Introduction
An increasing proportion of patients with
early-stage breast cancer are treated with taxanes as postoperative
adjuvant chemotherapy. Anthracycline-based chemotherapies are
effective and are also widely used as the main adjuvant
chemotherapies (1–3); however, those treatments are associated
with well-known adverse reactions, such as hematotoxicity, nausea,
vomiting and hair loss (4,5). The use of 5HT3 receptor antagonists
allows some degree of control of the symptoms of late-onset nausea
and vomiting (6–8). However, patients on anthracycline-based
chemotherapy experience fatigue and a decrease in their activity
and daily quality of life (QOL), and these problems may be
difficult to control (9–11). Moreover, although the host immune
function of breast cancer patients on chemotherapy affects the
recurrence rate (12),
anthracycline-based chemotherapy is reported to adversely affect
immune function (13). Accordingly,
there is a need for treatments that can maintain and/or improve the
QOL and immune function of breast cancer patients receiving
anthracycline-based chemotherapy.
Lentinula edodes mycelia extract (LEM) is a
dried powder of a hot water extract of the mycelia of Lentinula
edodes (14). Oral LEM
demonstrated in vivo activity in promoting antitumor effect
and improving host immune function (14–19). In
gastrointestinal cancer patients undergoing chemotherapy, oral LEM
exerted an inhibitory effect on the incidence of adverse events
(AEs), such as chemotherapy-induced nausea, and also improved
immune function, by increasing natural killer (NK) cell activity,
resulting in improvement of the patients' QOL (20,21). LEM
also improved the QOL and immune function of breast cancer patients
undergoing postoperative adjuvant hormone therapy (22).
The results of a study on breast cancer patients who
were administered 5-fluorouracil (5-FU) at 500 mg/m2,
cyclophosphamide at 500 mg/m2 and epirubicin at 75
mg/m2 (FEC75 regimen) every 3 weeks as postoperative
adjuvant chemotherapy were previously reported (23). In that study, oral LEM was
co-administered during the second course of chemotherapy and the
QOL and immune function of the second course were compared to those
of the first course. The results demonstrated that the decrease in
QOL as estimated by the QOL Questionnaire for Cancer Patients
Treated with Anticancer Drugs (QOL-ACD) (24) and the decrease in NK cell activity
caused by the FEC75 regimen were improved by the co-administration
of LEM in the second course of the chemotherapy compared with the
first course, demonstrating the usefulness of LEM. However, that
study was a single-group open trial, and administration of LEM was
limited to the 3 weeks of the second course of chemotherapy. Due to
that design, the possibility that the improvement seen during the
second course was due to the patients becoming accustomed to the
chemotherapy cannot be ruled out. Moreover, at the time of that
study, the use of 5HT3 receptor antagonists for nausea and vomiting
was not yet commonplace, and they were not used in the study.
Therefore, it is not clear whether the improving effect of LEM on
the QOL could also be achieved with the currently widely used 5HT3
receptor antagonists. For those reasons, the present
placebo-controlled randomized double-blind study was designed and
conducted to evaluate the effectiveness of LEM in improving the QOL
and the immune function and controlling the AEs caused by
postoperative adjuvant anthracycline-based chemotherapy
administered to early-stage breast cancer patients who were also
administered 5HT3 receptor antagonists as supportive therapy.
Patients and methods
Patients
In the present study, patients who met the following
criteria were enrolled: The patients were female, aged ≥20 years,
were diagnosed with breast cancer, and were scheduled for
anthracycline-based adjuvant chemotherapy as postoperative adjuvant
therapy. The patients had maintained principal organ function for
chemotherapy, with a performance status of 0 or 1, and had a life
expectancy of >3 months. Patients who were pregnant, were at
risk of becoming pregnant, were breastfeeding or had received prior
treatment within 4 weeks of the entry to this study, were excepted.
All the patients were fully informed on the aims and methods of the
trial prior to participation and provided written consent for
participation in the trial.
Drug
The LEM used was manufactured by Kobayashi
Pharmaceutical Co. Ltd. (Osaka, Japan). The manufacturing process
was as previously reported (14).
Briefly, Lentinula edodes mycelia (strain NITE SD 0043,
registered at the National Institute of Technology and Evaluation
in Japan) were first inoculated onto solid medium consisting mainly
of bagasse of sugarcane and defatted rice polishings, and cultured
until the mycelia had spread. The culture was then extracted using
hot water. The dissolved component was dried to obtain a dry
powder. This dry powder was used as the LEM in the present trial.
The LEM group patients were dispensed 230-mg test tablets that
contained 150 mg of LEM, and were instructed to ingest 6 tablets in
the morning and another 6 in the evening. The placebo group
patients were dispensed 230-mg placebo tablets that contained
caramel color, dextrin and maltitol, and were instructed to ingest
6 tablets in the morning and 6 in the evening. Both the test and
the placebo tablets were manufactured by Kobayashi Pharmaceutical
Co. Ltd.
Study design
This was a placebo-controlled randomized
double-blind study that evaluated the effectiveness of LEM during
the first 2 courses of adjuvant anthracycline-based chemotherapy
administered as postoperative adjuvant therapy (UMIN000004614). The
trial protocol was approved by the Institutional Review Boards of
Yamaguchi University, Shimonoseki Medical Center and Kinki
University, and the trial was conducted based on the ethical
principles established in the Declaration of Helsinki. The patients
were allocated to an LEM group or a placebo group by a block
randomization method with central randomization. The
anthracycline-based chemotherapy included any of the following:
5-FU, 500 mg/m2; cyclophosphamide, 500 mg/m2;
and epirubicin, 100 mg/m2 (FEC100 regimen),
cyclophosphamide, 600 mg/m2 and epirubicin, 90
mg/m2 (EC regimen), 5-FU, 500 mg/m2;
cyclophosphamide, 500 mg/m2; and
doxorubicin/pirarubicin, 50 mg/m2 (FAC regimen), or
cyclophosphamide, 600 mg/m2; and
doxorubicin/pirarubicin, 60 mg/m2 (AC regimen). The
inclusion/non-inclusion of 5-FU in the regimens was used as an
allocation factor. For each of these regimens, 3 weeks comprised a
single course and patients received the chemotherapies on day 1 in
each course. During the first 2 courses, LEM (daily dose of 1,800
mg) or placebo tablets were ingested daily over the 3 weeks of both
courses, for a total of 6 weeks.
