Introduction
The definition of synchronous bilateral breast
carcinomas (BBC) varies in the literature. Some investigators
regard tumors in both breasts as synchronous if they are diagnosed
within an interval of 12 months, whereas others regard them as
synchronous if they occur within 6 or 3 months (1–3). From a
biological point of view, 12 months is considered the most
reasonable time period (4,5). The incidence of synchronous BBCs has
increased with the use of magnetic resonance imaging screening of
the contralateral breast in women with newly diagnosed breast
cancer (6). A total of 30% of BBCs
occur synchronously, which constitutes <2% of all breast cancers
(7). Breast cancer patients have a
2- to 6-fold higher risk for developing contralateral breast cancer
compared to the risk of developing breast cancer for women in the
general population (8,9). Risk factors for BBC include young age,
family history (e.g., BRCA1/2 germline mutations), lobular
type of cancer, and multicentric tumors (8,10–12).
Before a diagnosis of BBC can be established,
contralateral metastatic spread has to be excluded. Synchronous and
metachronous BBC with highly concordant genetic profiles strongly
suggest contralateral metastasis (13). On the other hand, presence of a
carcinoma in situ component in an invasive cancer suggests a
primary tumor. The distinction between BBC and breast-to-breast
metastasis is important and forms the basis for the choice of
therapy and ultimately also for patient outcome. Recent studies
using genome-wide genomic profiling methods have facilitated the
molecular characterization of synchronous and metachronous BBCs and
have made it possible to rule out whether they are separate tumors
or have a common origin. Thus far, available data indicate that the
majority of BBC evolve independently and have distinct genotypes
(13,14).
In the present study, we describe a unique case of
synchronous BBC in a patient with an invasive lobular carcinoma
(ILC) of solid type in the right breast and an adenoid cystic
carcinoma (ACC) in the left breast. Genomic profiling revealed that
the tumors had few but distinctly different genomic imbalances and
that only the ACC expressed the MYB-NFIB gene fusion. These
observations are consistent with an independent origin of the two
tumors.
Case report
Clinical history
The patient was a 59-year-old previously healthy
woman with no known family history of breast or ovarian cancer. In
January 2015, she had a routine mammography screening at which
bilateral breast tumors were detected. Fine needle aspiration
cytology (FNAC) of the right breast lesion confirmed the presence
of an adenocarcinoma, whereas the FNAC of the left breast lesion
was inconclusive and showed only epithelial atypia. A subsequent
core biopsy of the left lesion confirmed the presence of an
invasive carcinoma, possibly an ACC. Both tumors were located
cranially close to the mamilla. In March 2015, bilateral partial
mastectomies were performed combined with bilateral axillary
sentinel node biopsies. Both tumors were clinically staged as
T2N0M0, anatomic stage/prognostic group IIA. The patient was
subsequently subjected to a multi-disciplinary conference for
post-operative oncologic adjuvant treatment. She received
post-operative, bilateral radiotherapy of the mammary glands with
2.66 Gy/fraction in 16 fractions, and endocrine treatment with an
aromatase inhibitor (1 mg/day of anastrozole during a 5-year
period).
Two years after diagnosis, the patient was
relapse-free with no clinical, mammographic or ultra-sound evidence
of disease in the mammary glands. In June 2016, FNAC of both
breasts revealed normal breast tissues without signs of cancer. The
study was approved by the Local Scientific Ethics Committee in
Gothenburg (Dnr: 287-15). The requirement for informed consent was
waived by the ethical committee since the patient material was
stripped from direct subject identifiers.
