1992 | SEER | H, P | (20) |
| Lee et al | 1998–2003 | WHI | P | (21) |
| Kabat et
al | 1993–1998,
1998–2005 | WHI | H, P | (22) |
| Kwan et
al | 1997–2000,
2006–2009 | LACE | P | (23) |
| Ma et al | 1994–1998 | United States | H, P | (24) |
| Kawai et
al | 2004–2010 | CSS | H, P | (25) |
| Lara-Medina et
al | 1998–2008 | Hispanic | P | (26) |
| Millikan et
al | 1993–1996,
1996–2001 | CBCS | P | (27) |
| Phipps et
al | 1993–1998 | WHI | H, P | (28) |
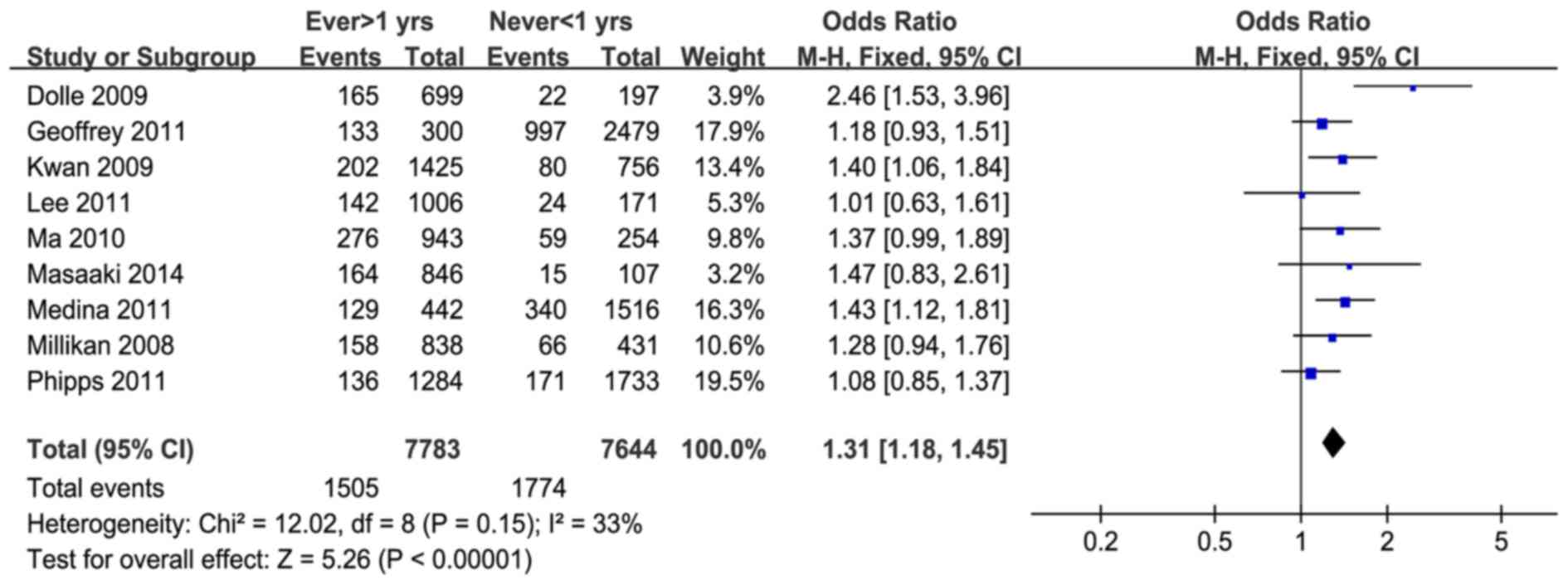
When all the data were synthesized, no significant
heterogeneity was detected. For the studies using other subtypes of