Measurement
In all the tests, measurements were made at five
different time points: On day 1 (prior to administration of
chemotherapy), day 8 (after administration of chemotherapy for 1
week) and day 22 (after administration of chemotherapy for 3 weeks
and prior to administration of chemotherapy in the second course)
in the first course, and on day 8 (after administration of
chemotherapy for 1 week) and day 22 (after administration of
chemotherapy for 3 weeks) in the second course.
A survey of QOL was measured by the Functional
Assessment of Cancer Therapy (FACT) for patients receiving
Biological Response Modifiers (BRM), version 4 (FACIT.org) (25), and
was evaluated from scores on the basis of the Scoring &
Interpretation Materials of the questionnaire provided by
FACIT.org. AEs were measured by the Common Terminology
Criteria for Adverse Events (CTCAE) v.4.0-Japan Clinical Oncology
Group edition (http://ctep.cancer.gov/protocol
Development/electronic_applications/ctc.htm#ctc_40).
Among immune parameters, the Th1/Th2 balance was
measured by flow cytometric analysis of the proportion of
interleukin (IL)-4-positive and interferon (IFN)-γ-positive cells
among CD4-positive lymphocytes in the peripheral blood. Cell
surface CD4 was detected with anti-CD4 PC-5-labeled antibody
(dilution, 1:20; catalog no. 566004, BD Japan, Tokyo, Japan).
Intracellular IL-4 and IFN-γ were detected with anti-IL-4
phycoerythrin (PE)-labeled antibody (dilution, 1:20; catalog no.
340451) and anti-IFN-γ fluorescein isothiocyanate (FITC)-labeled
antibodies (dilution, 1:20; catalog no. 340449, BD Japan),
respectively. Regulatory T cells (Tregs) were measured by flow
cytometric analysis of the proportion of forkhead box p3
(FoxP3)-positive cells among CD4-positive lymphocytes in the
peripheral blood. Cell surface CD4 and CD25 were detected using
anti-CD4 FITC-labeled and anti-CD25 PE-Cy5.5-labeled (dilution,
1:20; catalog no. 560503, BD Japan) antibodies, respectively. In
addition, intracellular FoxP3 was detected using anti-FoxP3
PE-labeled antibody (dilution, 1:20; catalog no. 560046, BD
Japan).
NK cell activity was measured using a
51Cr release assay. Peripheral blood mononuclear cells
(PBMCs) were isolated using Ficoll solution. The isolated PBMCs
were rinsed in RPMI-1640 medium containing 10% fetal bovine serum
(FBS), adjusted to 1×106 cells/ml, and used as effector
cells. K562 cells (DS Pharma Biomedical Co. Ltd., Osaka, Japan), a
human immortalized myelogenous leukemia cell line, were used for
adjusting the concentration of the target cells. A total of 100 µCi
of 51Cr were added to the K562 cells and the cells were
then cultured for 1 h at 37°C. After rinsing twice with
phosphate-buffered saline, the cell number was adjusted to
1×106 cells/ml in RPMI-1640 medium containing 10% FBS,
and these cells were used as the target cells. A mixed culture of
effector and target cells was performed for 3.5 h at an
effector/target (E/T) ratio of 20, after which time 51Cr
released from dead target cells was measured using a
γ-scintillation counter. In the maximum dissociation control, 1
N-HCl was added in the place of effector cells, and 51Cr
released from dead target cells was measured with a γ-scintillation
counter. In the spontaneous dissociation control, RPMI-1640 medium
containing 10% FBS was added in the place of effector cells. The
activity of NK cells was calculated as follows: Volume of released
51Cr at the time of effector cell addition minus that in
the spontaneous dissociation control/volume of released
51Cr in the maximum dissociation control minus that in
the spontaneous dissociation control.
Statistical analysis
Measurements are shown as means ± standard error.
Variation within each group was assayed by the Kruskal-Wallis test
with the Steel test for QOL scores, and by repeated one-way
analysis of variance with Bonferroni's correction for immune
parameters.