Histopathological and
immunohistochemical findings
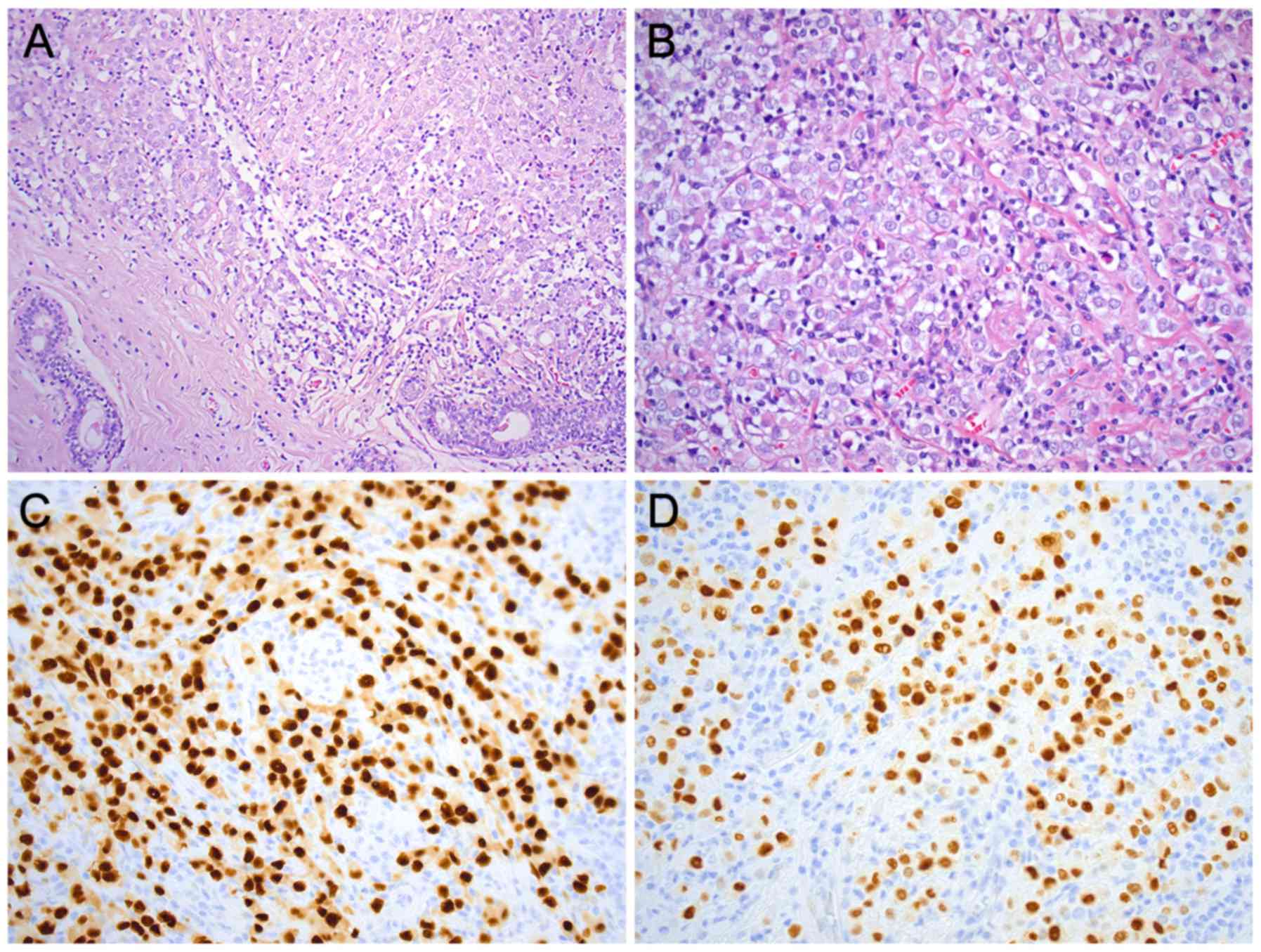
Microscopic examination of the 26-mm large lesion in
the right breast revealed an ILC of solid type, grade 2 (BRE-score
7: tubulus formation 3, nuclear pleomorphism 3, and mitotic
activity 1) (Fig. 1A and B).
Immunohistochemically, the tumor was negative for E-cadherin and
positive for estrogen (95%) and progesterone (80%) receptors
(Fig. 1C and D). The Ki-67 index was
20% and HercepTest was negative. No metastases were found in the
sentinel node from the right axilla.