breast cancer as the control arm, heterogeneity was not significant
(P=0.15, I2=33%). These results demonstrated that OC use
increased the risk of TNBC among these women (pooled OR = 1.31, 95%
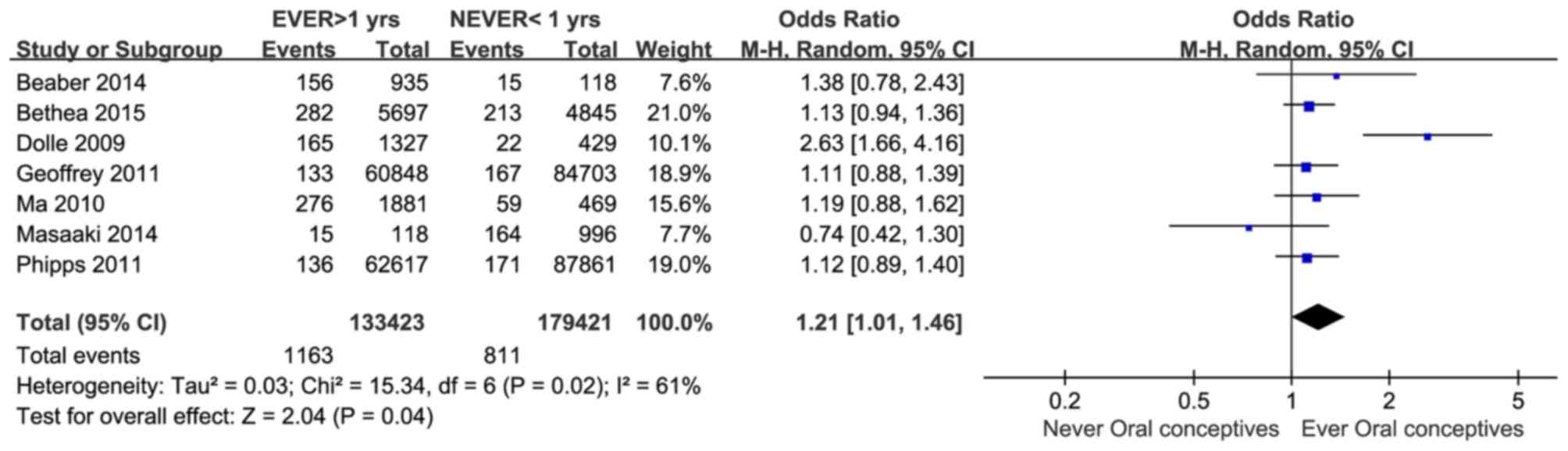
CI = 1.18–1.45; Z=5.26, P<0.00001) (Fig. 1). Using data from healthy individuals
as the control arm, the association between OC use and TNBC was
further confirmed (OR = 1.21, 95% CI = 1.01–1.46; Z=2.04, P=0.04)
(Fig. 2).
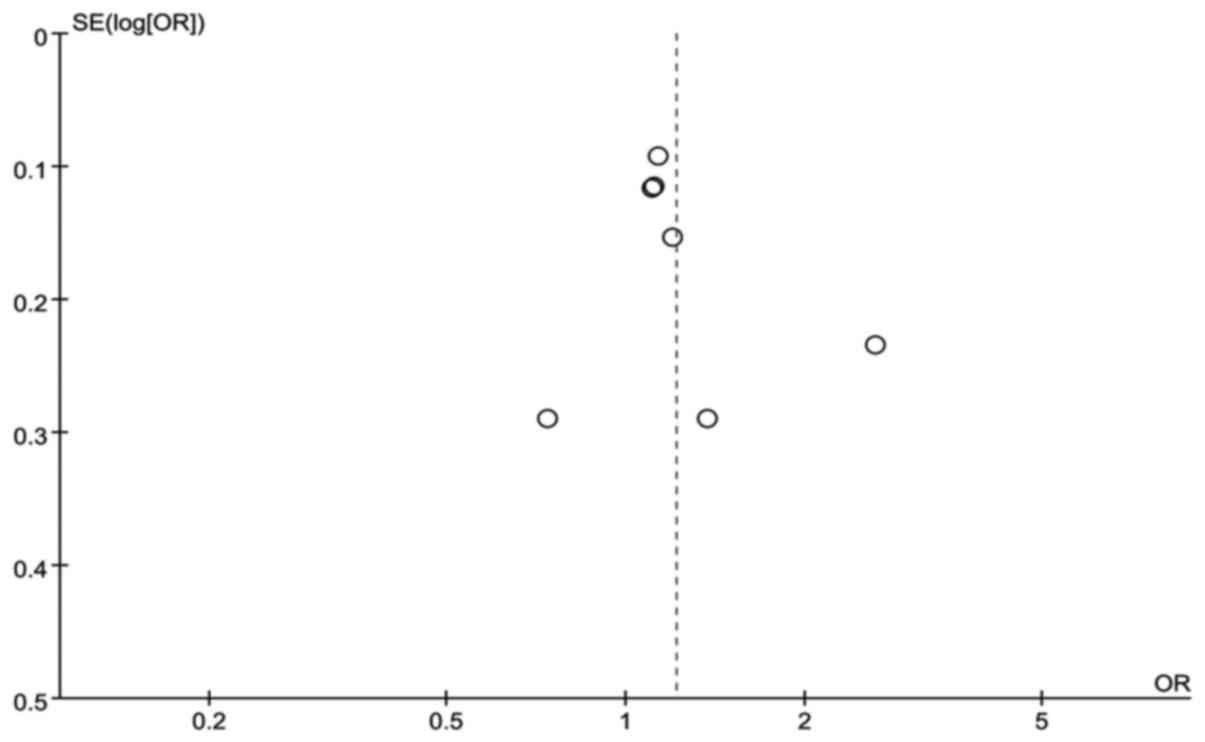
Publication bias was assessed by funnel plots
(Figs. 3 and 4); the data were distributed in the left
and right parts of the graph, and there was no obvious asymmetry.