Results
Patients
Between 2011 and 2014, 47 female breast cancer
patients with a performance status of 0 or 1 (determined according
to the Eastern Cooperative Oncology Group performance status in the
Common Toxicity Criteria, v.2.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_app-lications/docs/ctcv20_4-30-992.pdf)
were enrolled in the present study by the Department of Digestive
Surgery and Surgical Oncology at Yamaguchi University, the
Shimonoseki Medical Center of Japan Community Health Care
Organization, and the Departments of Surgery and Medical Oncology
at Kinki University Hospital, and scheduled to receive
postoperative adjuvant anthracycline-based chemotherapy. The
patients were allocated to the LEM group (n=23) or the placebo
group (n=24) by a block randomization method. The patient
background information is summarized in Table I. The age of the patients in the LEM
group ranged from 31 to 85 years (median, 62 years) and, although
none of the patients had distant metastasis, 12 were positive for
lymph node metastasis. The age of the patients in the placebo group
ranged from 42 to 77 years (median, 57.5 years) and, although none
of the cases had distant metastasis, 8 patients were positive for
lymph node metastasis.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| LEM, n | Placebo, n |
|---|
| Entry | 23 | 24 |
| Age, years |
|
|
| Mean
(range) | 62.0 (31–85) | 57.5 (42–77) |
| Tumor stage |
|
|
| 0 | 0 | 0 |
| 1 | 6 | 9 |
| 2 | 14 | 12 |
| 3 | 3 | 3 |
| 4 | 0 | 0 |
| T stage |
|
|
|
Tis | 0 | 0 |
| T0 | 2 | 0 |
| T1 | 10 | 10 |
| T2 | 10 | 12 |
| T3 | 1 | 0 |
| T4 | 0 | 0 |
|
Unknown | 0 | 2 |
| N stage |
|
|
| N0 | 11 | 16 |
| N1 | 10 | 5 |
| N2 | 2 | 2 |
| N3 | 0 | 1 |
| Treatments |
|
|
| FEC/FAC
therapy | 10 | 12 |
| AC/EC
therapy | 13 | 12 |
QOL
Patients with missing QOL score data for ≥2 time
points were excluded, leaving 22 patients in the LEM group and 21
patients in the placebo group for analysis of the QOL values. The
changes in the QOL scores of FACT-BRM, FACT-general (G), physical
well-being (PWB) and functional well-being (FWB) during the study
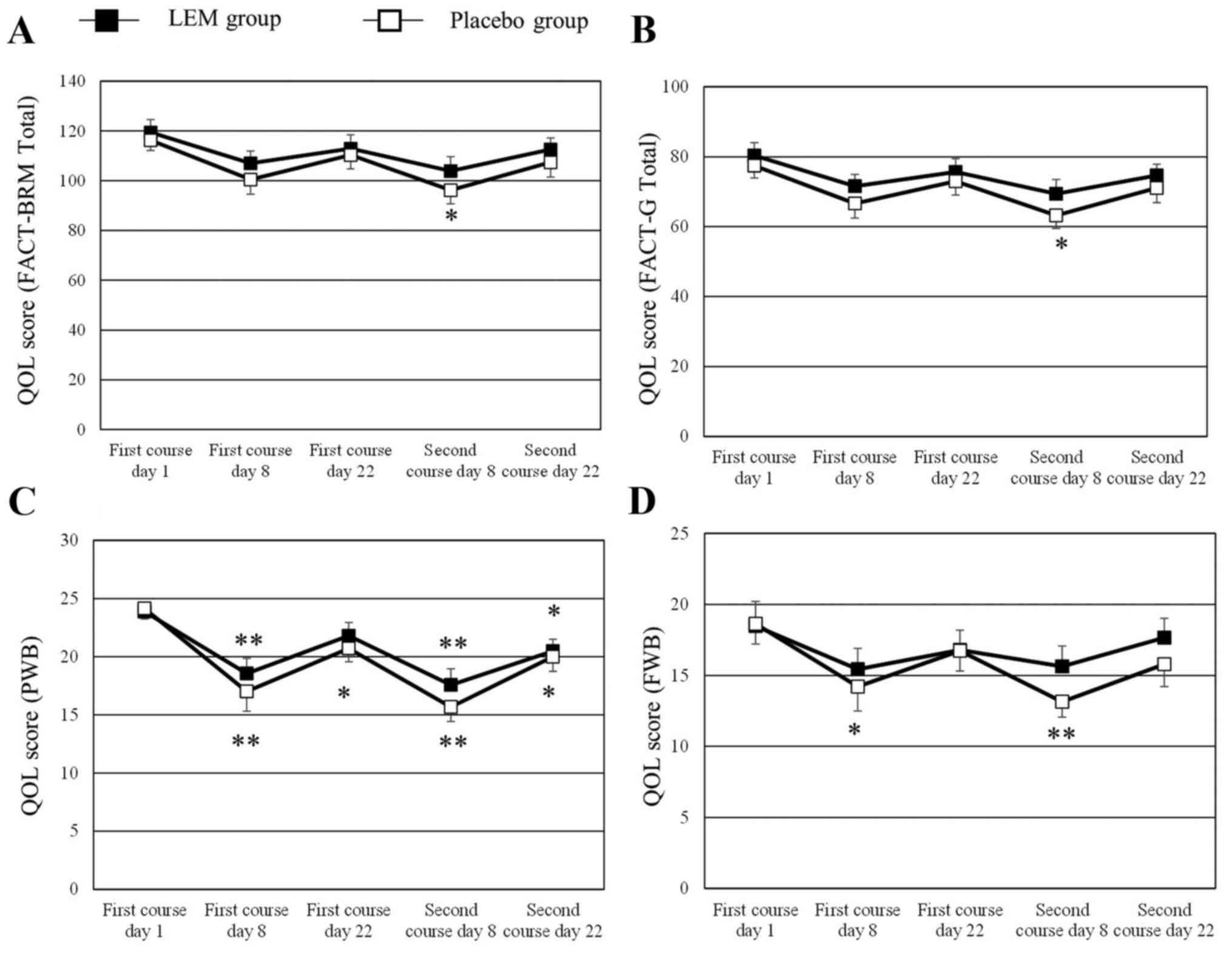
in graphic form are shown in Fig. 1.