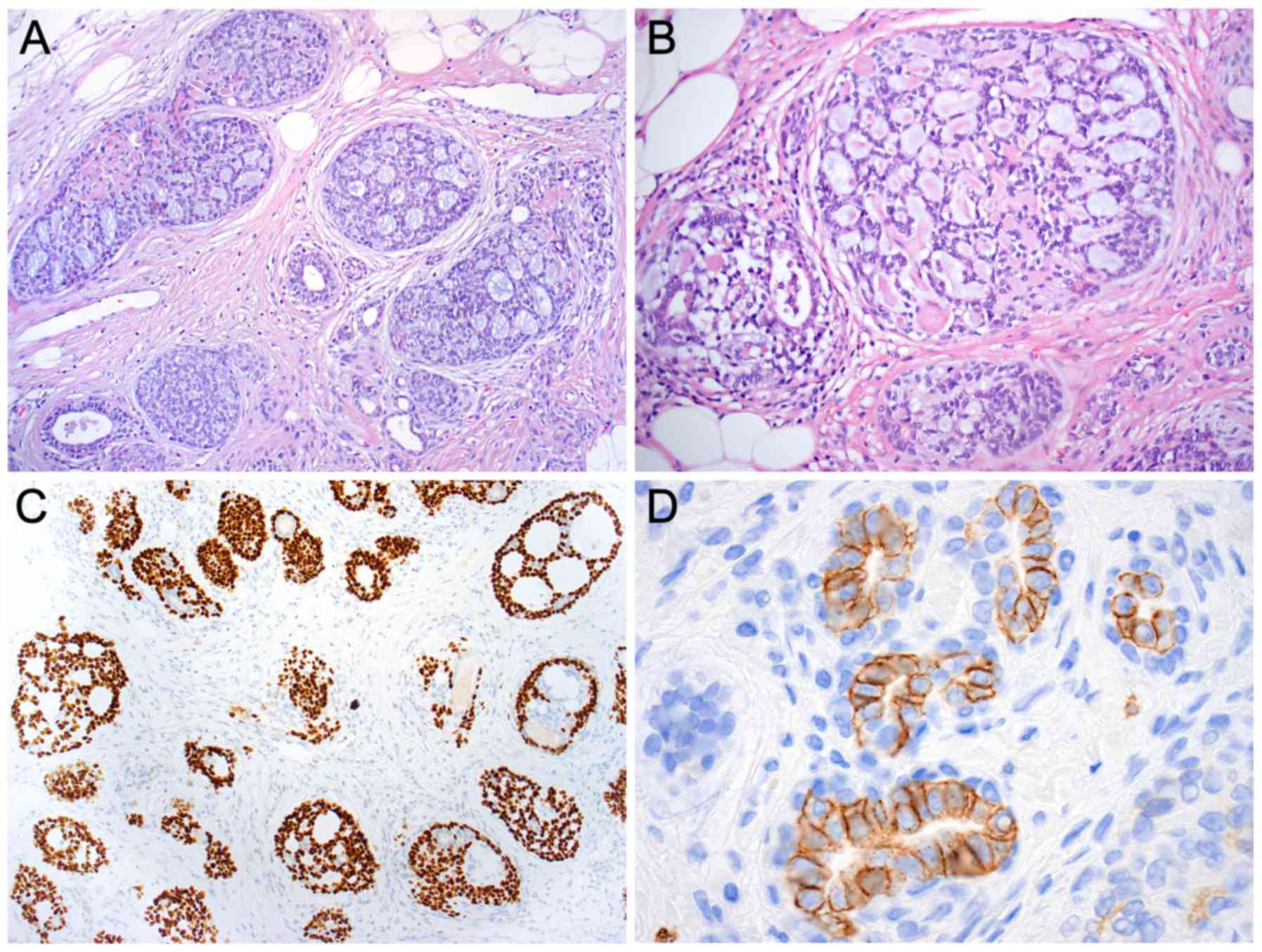
Microscopic examination of the lesion in the left
breast revealed an uncommon type of breast cancer, i.e., an ACC
measuring 23 mm at its largest diameter. Histologically, the tumor
was composed of epithelial, basaloid, and myoepithelial cells
forming typical tubular and cribriform structures (Fig. 2A and B). Combined Alcian blue-PAS
staining showed clear blue-stained mucin in the luminal spaces and
there was eosinophilic material in the pseudolumina.
Immunohistochemically, the tumor was triple negative (estrogen and
progesterone receptors and HercepTest were negative) and had a low
Ki-67 proliferation index (10%). The tumor was positive for
E-cadherin, p63 (Fig. 2C), α-SMA,
CD10, and KIT (CD117) (Fig. 2D).
Analysis of the sentinel node from the left axilla showed no signs
of metastases.
Genomic profiles of the ACC and
ILC
Genome-wide array-based comparative genomic
hybridization (arrayCGH) analysis of DNAs isolated from the
formalin-fixed paraffin-embedded (FFPE) blocks of the ACC and ILC
lesions (containing >75% tumor cells) was performed with the
Human Genome CGH Microarray 244K oligonucleotide arrays (G4411B;
Agilent Technologies, Palo Alto, CA, USA) as previously described
(15,16). Data analysis was performed with the
Nexus Copy Number software version 8.0 (BioDiscovery Inc., El
Segundo, CA, USA). Regions partially or completely covered by a
previously reported copy number variation were excluded from the
analysis.
