Introduction
Nasopharyngeal carcinoma (NPC) is disproportionately
common in southern China (1), where
the annual incidence is 15–50/100,000 individuals (2). Radiotherapy (RT) is the primary
treatment for NPC. While advances in tumor imaging and RT
technologies have increased 5-year disease-free survival (DFS) to
77% for early-stage disease (3),
distant metastasis remains a major obstacle to further improvements
in survival (4).
The immune checkpoints proteins programmed death-1
(PD-1) and programmed death ligand-1 (PD-L1) have been reported to
be important immunotherapy targets. The antitumor efficacy of
blocking these targets has been confirmed in vitro as well
as in vivo. Blocking the PD-1/PD-L1 signaling pathway
represents a promising immunotherapeutic strategy to enhance the
ability of the immune system to target cancer cells (5,6).
PD-L1 is expressed in a variety of tumor types,
including esophageal, gastrointestinal tract, pancreatic, breast,
lung and kidney carcinomas (7,8). Several
studies have indicated that tumor PD-L1 expression is associated
with poor prognosis (9,10); however, it remains unclear whether
such an association exists in NPC. The aim of the present study was
to investigate the associations between tumor PD-L1 expression and
prognosis in NPC.
Patients and methods
Patients and sample collection
Two cohorts of patients with NPC were enrolled: 62
patients with primary biopsy-confirmed NPC diagnosed between
January 2009 and January 2012 at the Second Affiliated Hospital of
Nanchang University (Nanchang, China) and 58 patients with primary
biopsy-confirmed NPC diagnosed between January 2010 and May 2015 at
the People's Hospital of Fuzhou (Fuzhou, China). Formalin-fixed
paraffin-embedded tissues were obtained from the initial diagnostic
biopsy specimens. Information on the clinical course of each
patient, including sex, age, tumor staging and smoking history, was
obtained from medical records. Complete follow-up data were
available for all 62 patients treated at the Second Affiliated
Hospital of Nanchang University. Data on disease and vital status
for these patients were obtained using a prospectively-maintained
hospital tumor registry. The study protocol was approved by the
Medical Ethics Committees of the Second Affiliated Hospital of
Nanchang University and the People's Hospital of Fuzhou.
Immunohistochemistry
Immunohistochemical staining for the PD-L1 protein
was performed using sections prepared from the formalin-fixed
diagnostic samples. Briefly, 4-µm sections were deparaffinized in
xylene and rehydrated through a graded ethanol series to distilled
water. After processing via routine procedures, the sections were
incubated overnight at 4°C with rabbit anti-PD-L1 antibody (E1L3
N™, catalog no. 13684, Cell Signaling Technology Inc., Shanghai,
China) diluted 1:100 in blocking solution, followed by incubation
with a horseradish peroxidase-conjugated secondary antibody
(MaxVision Immunohistochemical Detection kit, rabbit/mouse, 1:100
dilution, catalog no. 5001, MaxVision Biosciences, Inc., Fuzhou,
China) according to the manufacturer's instructions. After washing,
color was developed by incubation in 3,3′-diaminobenzidine
tetrahydrochloride, followed by hematoxylin counterstaining.
The staining was independently assessed by two
surgical pathologists using a semi-quantitative scale that ranges
from 0 to 100% for the proportion of PD-L1-positive cancer cells.
The mean score for replicate samples was recorded. Two groups of
patients were created based on receiver operating characteristic
(ROC) curves. According to the results, 20% was set as the cut-off
value for dividing the samples into high PD-L1 and low PD-L1 group
for DFS. The areas under the ROC curves were 0.827.
Statistical analysis
The associations between PD-L1 expression and
clinicopathological characteristics were assessed using Pearson's
χ2 test. Overall survival (OS) and DFS were compared using the
Kaplan-Meier method and log-rank tests. Cox proportional hazards
analysis was performed to calculate hazard ratios (HRs) and 95%
confidence intervals (CIs) to evaluate the associations between
tumor PD-L1 expression and survival outcome. Multivariate survival
analysis was conducted for all of the parameters using the Cox
regression model. All analyses were performed using SPSS software,
version 13.0 (SPSS Inc., Chicago, IL, USA). Two-sided P-values
<0.05 were considered to indicate statistically significant
differences.