The numerical changes in all the QOL scores tested are shown in
Table II. The FACT-BRM total score
and the FACT-G total score had significantly decreased from the
baseline scores in the placebo group on day 8 of the second course
of chemotherapy, whereas the scores had not decreased in the LEM
group. The FACT-BRM Trial Outcome Index had decreased from the
baseline score in the placebo group on day 8 of both the first and
the second courses of chemotherapy, whereas in the LEM group the
score was only decreased on day 8 of the second course of
chemotherapy. In the subscales, the PWB score had significantly
decreased from the baseline value in both groups on day 8 of both
courses of chemotherapy. In the LEM group, the decreased PWB score
on day 8 of the first course of chemotherapy had recovered on day
22 of that course, while the score in the placebo group had not
recovered. The FWB score had significantly decreased from the
baseline value in the placebo group on day 8 of both courses of
chemotherapy, whereas the score did not decrease in the LEM group
during either of the chemotherapy courses. The BRM physical
subscale (BRMP) score had significantly decreased from the baseline
value in the placebo group on day 8 of both courses of
chemotherapy, while the score was not decreased on day 8 of the
first course of chemotherapy in the LEM group. No significant
changes were observed in any of the other subscale scores during
either of the two chemotherapy courses.
 | Table II.Effect of LEM vs. placebo on QOL
scores during the study course. |
Table II.
Effect of LEM vs. placebo on QOL
scores during the study course.
|
|
|
| First course | Second course |
|---|
|
|
|
|
|
|
|---|
| Scores | Groups | Subject no. | Day 1 | Day 8 | Day 22 | Day 8 | Day 22 |
|---|
| FACT-BRM total
score | LEM | 21 | 119.5±5.1 | 107.0±4.9 | 112.9±5.5 | 103.9±5.7 | 112.6±4.6 |
|
| Placebo | 21 | 116.2±4.1 | 100.5±5.9 | 110.4±5.6 |
96.2±5.4a | 107.4±5.9 |
| FACT-G total
score | LEM | 21 | 80.4±3.6 | 71.6±3.4 | 75.6±3.8 | 69.4±4.1 | 74.7±3.2 |
|
| Placebo | 21 | 77.4±3.5 | 66.6±4.2 | 73.0±4.0 |
63.2±3.7a | 71.1±4.2 |
| FACT-BRM TOI | LEM | 21 | 81.4±3.7 | 69.4±3.9 | 75.9±4.0 |
67.7±4.1a | 76.0±3.5 |
|
| Placebo | 21 | 81.6±2.4 |
65.1±4.3b | 74.8±3.8 |
61.8±3.8b | 72.1±4.3 |
| PWB | LEM | 22 | 23.9 ±0.8 |
18.6±1.3b | 21.8±1.1 |
17.6±1.4b |
20.5±1.0a |
|
| Placebo | 21 | 24.1±0.9 |
17.0±1.7b |
20.7±1.2a |
15.7±1.3b |
20.0±1.3a |
| SWB | LEM | 21 | 20.8±1.1 | 20.6±1.3 | 20.5±1.3 | 20.1±1.3 | 19.6±1.3 |
|
| Placebo | 21 | 19.4±1.6 | 20.2±1.5 | 19.0±1.7 | 19.6±1.5 | 19.0±1.6 |
| EWB | LEM | 22 | 17.3±1.0 | 17.0±1.1 | 16.5±1.2 | 16.1±1.4 | 16.9±1.1 |
|
| Placebo | 21 | 15.3±1.1 | 15.2±1.2 | 16.6±0.9 | 14.8±1.1 | 16.3±1.0 |
| FWB | LEM | 22 | 18.5±1.7 | 15.4±1.5 | 16.8±1.4 | 15.6±1.4 | 17.7±1.4 |
|
| Placebo | 21 | 18.6±1.4 |
14.2±1.7a | 16.7±1.4 |
13.1±1.1b | 15.8±1.6 |
| BRMP | LEM | 21 | 22.5±1.0 | 20.4±0.7 | 20.8±0.9 |
18.7±1.1a | 20.8±0.8 |
|
| Placebo | 21 | 22.1±1.0 |
19.3±1.0a | 21.5±0.8 |
18.8±1.0a | 20.9±1.1 |
| BRMCE | LEM | 21 | 16.6±1.1 | 15.0±1.2 | 16.5±1.3 | 15.8±1.0 | 17.1±1.0 |
|
| Placebo | 21 | 16.7±1.0 | 14.6±1.2 | 15.9±1.2 | 14.2±1.3 | 15.4±1.2 |
Toxicity
No AEs due to LEM occurred during this study.
Table III summarizes the grade ≥3
AEs (CTCAE v.4) that occurred due to anthracycline-based
chemotherapy. Of these AEs, the following occurred in the LEM
group: Anorexia (n=1), nausea (n=1), febrile neutropenia (n=1) and
decrease in neutrophil/lymphocyte ratio (n=5). The AEs in the
placebo group were as follows: Febrile neutropenia (n=2), decrease
in neutrophil/lymphocyte ratio (n=4), hand-foot skin reaction (n=1)
and erythroderma (n=1). No differences in AEs were observed between
the two groups. However, the 2 placebo group patients with febrile
neutropenia were unable to complete the second course of treatment
and dropped out of the study. There were no dropouts in the LEM
group.
 | Table III.Adverse events with LEM vs. placebo
treatment. |
Table III.
Adverse events with LEM vs. placebo
treatment.