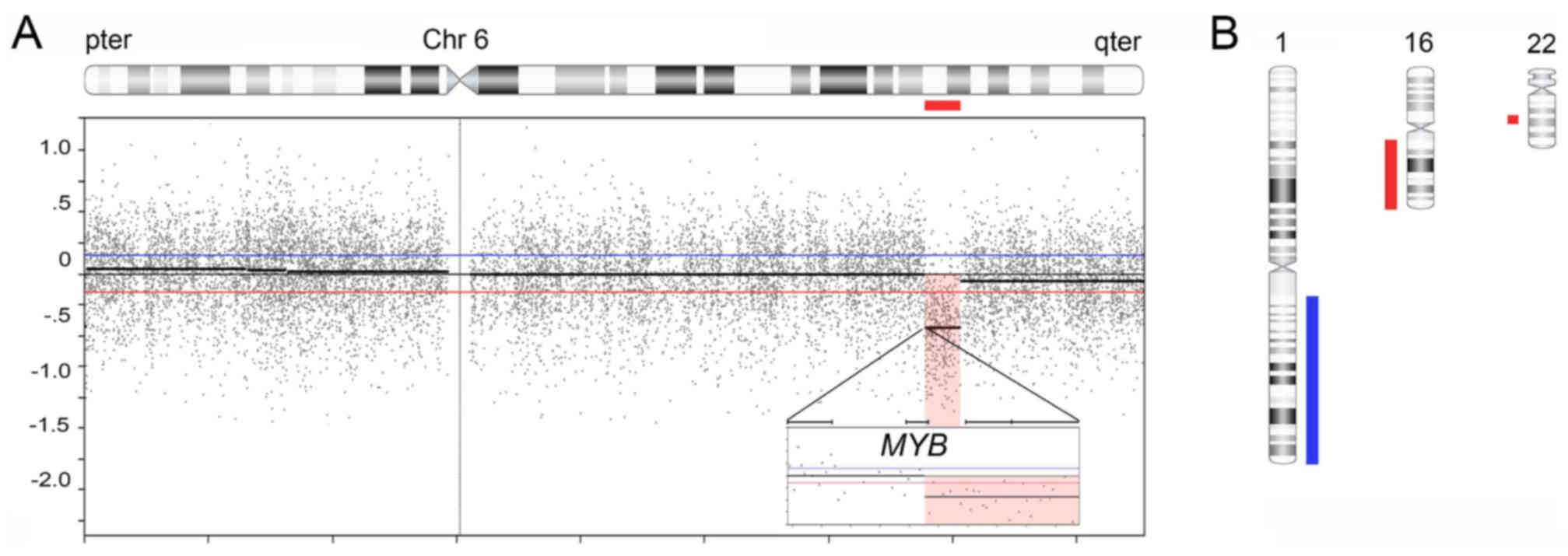
ArrayCGH analysis of the ACC revealed a single
genomic imbalance, that is a 5.7 Mb deletion in 6q23.2-q24.1. The
centromeric breakpoint was located in the 3′-part of the MYB
gene with deletion of the last coding exon of MYB including
the 3′-UTR and flanking sequences (Fig.
3A). The telomeric breakpoint was in an intergenic region in
6q24.1.
The ILC had also relatively few but different
genomic imbalances compared to the ACC. It was characterized by
gain of a 104.3 Mb segment in 1q21.1-qter, loss of a 43.8 Mb
segment in 16q11.2-qter, and loss of a 4.8 Mb segment in
22q12.2-q12.3 (Fig. 3B). There was
no evidence of amplifications or homozygous deletions in any of the
tumors.