Results
Clinicopathological
characteristics
The 120 patients in this cohort ranged in age from
17 to 69 years (median, 48 years). A total of 78% (79/102) of the
patients had stage I–III and 22% (23/102) had stage IV NPC
according to the 1997 American Joint Committee on Cancer staging
system (11). All the cases were
classified as undifferentiated non-keratinizing NPC. The estimated
5-year OS and DFS rates for the group eligible for survival
analysis (the 62 patients treated at the Second Affiliated Hospital
of Nanchang University) were 87.5 and 70.1%, respectively. Other
clinicopathological characteristics are summarized in Table I.
 | Table I.Associations between PD-L1 expression
and the clinicopathological characteristics of nasopharyngeal
carcinoma. |
Table I.
Associations between PD-L1 expression
and the clinicopathological characteristics of nasopharyngeal
carcinoma.
|
| PD-L1 expression |
|
|---|
|
|
|
|
|---|
| Clinicopathological
characteristics | High | Low | P-valuea |
|---|
| Sex |
|
|
|
| Male | 40 | 46 |
|
|
Female | 14 | 20 | 0.597 |
| Age, years |
|
|
|
| ≤45 | 9 | 26 |
|
|
>45 | 45 | 40 | 0.006 |
| Smoking history |
|
|
|
| No | 26 | 28 |
|
| Yes | 18 | 19 | 0.963 |
| Histology |
|
|
|
|
Differentiated | 29 | 26 |
|
|
Undifferentiated | 25 | 40 | 0.118 |
| AJCC stage
(2010) |
|
|
|
|
I–III | 32 | 47 |
|
| IV | 15 | 8 | 0.036 |
| T stage |
|
|
|
|
T1/T2 | 17 | 30 |
|
|