| Adverse events,
n | LEM | Placebo |
|---|
| Anorexia | 1 | 0 |
| Nausea | 1 | 0 |
| Febrile
neutropenia | 1 | 2 |
|
Neutrophil/lymphocyte decrease | 5 | 4 |
| Hand-foot skin
reaction | 0 | 1 |
| Erythroderma | 0 | 1 |
Immune parameters
Patients with missing immune parameter data for ≥2
time points were excluded, leaving 23 patients in the LEM group and
19 patients in the placebo group for analysis of the immune
parameters. The data regarding the changes in immune parameters
during the study are summarized in Table IV. NK cell activity significantly
decreased from the baseline value in both groups on day 8 of both
the first and second courses of chemotherapy. In the LEM group, NK
cell activity also decreased on day 22 of the second course. The
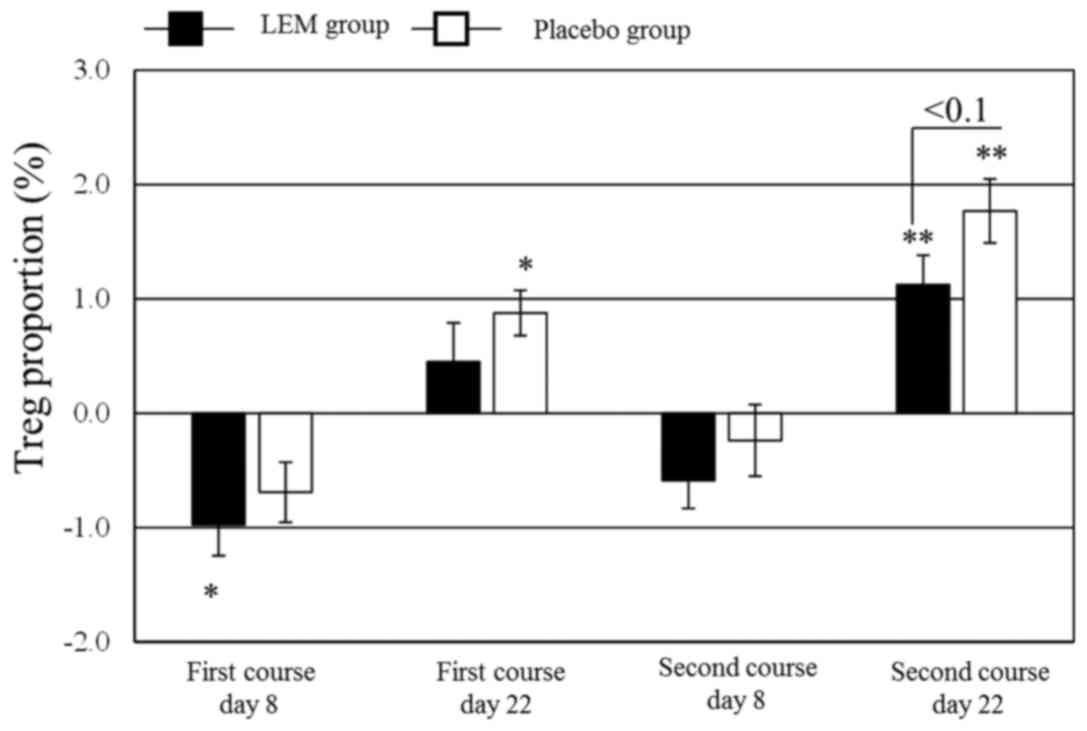
proportion of Tregs in the peripheral blood CD4+ cells
(FoxP3+ CD25+/CD4+) was significantly
decreased in the LEM group on day 8 of the first chemotherapy
course. In the placebo group, that proportion was significantly
increased from the baseline value on day 22 of both chemotherapy
courses. Even in the LEM group, the proportion was significantly
increased from the baseline value on day 22 of the second
chemotherapy course, but the degree of that increase tended to be
lower compared with that in the placebo group (Fig. 2). The Th1/Th2 balance exhibited no
significant changes in either the LEM or the placebo group.
 | Table IV.Effect of LEM vs. placebo on
immunological parameters during the study course. |
Table IV.
Effect of LEM vs. placebo on
immunological parameters during the study course.
|
|
|
| First course | Second course |
|---|
|
|
|
|
|
|
|---|
| Parameters | Groups | Subject no. | Day 1 | Day 8 | Day 22 | Day 8 | Day 22 |
|---|
| NK cell activity
(%) | LEM | 23 | 32.5±3.7 |
19.1±2.9b |
23.4±2.4b |
13.6±1.7b |
25.2±2.7a |
|
| Placebo | 19 | 27.4±3.1 |
16.2±1.9b | 20.4±1.8 |
10.8±2.2b | 23.2±2.9 |
| Treg
(Foxp3+ CD25+/CD4+ | LEM | 23 | 6.2±0.3 |
5.2±0.3a | 6.6±0.4 | 5.5±0.2 |
7.3±0.4b |
| cells) (%) | Placebo | 19 | 5.8±0.3 | 5.1±0.3 |
6.6±0.4a | 5.6±0.4 |
7.6±0.4b |
| Th1/Th2 balance
(IFN-γ+/IL-4+ | LEM | 23 | 10.1±1.5 | 8.7±1.3 | 9.2±1.1 | 7.7±0.9 | 8.1±1.0 |
| in CD4+
cells) | Placebo | 19 | 10.3±1.4 | 9.5±1.2 | 10.1±1.1 | 8.7±1.1 | 10.5±1.3 |
Discussion
Anthracycline-based chemotherapies are effective and
are widely used as the main postoperative adjuvant chemotherapy for
patients with early-stage breast cancer (1–3).
However, those treatments are accompanied by a high incidence of
nausea and vomiting (4,5), severely compromising the patients' QOL.
The use of 5HT3 receptor antagonists has become commonplace in
recent years, enabling a certain level of control of these
symptoms. However, fatigue and decreased physical QOL remain
challenging issues in breast cancer patients receiving
postoperative adjuvant chemotherapy, and methods for alleviating
these symptoms are needed (9). In
our earlier single-group open study, in which LEM was
coadministered to breast cancer patients who were treated with the
FEC75 regimen, the QOL-ACD (24) was
used to evaluate the effect of LEM on the patients' QOL. The
results indicated that coadministration of LEM was effective in
improving the patients' physical scale in response to the FEC75
regimen. LEM is a BRM agent; thus, in the present study, the QOL
was evaluated using the FACT-BRM (25), which was developed for assessing the
effects of BRM on the QOL of cancer patients.