This demonstrated that publication bias was not significant, and
the study heterogeneity was unlikely due to publication bias.
Discussion
Previous studies indicated that the use of OCs may
increase the risk of TNBC; however, the conclusions of these
studies were conflicting. Indeed, Dolle et al (20), Beaber et al (18) and Kwan et al (23) reported that OC use increases the risk
of TNBC; however, Marchbanks et al (29), Kawai et al (25) and Kabat et al (22) did not confirm this association.
The onset of TNBC is affected by multiple factors.
The results of this analysis demonstrated that OC use increases the
risk of TNBC. This result suggested that i) the pathological
mechanism underlying OC use increasing the risk of TNBC requires
further investigation; and ii) women who use OCs should be more
closely followed regarding TNBC screening and prevention. It was
previously demonstrated that TNBC was associated with family
history (30,31), race (32,33),
obesity (15,34) and menopausal status (35). These conclusions indicated that TNBC
exhibits a certain association with hormone status. OCs are likely
to exert a certain effect on hormone levels; thus, it is reasonable
to infer that OC use is a likely risk factor for TNBC, with several
hypotheses explaining this phenomenon. One of the mechanisms
through which OC use affects breast cancer in younger women is the
combination of estrogen and ER positivity in the mammary gland
developing cancer, which promotes tumor growth and angiogenesis.
The second mechanism is that estrogen increases the density of
vascular distribution and recruitment of stromal cells
systematically, which promotes tumor growth in ER− as
well as ER+ cases (36).
The second mechanism may play a leading role in breast
carcinogenesis.
The present meta-analysis analyzed the strength of
association between OC use and TNBC, compared with non-TNBC cases
or the healthy population. The non-TNBC control group demonstrated
that OC use may cause an increase in the risk of TNBC, and the
healthy population further confirmed this conclusion.
The present systematic review and meta-analysis had
several limitations. First, the number of TNBC cases was relatively
small and the history of OC use was dichotomous. This
classification is relatively crude, and cannot explain the
association between exposure dose of OCs and TNBC. Second, the use
of hormone replacement therapy was not controlled in this analysis.
Therefore, the population under investigation may have obvious
endocrinology heterogeneity. In order to obtain a more accurate
strength of association between TNBC and OC use, this analysis
requires more studies to appropriately control the abovementioned
potential confounding factors. In addition, the study classified
the use of OCs into never or ever users, whereas more detailed
information such as types or dose of OCs was not taken into
consideration. Because of the relatively limited number of studies
and heterogeneous information obtained by different analyses,
information on confounding factor control and heterogeneity are
difficult to obtain.
Even with these limitations, the present study
provides a new perspective on the association between TNBC and
physiological and pathological conditions. In order to ensure the
accuracy of the analysis, a funnel plot was used to analyze the
publication bias of the included studies, with the results showing
that publication bias was not significant.
The identification of risk factors for TNBC may
provide a reference for the screening and prevention of the target
population. Overall, the results of this meta-analysis indicate
that women who use OCs are more likely to develop TNBC compared
with women who do not. Women who use OCs, particularly combined
with other risk factors of TNBC, should be followed more closely.