T3/T4 | 30 | 25 | 0.063 |
| N stage |
|
|
|
|
N0/N1 | 22 | 17 |
|
|
N2/N3 | 25 | 38 | 0.100 |
PD-L1 protein is frequently expressed
in NPC
To investigate whether PD-L1 is expressed in NPC
similar to its expression in other tumors, PD-L1 protein expression
was assessed using immunohistochemistry in tumor samples from 120
patients with NPC. The PD-L1 protein was found to be frequently
expressed in NPC. PD-L1 expression was detected in 71% (85/120) of
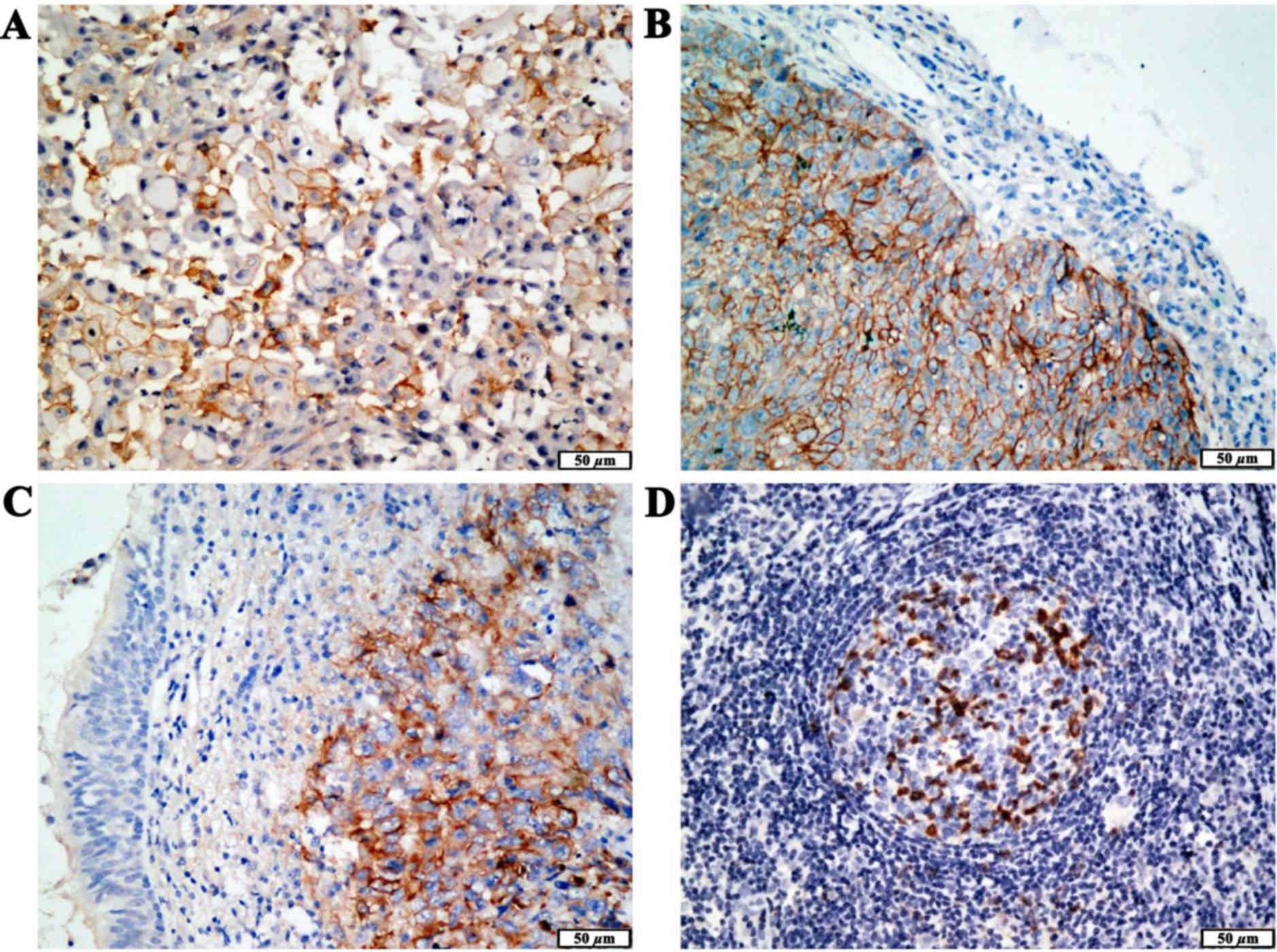
the tumor samples. The PD-L1 protein was localized to the cell
membrane of the primary tumor cells (Fig. 1A) and metastatic lymph nodes
(Fig. 1D). Moreover, PD-L1 protein
expression was significantly higher in the tumor cells compared
with that in adjacent non-cancerous tissues (Fig. 1B and C).
Associations between PD-L1 expression
and the clinicopathological characteristics of NPC
To identify the clinical relevance of PD-L1
expression in NPC, the associations between PD-L1 expression and
clinicopathological characteristics of NPC, such as sex, age,
smoking history, histology and TNM stage, were examined. The 120
patients were further classified into the high PD-L1 (n=54) and low
PD-L1 (n=66) groups based on the tumor PD-L1 expression level. The
results revealed that high PD-L1 expression was significantly
associated with age (P=0.006) and stage (P=0.036), but not the
other clinical characteristics examined (Table I).