The FACT-BRM total score was significantly decreased
from the baseline score in the placebo group on day 8 of the second
course of chemotherapy, whereas in the LEM group this score was not
decreased. The placebo group also exhibited significantly reduced
scores for the PWB, BRMP and FWB subscales on day 8 of both the
first and second courses of chemotherapy in comparison with the
baseline scores, and the PWB had not recovered to the baseline
score on day 22 of both courses. The PWB questions include one
regarding nausea and vomiting. In the present study, only 1 patient
experienced grade ≥3 severe nausea and vomiting, and the nausea and
vomiting were controlled to a certain extent by the use of 5HT3
receptor antagonists. However, the PWB scores had not recovered in
either course on day 22 in the placebo group, suggesting that the
patients' physical QOL must be improved. In the LEM group, the PWB
scores were decreased on day 8 of both chemotherapy courses
compared with their baseline scores, as well as compared with the
placebo group, but they had recovered by day 22 of the first
course. Furthermore, unlike in the placebo group, the BRMP score,
which includes questions regarding fatigue, was not decreased on
day 8 of the first chemotherapy course in the LEM group. Moreover,
the FWB score was not decreased in the LEM group. These results
indicate that coadministration of LEM with anthracycline-based
chemotherapies was effective in alleviating decreases in the QOL,
particularly regarding the patients' physical condition and
functional activity.
An attempt to ameliorate the chemotherapy-induced
fatigue and decrease in the physical QOL in breast cancer patients
by means of resistance exercise was reported (26), but no reliable protocols have been
established to date. LEM was also reported to improve the QOL in
breast cancer patients who receive postoperative hormone therapy
(22). Combined with the results of
the present study, these data suggest that coadministration of LEM
may be a useful method for better preserving the QOL of
postoperative breast cancer patients undergoing a broad range of
drug therapies.
The host immune function of breast cancer patients
was reported to affect the prognosis of such patients receiving
anticancer chemotherapy (27). The
present study investigated the mechanism through which
anthracycline-based chemotherapies affect NK cell activity, Tregs
and the Th1/Th2 balance, and evaluated the effect of coadministered
LEM on these immune parameters (Table
IV). In our earlier single-group open study on postoperative
breast cancer patients who were treated with the FEC75 regimen, NK
cell activity was found to be decreased after 1 week of
chemotherapy, but coadministration of LEM inhibited that decrease.
However, in the present study, NK cell activity was significantly
reduced on day 8 of both the first and second chemotherapy courses
in both the placebo and the LEM groups, and we were unable to
demonstrate that coadministration of LEM prevented that decrease.
Further investigation into the effect of coadministration of LEM on
NK cell activity is required. In the present study, no significant
change was observed in the Th1/Th2 balance in either of the two
groups, which is in agreement with the results of our earlier
study. Th1/Th2 balance analysis is a T-cell subset analysis that
evaluates the proportion of IFN-γ-producing and IL-4-producing
cells among CD4+ lymphocytes. In recent years, it has
become widely recognized that, for induction of anticancer
immunity, the proportion of Tregs
(FoxP3+/CD25+ cells) in CD4+
lymphocytes provides a more important index of the tumor immune
status compared with the Th1/Th2 balance. That is, an increase in
Tregs in the tumor-bearing state is known to result in a decrease
in tumor immune function (28–30). In
the present study, the proportion of Tregs was significantly
increased over the baseline value in the placebo group on day 22 of
both the first and second chemotherapy courses, while the
lymphocyte count was found to be decreased on day 8 of both
courses. Therefore, it may be surmised that the lymphocyte count
decreased transiently due to the anthracycline-based chemotherapy,
and then, during the ensuing recovery process, the
immunosuppressive Tregs recovered earlier among the subsets of
CD4+ cells. On the other hand, in the LEM group, the
Treg proportion on day 8 of course 1 was significantly reduced
compared with the baseline value, and this reduction was maintained
on day 22 of course 1. In addition, on day 22 of course 2, as in
the placebo group, the Treg proportion increased significantly
compared with the baseline value, but the degree of that increase
tended to be lower compared with the placebo group. In vivo,
LEM mitigated the immunosuppression mediated by the tumor-bearing
host's Tregs and induced antitumor immunity (18,19).
Moreover, in a study on patients receiving cancer immune-cell
therapy, the increase in Tregs accompanying cancer progression was
reported to be improved (31). It is
known that certain chemotherapies may reduce Tregs (32), and low-dose cyclophosphamide, which
is a drug used in anthracycline-based regimens, is known to
somewhat selectively reduce Tregs (33,34). In
the present study, the dose of cyclophosphamide was not as low as
in the former reports (33,34), and it is perhaps for that reason that
the total lymphocyte count appeared to be decreased non-selectively
in both groups. In the LEM group, the data suggested that LEM may
promote the reduction of Tregs on day 8 of course 1 and inhibit the
increase in Tregs on day 22 of course 2 after the recovery process.
With regard to the association between the prognosis of breast
cancer patients and Tregs, a number of reports have verified the
association between Tregs in the tumor-infiltrating lymphocyte
population and prognosis. However, the association of Tregs with
prognosis remains controversial, possibly due to variations in the
tumor microenvironment (33,35,36).
With regard to the Treg proportion among CD4+ cells in
the peripheral blood of breast cancer patients administered
postoperative adjuvant therapy who were evaluated in this study, it
was reported that this proportion decreases temporarily after
surgery, and then rebounds after postoperative adjuvant
chemotherapy (37). It may be
hypothesized that, in breast cancer patients, suppression of immune
function occurs following postoperative adjuvant therapy, similar
to that occurring under tumor-bearing conditions. Based on the
results of the present study, indicating that coadministration of
LEM has the potential to improve the suppression of immune function
that occurs following adjuvant chemotherapy, more detailed studies
on this subject are anticipated in the future.