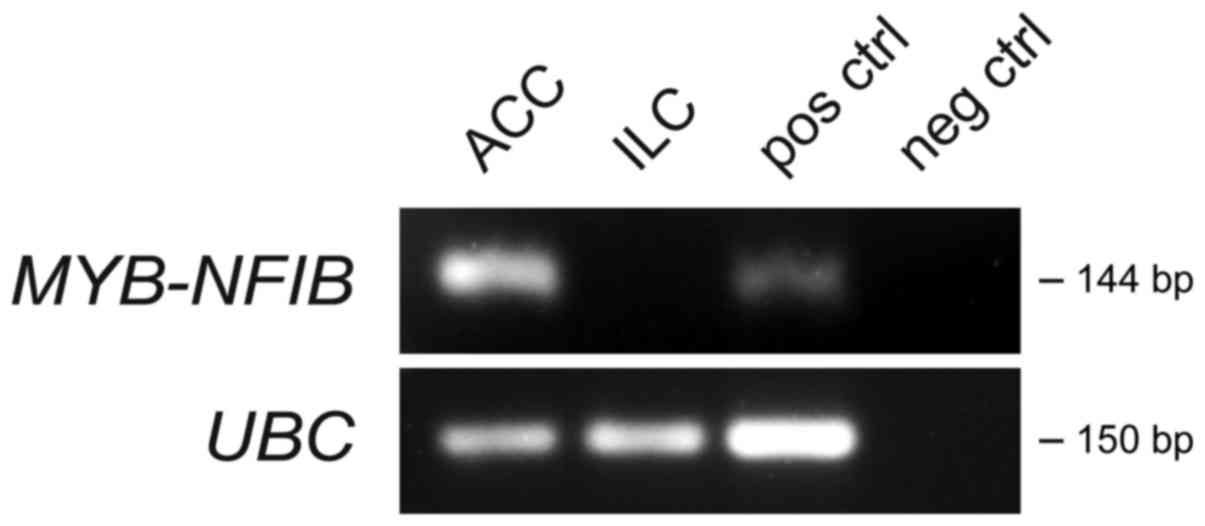
To further characterize the ACC and ILC genomically,
we screened both tumors for expression of the ACC-specific
MYB-NFIB gene fusion (17,18).
Reverse transcription polymerase chain reaction (RT-PCR) analysis
was conducted on RNAs isolated from the FFPE blocks of both tumors
using PCR-primers located in MYB exon 14 and NFIB
exons 8a, 8c, and 9 as previously described (19). As shown in Fig. 4, the ACC was strongly positive for
the MYB-NFIB fusion whereas the ILC was negative.
Discussion
The present study describes a unique case of
synchronous BBC with two histologically different carcinomas. At
the time of diagnosis the patient had an ILC in the right breast
and an ACC in the left breast. The tumors were detected by routine
mammography screening. The histopathological diagnoses of both
lesions were unequivocal (Figs. 1
and 2) and there was no evidence of
ILC or carcinoma in situ component in the surgical specimen
from the left breast. To the best of our knowledge this is the
first case of bilateral, simultaneously occurring ACC and ILC of
the breast. Morphologically and immunohistochemically, the two
tumors showed the typical picture and immunoprofile consistent with
the respective histological subtype. Thus, the ILC was estrogen and
progesterone receptor positive and E-cadherin negative whereas the
ACC was triple negative and strongly positive for KIT.
Genome-wide genomic profiling of the tumors provided
additional evidence in support of an independent origin of the
BBCs. Thus, the ACC had an interstitial 6q deletion with a
centromeric breakpoint located in the 3′-part of MYB,
resulting in loss of the last coding exon of MYB including
its 3′-UTR. The deletion, which spanned a 5.7 Mb segment in
6q23.2-q24.1, was the sole genomic imbalance. Previous findings
have unequivocally shown that rearrangements of MYB is the
main genomic hallmark of ACC (15,17,18,20,21). The
most common MYB alteration in ACC is a MYB-NFIB gene
fusion generated by a t(6;9) translocation (22,23). In
the resulting fusion gene, the 3′-part of MYB is replaced by
the 3′-part of NFIB leading to the overexpression of
MYB (17,18). Activation of MYB through gene
fusion or juxtaposition of strong enhancer elements to MYB
occurs in 80–90% of ACCs (18,24,25)
irrespective of anatomical localization (salivary gland, breast,
skin, lacrimal gland, tracheobronchial tree, digestive tract,
prostate, and female genital tract) (17,18,26,27). In
the breast, >90% of ACCs have MYB activation (21,27). In
keeping with this observation the present ACC was also strongly
positive for the MYB-NFIB fusion (Fig. 4). The ubiquitously expressed gene
UBC was used as a positive control for the PCR reaction.
In contrast to head and neck ACCs, breast ACCs are
usually low-grade tumors with an indolent clinical course. A major
reason for this difference is that breast ACCs have very few, if
any, genomic alterations other than MYB
rearrangements/activation (as identified in the present case),
whereas head and neck ACCs have a much higher frequency of genomic
imbalances some of which are associated with an aggressive clinical
behavior and a poor prognosis (15,27).