Associations between PD-L1 expression
and prognosis in NPC
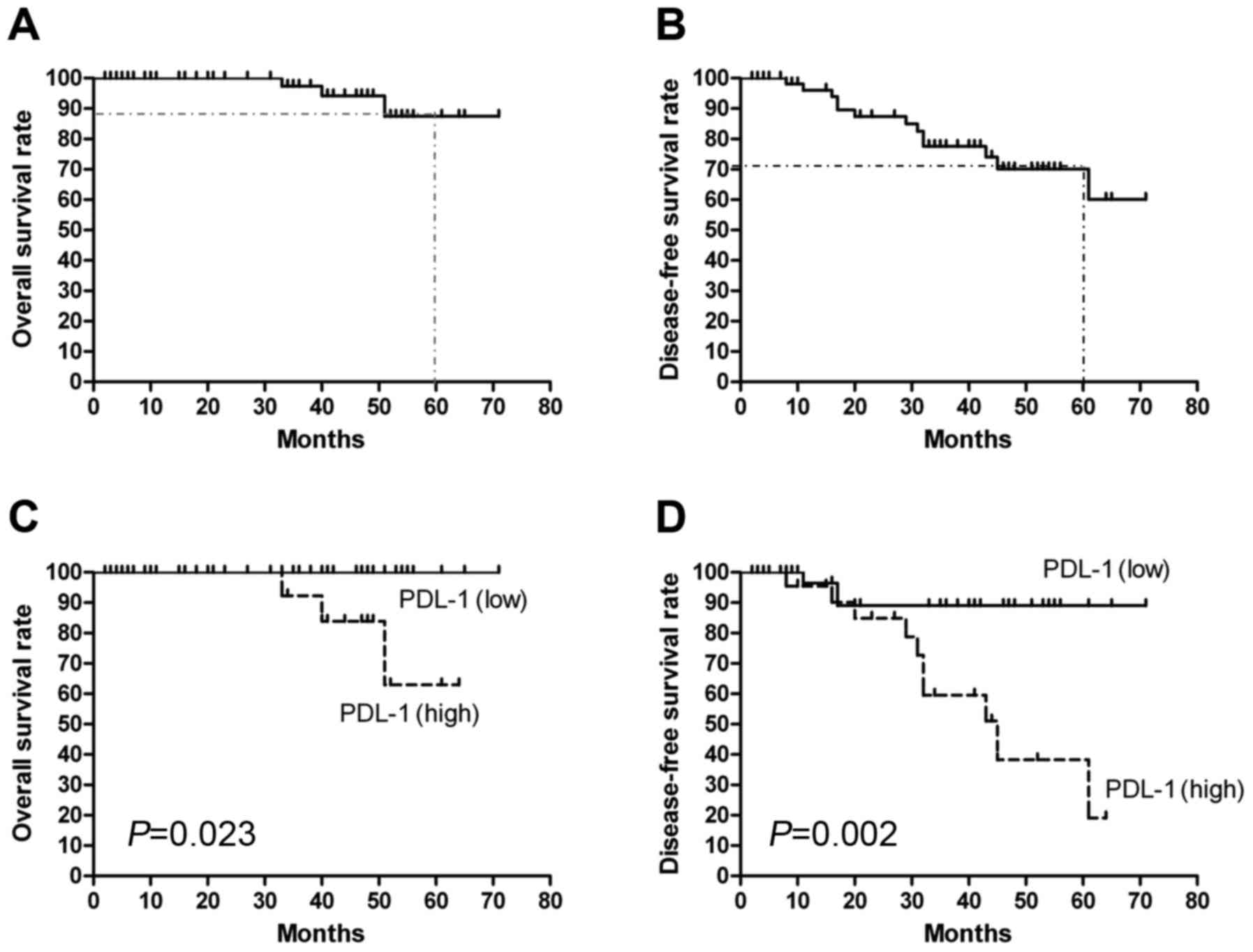
Estimated 5-year OS and DFS for the group eligible
for survival analysis (the 62 patients treated at Second Affiliated
Hospital of Nanchang University) were 87.5 and 70.1%, respectively
(Fig. 2A and B). The OS and DFS
curves for the high PD-L1 and low PD-L1 groups are shown in
Fig. 2C and D. As expected, patients
with high PD-L1 expression had significantly poorer OS (P=0.023)
and DFS (P=0.002) compared with patients with low PD-L1 expression.
Univariate Cox proportional regression analysis revealed that T
stage (HR=4.081, 95% CI: 1.130–18.14; P=0.048) and PD-L1 expression
(HR=0.163, 95% CI: 0.044–0.600; P=0.006) were prognostic factors
for DFS (Table II). A multivariate
analysis using the Cox regression model revealed that T stage
(HR=8.190, 95% CI: 1.355–18.152; P=0.023) and PD-L1 expression
level (HR=0.124, 95% CI: 0.031–0.509; P=0.001) served as
independent prognostic factors for DFS.
 | Table II.Univariate analysis of the
associations between the clinicopathological characteristics of
nasopharyngeal carcinoma and disease-free survival. |
Table II.