In conclusion, postoperative breast cancer patients
who receive anthracycline-based chemotherapy experience decreased
QOL and immune function. However, coadministration of LEM may
ameliorate those decreases in QOL and immune function. Accordingly,
oral LEM may prove to be a useful supportive care option for such
postoperative breast cancer patients.
Acknowledgements
The present study received a research grant from the
Osaka Cancer Research Foundation.
References
|
1
|
Group (EBCTCG): Effects of chemotherapy
and hormonal therapy for early breast cancer on recurrence and
15-year survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar
|
|
2
|
French Adjuvant Study Group: Benefit of a
high-dose epirubicin regimen in adjuvant chemotherapy for
node-positive breast cancer patients with poor prognostic factors:
5-year follow-up results of French Adjuvant Study Group 05
randomized trial. J Clin Oncol. 19:602–611. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Turner N, Biganzoli L and Di Leo A:
Continued value of adjuvant anthracyclines as treatment for early
breast cancer. Lancet Oncol. 16:e362–e369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Riccardi A, Brugnatelli S, Giordano M,
Danova M, Pugliese P, Tinelli C, Klersy C, Richetti A, Fava S,
Nastasi G, et al: Myeloprotective effect of early primary
granulocyte-colony stimulating factor during six courses of
intensified 5-fluorouracil, epirubicin and cyclophosphamide
(120FEC) chemotherapy for advanced breast cancer. Cooperative group
of study and treatment of breast cancer. Tumori. 84:540–546.
1998.PubMed/NCBI
|
|
5
|
Baltali E, Günel N, Onat DA, Atahan IL,
Akçali Z, Büyukünal E and Firat D: Neoadjuvant chemotherapy in
locally advanced breast cancer: A preliminary report. Turkish
oncology study group. Tumori. 85:483–487. 1999.PubMed/NCBI
|
|
6
|
Takeuchi H, Saeki T, Aiba K, Tamura K,
Aogi K, Eguchi K, Okita K, Kagami Y, Tanaka R, Nakagawa K, et al:
Japanese society of clinical oncology clinical practice guidelines
2010 for antiemesis in oncology: Executive summary. Int J Clin
Oncol. 21:1–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohzawa H, Miki A, Hozumi Y, Miyazaki C,
Sagara Y, Tanaka Y, Shiba S, Joutoku H, Sakuragi M, Takehara M, et
al: Comparison between the antiemetic effects of palonosetron and
granisetron in breast cancer patients treated with
anthracycline-based regimens. Oncol Lett. 9:119–124.
2015.PubMed/NCBI
|
|
8
|
Rapoport BL, Jordan K, Boice JA, Taylor A,
Brown C, Hardwick JS, Carides A, Webb T and Schmoll HJ: Aprepitant
for the prevention of chemotherapy-induced nausea and vomiting
associated with a broad range of moderately emetogenic
chemotherapies and tumor types: A randomized, double-blind study.
Support Care Cancer. 18:423–431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berger AM, Lockhart K and Agrawal S:
Variability of patterns of fatigue and quality of life over time
based on different breast cancer adjuvant chemotherapy regimens.
Oncol Nurs Forum. 36:563–570. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kornblith AB, Lan L, Archer L, Partridge
A, Kimmick G, Hudis C, Winer E, Casey R, Bennett S, Cohen HJ and
Muss HB: Quality of life of older patients with early-stage breast
cancer receiving adjuvant chemotherapy: A companion study to cancer
and leukemia group B 49907. J Clin Oncol. 29:1022–1028. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martín M, Lluch A, Seguí MA, Ruiz A, Ramos
M, Adrover E, Rodriguez-Lescure A, Grosse R, Calvo L,
Fernandez-Chacón C, et al: Toxicity and health-related quality of
life in breast cancer patients receiving adjuvant docetaxel,
doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin
and cyclophosphamide (FAC): Impact of adding primary prophylactic
granulocyte-colony stimulating factor to the TAC regimen. Ann
Oncol. 17:1205–1212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Apetoh L, Ghiringhelli F, Tesniere A,
Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E,
Saulnier P, et al: Toll-like receptor 4-dependent contribution of
the immune system to anticancer chemotherapy and radiotherapy. Nat
Med. 13:1050–1059. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mozaffari F, Lindemalm C, Choudhury A,
Granstam-Björneklett H, Helander I, Lekander M, Mikaelsson E,
Nilsson B, Ojutkangas ML, Osterborg A, et al: NK-cell and T-cell
functions in patients with breast cancer: effects of surgery and
adjuvant chemo- and radiotherapy. Br J Cancer. 97:105–111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawanishi T, Ikeda-Dantsuji Y and Nagayama
A: Effects of two basidiomycete species on interleukin 1 and
interleukin 2 production by macrophage and T cell lines.
Immunobiology. 215:516–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugano N, Hibino Y, Choji Y and Maeda H:
Anticarcinogenic actions of water-soluble and alcohol-insoluble
fractions from culture medium of Lentinus edodes mycelia. Cancer
Lett. 17:109–114. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sugano N, Choji Y, Hibino Y, Yasumura S
and Maeda H: Anticarcinogenic action of an alcohol-insoluble
fraction (LAP1) from culture medium of Lentinus edodes mycelia.
Cancer Lett. 27:1–6. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu M, Li J, Kong F, Lin J and Gao Y:
Induction of immunomodulating cytokines by a new
polysaccharide-peptide complex from culture mycelia of Lentinus
edodes. Immunopharmacology. 40:187–198. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanaka K, Matsui Y, Ishikawa S, Kawanishi
T and Harada M: Oral ingestion of Lentinula edodes mycelia extract
can restore the antitumor T cell response of mice inoculated with
colon-26 cells into the subserosal space of the cecum. Oncol Rep.