However, studies of the mutational landscape of breast and salivary
gland ACCs have revealed a similar mutational profile with
mutations targeting chromatin remodelling, cell adhesion, RNA
biology, ubiquitination, and canonical signaling pathway genes
(20,21,28).
Furthermore, breast and salivary ACCs show very similar histologies
with luminal, basaloid, and myoepithelial cells arranged in tubular
and cribriform structures with or without the presence of solid
structures (29–31).
ArrayCGH analysis of the present ILC revealed a
genomic profile that was completely different from that of the
breast ACC. The ILC had no rearrangements of 6q, did not express
the MYB-NFIB gene fusion, and showed gain of 1q21.1-qter,
loss of 16q11.2-qter, and 22q12.2-q12.3 as the sole genomic
imbalances. Notably, concurrent gains of 1q and losses of 16q are
recurrent alterations in ILC (32–34).
Taken together, our studies clearly demonstrate that the
synchronous BBCs had different histopathologic and genomic
characteristics and had developed independently of each other
consistent with the classical molecular pathways known for sporadic
ACC and ILC (18,34,35).
Previous findings have shown that synchronous BBCs
are often of the same histological type and show an association
between hormone receptor status and tumor grade (1,4). Despite
these similarities, synchronous BBCs are commonly considered as two
separate primary tumors evolving in a similar microenvironment and
with the same genetic background (13,14,36,37).
Notably, there are a number of cases on record with histologically
different synchronous bilateral breast tumors. Thus, there is a
rare case of pleomorphic adenoma of the breast and a synchronous
invasive ductal breast carcinoma in a 58-year-old woman (38). Da Silva et al have also
described an interesting case of ACC with synchronous tubular
adenosis (39). Although the two
tumors occurred in the same breast, genomic analysis indicated that
they had an independent origin. The fact that synchronous BBCs are
not always identical tumors suggests that they ideally should be
treated individually in line with the concept of personalized
cancer medicine.
In summary, we describe a unique case of synchronous
BBC in a woman with an ACC in the left breast and an ILC in the
right breast. Molecular analyses revealed that the two tumors had
different genomic profiles and that the ACC expressed the
tumor-type specific MYB-NFIB gene fusion. Taken together,
our findings strongly indicate that the two tumors had originated
independently of each other and that the MYB-NFIB fusion is
a specific biomarker for breast ACC.
Acknowledgements
The present study was supported by the Swedish
Cancer Society, and BioCARE, a National Strategic Cancer Research
Program at the University of Gothenburg.
References
|
1
|
Hungness ES, Safa M, Shaughnessy EA, Aron
BS, Gazder PA, Hawkins HH, Lower EE, Seeskin C, Yassin RS and
Hasselgren PO: Bilateral synchronous breast cancer: Mode of
detection and comparison of histologic features between the 2
breasts. Surgery. 128:702–707. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hartman M, Czene K, Reilly M, Adolfsson J,
Bergh J, Adami H-O, Dickman PW and Hall P: Incidence and prognosis
of synchronous and metachronous bilateral breast cancer. J Clin
Oncol. 25:4210–4216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
MacGrogan G, Tot T, Rakha E and Morrow M:
Bilateral breast carcinoma and nonsynchronous breast carcinomaWHO
Classification of Tumours of the Breast. Lakhani SR, Ellis OI,
Schnitt SJ, Tan PH and van de Vijver MJ: 4. 4th edition. IARC;
Lyon: pp. 69–70. 2012
|
|
4
|
Huo D, Melkonian S, Rathouz PJ, Khramtsov
A and Olopade OI: Concordance in histological and biological
parameters between first and second primary breast cancers. Cancer.
117:907–915. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holm M, Tjønneland A, Balslev E and Kroman
N: Prognosis of synchronous bilateral breast cancer: A review and
meta-analysis of observational studies. Breast Cancer Res Treat.
146:461–475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brennan ME, Houssami N, Lord S, Macaskill
P, Irwig L, Dixon JM, Warren RM and Ciatto S: Magnetic resonance
imaging screening of the contralateral breast in women with newly
diagnosed breast cancer: Systematic review and meta-analysis of
incremental cancer detection and impact on surgical management. J
Clin Oncol. 27:5640–5649. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Senkus E, Szade J, Pieczyńska B, Zaczek A,
Pikiel J, Sosińska-Mielcarek K, Karpińska A and Jassem J: Are
synchronous and metachronous bilateral breast cancers different? An
immunohistochemical analysis aimed at intrinsic tumor phenotype.