In addition, the mechanism underlying the increased risk of breast
cancer, such as TNBC, also requires further investigation.
The results of the present study should draw more
attention to the disease screening and follow-up of TNBC patients
and the use of OCs, particularly as risk factors of TNBC.
Acknowledgements
The present study was supported by a grant from the
Outstanding Scientific Fund of Wuhan University (no.
2042014kf0162).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rakha EA and Ellis IO:
Triple-negative/basal-like breast cancer: review. Pathology.
41:40–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huo D, Ikpatt F, Khramtsov A, Dangou JM,
Nanda R, Dignam J, Zhang B, Grushko T, Zhang C, Oluwasola O, et al:
Population differences in breast cancer: survey in indigenous
African women reveals over-representation of triple-negative breast
cancer. J Clin Oncol. 27:4515–4521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lv M, Li B, Li Y, Mao X, Yao F and Jin F:
Predictive role of molecular subtypes in response to neoadjuvant
chemotherapy in breast cancer patients in Northeast China. Asian
Pac J Cancer Prev. 12:2411–2417. 2011.PubMed/NCBI
|
|
5
|
Dawood S, Lei X, Litton JK, Buchholz TA,
Hortobagyi GN and Gonzalez-Angulo AM: Impact of body mass index on
survival outcome among women with early stage triple-negative
breast cancer. Clin Breast Cancer. 12:364–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Giorgi U, Rosti G, Frassineti L, Kopf
B, Giovannini N, Zumaglini F and Marangolo M: High-dose
chemotherapy for triple negative breast cancer. Ann Oncol.
18:202–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brady-West DC and McGrowder DA: Triple
negative breast cancer: therapeutic and prognostic implications.
Asian Pac J Cancer Prev. 12:2139–2143. 2011.PubMed/NCBI
|
|
8
|
Choi J, Jung WH and Koo JS:
Clinicopathologic features of molecular subtypes of triple negative
breast cancer based on immunohistochemical markers. Histol
Histopathol. 27:1481–1493. 2012.PubMed/NCBI
|
|
9
|
Vaklavas C and Forero-Torres A: How do I
treat ‘triple-negative’ disease. Curr Treat Options Oncol.
12:369–388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Ruijter TC, Veeck J, De Hoon JP, van
Engeland M and Tjan-Heijnen VC: Characteristics of triple-negative
breast cancer. J Cancer Res Clin Oncol. 137:183–192. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Irvin WJ Jr and Carey LA: What is
triple-negative breast cancer? Eur J Cancer. 44:2799–2805. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brenton JD, Carey LA, Ahmed AA and Caldas
C: Molecular classification and molecular forecasting of breast
cancer: ready for clinical application? J Clin Oncol. 23:7350–7360.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pierobon M and Frankenfeld CL: Obesity as
a risk factor for triple-negative breast cancers: a systematic
review and meta-analysis. Breast Cancer Res Treat. 137:307–314.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosenberg L, Zhang Y, Coogan PF, Strom BL
and Palmer JR: A case-control study of oral contraceptive use and
incident breast cancer. Am J Epidemiol. 169:473–479. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sutton AJ, Abrams KR, Jones DR, Sheldon TA
and Song F: Methods for meta-analysis in medical researchStatistics
in Medicine. Schwarzer and Guido: 22. John Wiley & Sons, Inc.;
pp. 3112–3114. 2003, View
Article : Google Scholar
|
|
18
|
Beaber EF, Malone KE, Tang MT, Barlow WE,
Porter PL, Daling JR and Li CI: Oral contraceptives and breast
cancer risk overall and by molecular subtype among young women.
Cancer Epidemiol Biomarkers Prev. 23:755–764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bethea TN, Rosenberg L, Hong CC, Troester
MA, Lunetta KL, Bandera EV, Schedin P, Kolonel LN, Olshan AF,
Ambrosone CB and Palmer JR: A case-control analysis of oral
contraceptive use and breast cancer subtypes in the African
American Breast Cancer Epidemiology and Risk Consortium. Breast
Cancer Res. 17:222015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dolle JM, Daling JR, White E, Brinton LA,
Doody DR, Porter PL and Malone KE: Risk factors for triple-negative
breast cancer in women under the age of 45 years. Cancer Epidemiol
Biomarkers Prev. 18:1157–1166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee E, McKean-Cowdin R, Ma H, Spicer DV,
Van Den Berg D, Bernstein L and Ursin G: Characteristics of
triple-negative breast cancer in patients with a BRCA1 mutation:
results from a population-based study of young women. J Clin Oncol.