27:325–332. 2012.PubMed/NCBI
|
|
19
|
Tanaka K, Ishikawa S, Matsui Y, Tamesada
M, Harashima N and Harada M: Oral ingestion of Lentinula edodes
mycelia extract inhibits B16 melanoma growth via mitigation of
regulatory T cell-mediated immunosuppression. Cancer Sci.
102:516–521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamaguchi Y, Miyahara E and Hihara J:
Efficacy and safety of orally administered Lentinula edodes mycelia
extract for patients undergoing cancer chemotherapy: A pilot study.
Am J Chin Med. 39:451–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okuno K and Uno K: Efficacy of orally
administered Lentinula edodes mycelia extract for advanced
gastrointestinal cancer patients undergoing cancer chemotherapy: A
pilot study. Asian Pac J Cancer Prev. 12:1671–1674. 2011.PubMed/NCBI
|
|
22
|
Suzuki N, Takimoto Y, Suzuki R, Arai T,
Uebaba K, Nakai M, Strong JM and Tokuda H: Efficacy of oral
administration of Lentinula edodes mycelia extract for breast
cancer patients undergoing postoperative hormone therapy. Asian Pac
J Cancer Prev. 14:3469–3472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagashima Y, Maeda N, Yamamoto S, Yoshino
S and Oka M: Evaluation of host quality of life and immune function
in breast cancer patients treated with combination of adjuvant
chemotherapy and oral administration of Lentinula edodes mycelia
extract. Onco Targets Ther. 6:853–859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kurihara M, Shimizu H, Tsuboi K, Kobayashi
K, Murakami M, Eguchi K and Shimozuma K: Development of quality of
life questionnaire in Japan: Quality of life assessment of cancer
patients receiving chemotherapy. Psychooncology. 8:355–363. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bacik J, Mazumdar M, Murphy BA, Fairclough
DL, Eremenco S, Mariani T, Motzer RJ and Cella D: The functional
assessment of cancer therapy-BRM (FACT-BRM): A new tool for the
assessment of quality of life in patients treated with biologic
response modifiers. Qual Life Res. 13:137–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmidt ME, Wiskemann J, Armbrust P,
Schneeweiss A, Ulrich CM and Steindorf K: Effects of resistance
exercise on fatigue and quality of life in breast cancer patients
undergoing adjuvant chemotherapy: A randomized controlled trial.
Int J Cancer. 137:471–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mozaffari F, Lindemalm C, Choudhury A,
Granstam-Björneklett H, Lekander M, Nilsson B, Ojutkangas ML,
Osterborg A, Bergkvist L and Mellstedt H: Systemic immune effects
of adjuvant chemotherapy with 5-fluorouracil, epirubicin and
cyclophosphamide and/or radiotherapy in breast cancer: A
longitudinal study. Cancer Immunol Immunother. 58:111–120. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sato E, Olson SH, Ahn J, Bundy B,
Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone
C, et al: Intraepithelial CD8+ tumor-infiltrating
lymphocytes and a high CD8+/regulatory T cell ratio are
associated with favorable prognosis in ovarian cancer. Proc Natl
Acad Sci USA. 102:18538–18543. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamaguchi T and Sakaguchi S: Regulatory T
cells in immune surveillance and treatment of cancer. Semin Cancer
Biol. 16:115–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanigawa K, Ito Y, Sakai M and Kobayashi
Y: Evaluation of quality of life and immune function in cancer
patients receiving combined immunotherapy and oral administration
of lentinula edodes mycelia extract. Gan To Kagaku Ryoho.
39:1779–1781. 2012.(In Japanese). PubMed/NCBI
|
|
32
|
Maeda K, Hazama S, Tokuno K, Kan S, Maeda
Y, Watanabe Y, Kamei R, Shindo Y, Maeda N, Yoshimura K, et al:
Impact of chemotherapy for colorectal cancer on regulatory T-cells
and tumor immunity. Anticancer Res. 31:4569–4574. 2011.PubMed/NCBI
|
|
33
|
Bates GJ, Fox SB, Han C, Leek RD, Garcia
JF, Harris AL and Banham AH: Quantification of regulatory T cells
enables the identification of high-risk breast cancer patients and
those at risk of late relapse. J Clin Oncol. 24:5373–5380. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ercolini AM, Ladle BH, Manning EA,
Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA,
Reilly RT and Jaffee EM: Recruitment of latent pools of
high-avidity CD8(+) T cells to the antitumor immune response. J Exp
Med. 201:1591–1602. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu S, Foulkes WD, Leung S, Gao D, Lau S,
Kos Z and Nielsen TO: Prognostic significance of FOXP3+
tumor-infiltrating lymphocytes in breast cancer depends on estrogen
receptor and human epidermal growth factor receptor-2 expression
status and concurrent cytotoxic T-cell infiltration. Breast Cancer
Res. 16:4322014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aruga T, Suzuki E, Saji S, Horiguchi S,
Horiguchi K, Sekine S, Kitagawa D, Funata N, Toi M, Sugihara K and
Kuroi K: A low number of tumor-infiltrating FOXP3-positive cells
during primary systemic chemotherapy correlates with favorable
anti-tumor response in patients with breast cancer. Oncol Rep.
22:273–278. 2009.PubMed/NCBI
|
|
37
|
Rech AJ, Mick R, Kaplan DE, Chang KM,
Domchek SM and Vonderheide RH: Homeostasis of peripheral FoxP3(+)
CD4 (+) regulatory T cells in patients with early and late stage
breast cancer. Cancer Immunol Immunother. 59:599–607. 2010.
View Article : Google Scholar : PubMed/NCBI
|