Int J Clin Exp Pathol. 7:353–363. 2013.PubMed/NCBI
|
|
8
|
Chen Y, Thompson W, Semenciw R and Mao Y:
Epidemiology of contralateral breast cancer. Cancer Epidemiol
Biomarkers Prev. 8:855–861. 1999.PubMed/NCBI
|
|
9
|
Singletary SE: Rating the risk factors for
breast cancer. Ann Surg. 237:474–482. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adami H-O, Hansen J, Jung B and Rimsten A:
Characteristics of familial breast cancer in Sweden: Absence of
relation to age and unilateral versus bilateral disease. Cancer.
48:1688–1695. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Polednak AP: Bilateral synchronous breast
cancer: A population-based study of characteristics, method of
detection, and survival. Surgery. 133:383–389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jobsen JJ, van der Palen J, Ong F,
Riemersma S and Struikmans H: Bilateral breast cancer, synchronous
and metachronous; differences and outcome. Breast Cancer Res Treat.
153:277–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song F, Li X, Song F, Zhao Y, Li H, Zheng
H, Gao Z, Wang J, Zhang W and Chen K: Comparative genomic analysis
reveals bilateral breast cancers are genetically independent.
Oncotarget. 6:31820–31829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fountzilas E, Kotoula V, Zagouri F,
Giannoulatou E, Kouvatseas G, Pentheroudakis G, Koletsa T, Bobos M,
Papadopoulou K, Samantas E, et al: Disease evolution and
heterogeneity in bilateral breast cancer. Am J Cancer Res.
6:2611–2630. 2016.PubMed/NCBI
|
|
15
|
Persson M, Andrén Y, Moskaluk CA, Frierson
HF Jr, Cooke SL, Futreal PA, Kling T, Nelander S, Nordkvist A,
Persson F and Stenman G: Clinically significant copy number
alterations and complex rearrangements of MYBNFIB in head and neck
adenoid cystic carcinoma. Genes Chromosomes Cancer. 51:805–817.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jee KJ, Persson M, Heikinheimo K,
Passador-Santos F, Aro K, Knuutila S, Odell EW, Mäkitie A, Sundelin
K, Stenman G and Leivo I: Genomic profiles and CRTC1-MAML2 fusion
distinguish different subtypes of mucoepidermoid carcinoma. Mod
Pathol. 26:213–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Persson M, Andrén Y, Mark J, Horlings HM,
Persson F and Stenman G: Recurrent fusion of MYBNFIB transcription
factor genes in carcinomas of the breast and head and neck. Proc
Natl Acad Sci USA. 106:18740–18744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andersson MK and Stenman G: The landscape
of gene fusions and somatic mutations in salivary gland neoplasms -
Implications for diagnosis and therapy. Oral Oncol. 57:63–69. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fehr A, Kovács A, Löning T, Frierson H Jr,
van den Oord J and Stenman G: The MYB-NFIB gene fusion-a novel
genetic link between adenoid cystic carcinoma and dermal
cylindroma. J Pathol. 224:322–327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ho AS, Kannan K, Roy DM, Morris LG, Ganly
I, Katabi N, Ramaswami D, Walsh LA, Eng S, Huse JT, et al: The
mutational landscape of adenoid cystic carcinoma. Nat Genet.
45:791–798. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martelotto LG, De Filippo MR, Ng CK,
Natrajan R, Fuhrmann L, Cyrta J, Piscuoglio S, Wen HC, Lim RS, Shen
R, et al: Genomic landscape of adenoid cystic carcinoma of the
breast. J Pathol. 237:179–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stenman G, Sandros J, Dahlenfors R,
Juberg-Ode M and Mark J: 6q- and loss of the Y chromosome - two
common deviations in malignant human salivary gland tumors. Cancer
Genet Cytogenet. 22:283–293. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nordkvist A, Mark J, Gustafsson H, Bang G
and Stenman G: Non-random chromosome rearrangements in adenoid
cystic carcinoma of the salivary glands. Genes Chromosomes Cancer.