29:4373–4380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kabat GC, Kim M, Phipps AI, Li CI, Messina
CR, Wactawski-Wende J, Kuller L, Simon MS, Yasmeen S,
Wassertheil-Smoller S and Rohan TE: Smoking and alcohol consumption
in relation to risk of triple-negative breast cancer in a cohort of
postmenopausal women. Cancer Causes Control. 22:775–783. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwan ML, Kushi LH, Weltzien E, Maring B,
Kutner SE, Fulton RS, Lee MM, Ambrosone CB and Caan BJ:
Epidemiology of breast cancer subtypes in two prospective cohort
studies of breast cancer survivors. Breast Cancer Res. 11:R312009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma H, Wang Y, Sullivan-Halley J, Weiss L,
Marchbanks PA, Spirtas R, Ursin G, Burkman RT, Simon MS, Malone KE,
et al: Use of four biomarkers to evaluate the risk of breast cancer
subtypes in the womens contraceptive and reproductive experiences
study. Cancer Res. 70:575–587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawai M, Malone KE, Tang MTC and Li CI:
Active smoking and the risk of estrogen receptor-positive and
triple-negative breast cancer among women ages 20 to 44 years.
Cancer. 120:1026–1034. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lara-Medina F, Pérez-Sánchez V,
Saavedra-Pérez D, Blake-Cerda M, Arce C, Motola-Kuba D,
Villarreal-Garza C, González-Angulo AM, Bargalló E, Aguilar JL, et
al: Triple-negative breast cancer in Hispanic patients: high
prevalence, poor prognosis, and association with menopausal status,
body mass index, and parity. Cancer. 117:3658–3669. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Millikan RC, Newman B, Tse CK, Moorman PG,
Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT,
et al: Epidemiology of basal-like breast cancer. Breast Cancer Res
Treat. 109:123–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Phipps AI, Chlebowski RT, Prentice R,
McTiernan A, Wactawski-Wende J, Kuller LH, Adams-Campbell LL, Lane
D, Stefanick ML, Vitolins M, et al: Reproductive history and oral
contraceptive use in relation to risk of triple-negative breast
cancer. J Natl Cancer Inst. 103:470–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marchbanks PA, Curtis KM, Mandel MG,
Wilson HG, Jeng G, Folger SG, McDonald JA, Daling JR, Bernstein L,
Malone KE, et al: Oral contraceptive formulation and risk of breast
cancer. Contraception. 85:342–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tariq K and Rana F: TNBC vs. non-TNBC: a
five-year retrospective review of differences in mean age, family
history, smoking history and stage at diagnosis at an inner city
university program. World J Oncol. 4:241–247. 2013.
|
|
31
|
Phipps AI, Buist DSM, Malone KE, Barlow
WE, Porter PL, Kerlikowske K and Li CI: Family history of breast
cancer in first-degree relatives and triple-negative breast cancer
risk. Breast Cancer Res Treat. 126:671–678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rosenberg L, Boggs DA, Wise LA,
Adams-Campbell LL and Palmer JR: Oral contraceptive use and
estrogen/progesterone receptor-negative breast cancer among African
American women. Cancer Epidemiol Biomarkers Prev. 19:2073–2079.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trivers KF, Lund MJ, Porter PL, Liff JM,
Flagg EW, Coates RJ and Eley JW: The epidemiology of
triple-negative breast cancer, including race. Cancer Causes
Control. 20:1071–1082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Phipps AI, Malone KE, Porter PL, Daling JR
and Li CI: Body size and risk of luminal, HER2-overexpressing, and
triple-negative breast cancer in postmenopausal women. Cancer
Epidemiol Biomarkers Prev. 17:2078–2086. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rose DP and Vona-Davis L: Interaction
between menopausal status and obesity in affecting breast cancer
risk. Maturitas. 66:33–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gupta PB, Proia D, Cingoz O, Weremowicz J,
Naber SP, Weinberg RA and Kuperwasser C: Systemic stromal effects
of estrogen promote the growth of estrogen receptor-negative
cancers. Cancer Res. 67:2062–2071. 2007. View Article : Google Scholar : PubMed/NCBI
|