10:115–121. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brayer KJ, Frerich CA, Kang H and Ness SA:
Recurrent fusions in MYBMYBL1 define a common, transcription
factor-driven oncogenic pathway in salivary gland adenoid cystic
carcinoma. Cancer Discov. 6:176–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drier Y, Cotton MJ, Williamson KE,
Gillespie SM, Ryan RJ, Kluk MJ, Carey CD, Rodig SJ, Sholl LM,
Afrogheh AH, et al: An oncogenic MYB feedback loop drives alternate
cell fates in adenoid cystic carcinoma. Nat Genet. 48:265–272.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brill LB II, Kanner WA, Fehr A, Andrén Y,
Moskaluk CA, Löning T, Stenman G and Frierson HF Jr: Analysis of
MYB expression and MYB-NFIB gene fusions in adenoid cystic
carcinoma and other salivary neoplasms. Mod Pathol. 24:1169–1176.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wetterskog D, Lopez-Garcia MA, Lambros MB,
A'Hern R, Geyer FC, Milanezi F, Cabral MC, Natrajan R, Gauthier A,
Shiu K, et al: Adenoid cystic carcinomas constitute a genomically
distinct subgroup of triple-negative and basal-like breast cancers.
J Pathol. 226:84–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wetterskog D, Wilkerson PM, Rodrigues DN,
Lambros MB, Fritchie K, Andersson MK, Natrajan R, Gauthier A, Di
Palma S, Shousha S, et al: Mutation profiling of adenoid cystic
carcinomas from multiple anatomical sites identifies mutations in
the RAS pathway, but no KIT mutations. Histopathology. 62:543–550.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sapino A, Sneige N and Eusebi V: Adenoid
cystic carcinomaWHO Classification of Tumours of the Breast.
Lakhani SR, Ellis OI, Schnitt SJ, Tan PH and van de Vijver MJ: 4.
4th edition. IARC; Lyon: pp. 56–57. 2012
|
|
30
|
Foschini MP, Morandi L, Asioli S, Giove G,
Corradini AG and Eusebi V: The morphological spectrum of salivary
gland type tumours of the breast. Pathology. 49:215–227. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stenman G, Licitra L, Said-Al-Naief N, van
Zante A and Yarbrough WG: Adenoid cystic carcinomaWHO
Classification of Head and Neck Tumours. 9. 4th edition. El-Naggar
AK, Chan JKC, Grandis JR, Takata T and Slootweg PJ: IARC; Lyon: pp.
164–165. 2017
|
|
32
|
Hungermann D, Schmidt H, Natrajan R, Tidow
N, Poos K, Reis-Filho JS, Brandt B, Buerger H and Korsching E:
Influence of whole arm loss of chromosome 16q on gene expression
patterns in oestrogen receptor-positive, invasive breast cancer. J
Pathol. 224:517–528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stacher E, Boldt V, Leibl S, Halbwedl I,
Popper HH, Ullmann R, Tavassoli FA and Moinfar F: Chromosomal
aberrations as detected by array comparative genomic hybridization
in early low-grade intraepithelial neoplasias of the breast.
Histopathology. 59:549–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lakhani SR, Rakha E and Simpson PT: WHO
Classification of Tumours of the Breast. Lakhani SR, Ellis OI,
Schnitt SJ, Tan PH and van de Vijver MJ: 4. 4th edition. IARC;
Lyon: pp. 40–42. 2012
|
|
35
|
Stenman G, Andersson MK and Andrén Y: New
tricks from an old oncogene: Gene fusion and copy number
alterations of MYB in human cancer. Cell Cycle. 9:2986–2995. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tse GMK, Kung FYL, Chan ABW, Law BKB,
Chang AR and Lo K-W: Clonal analysis of bilateral mammary
carcinomas by clinical evaluation and partial allelotyping. Am J
Clin Pathol. 120:168–174. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saad RS, Denning KL, Finkelstein SD, Liu
Y, Pereira TC, Lin X and Silverman JF: Diagnostic and prognostic
utility of molecular markers in synchronous bilateral breast
carcinoma. Mod Pathol. 21:1200–1207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Di Bonito M, Cantile M, Cerrone M, Liguori
G and Botti G: Synchronous pleomorphic adenoma and invasive ductal
carcinoma in distinct breasts. Breast J. 21:428–430. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Da Silva L, Buck L, Simpson PT, Reid L,
McCallum N, Madigan BJ and Lakhani SR: Molecular and morphological
analysis of adenoid cystic carcinoma of the breast with synchronous
tubular adenosis. Virchows Arch. 454:107–114. 2009. View Article : Google Scholar : PubMed/NCBI
|