Univariate analysis of the
associations between the clinicopathological characteristics of
nasopharyngeal carcinoma and disease-free survival.
| Clinicopathological
characteristics | Subset | Hazard ratio (95%
CI) | P-value |
|---|
| Gender | Male vs.
female | 1.322
(0.431–4.056) | 0.626 |
| Age, years | >45 vs. ≤45 | 1.747
(0.570–5.352) | 0.329 |
| Smoking
history | No vs. yes | 0.785
(0.207–2.973) | 0.722 |
| Histology | Undifferentiated
vs. differentiated | 1.595
(0.521–4.886) | 0.413 |
| AJCC stage | IV vs. I–III | 1.662
(0.533–5.181) | 0.381 |
| T stage | T3/T4 vs.
T1/T2 | 4.081
(1.130–18.14) | 0.048 |
| N stage | N0/N1 vs. ≤
N2/N3 | 0.530
(0.145–1.947) | 0.339 |
| Neoadjuvant
chemotherapy 1 | TPF vs.
TP/FP/other | 0.489
(0.134–1.792) | 0.281 |
| Neoadjuvant
chemotherapy 2 | Includes NDP vs.
DDP | 1.669
(0.451–6.181) | 0.443 |
| Neoadjuvant
chemotherapy 3 | Yes vs. no | 0.832
(0.270–2.562) | 0.749 |
| Radiotherapy | CCRT vs. RT | 0.461
(0.149–1.426) | 0.179 |
| Adjuvant
chemotherapy | 2-3 vs. 0–1
cycles | 0.370
(0.113–1.218) | 0.102 |
| PD-L1
expression | Negative vs.
positive | 0.163
(0.044–0.600) | 0.006 |
Discussion
Tumor immune evasion is considered to be a hallmark
of cancer (12). Blockade of immune
checkpoints represents a promising therapeutic approach to activate
antitumor immunity (13). PD-L1 is
recognized as an important immunosuppressive factor (14) and is upregulated in a variety of
Epstein-Barr virus (EBV)-associated malignancies (15), although its expression in NPC, an
EBV-associated malignancy with a higher metastatic potential
compared with other head and neck cancers, is poorly characterized
(16,17). The expression of PD-L1 is associated
with the treatment response to nivolumab, an anti-PD-1 antibody, in
a subset of tumor types (18). The
efficacy of immune-targeted therapies in EBV-associated
malignancies requires further investigation. Therefore, assessing
the expression of biomarkers such as PD-L1 may help identify
patients who will benefit from immunomodulatory agents. However,
variation in PD-L1 expression rates previously observed in a study
of various cancers has been attributed to differences in cut-off
values, antibodies, and study populations (19,20). In
lung cancer characterized by PD-L1 expression, the expression rates
varied from 24 to 60% using the same 5% cut-off value (21,22), and
from 21 to 95 % according to different cut-off values of 1, 10 and
50% (23,24). Thus, the use of PD-L1 expression as a
marker remains controversial due to the different antibodies used,
the method of assessment and the different thresholds.
In the present study, tumor PD-L1 expression and its
association with the clinicopathological characteristics and
survival outcomes of NPC were analyzed. The PD-L1 protein was found
to be frequently expressed in NPC and high tumor expression of
PD-L1 was prognostic for poor DFS. Fang et al (25) reported that 89% (16/18) of
EBV-associated NPC cases expressed PD-L1 in the malignant tumor
cells, which is consistent with the findings of our study.
Additionally, high expression of PD-L1 was also found to be
associated with poor clinical prognosis in renal cell carcinoma,
hepatocellular cancer and breast cancer (26–29).
Previous studies demonstrated that
constitutively-activated oncogenic pathways may upregulate PD-L1.
Parsa et al reported loss of phosphatase and tensin homolog
and resulting activation of the phosphoinositide 3-kinase pathway
significantly upregulates PD-L1 in glioma (30). Marzec et al found constitutive
activation of nucleophosmin/anaplastic lymphoma kinase induces
PD-L1 expression via signal transducer and activator of
transcription 3 (31). The mechanism
through which PD-L1 is upregulated in NPC has not been fully
elucidated. Fang et al observed that high levels of PD-L1
expression in EBV-infected NPC cells were associated with latent
membrane protein 1 (LMP1)-mediated oncogenic pathways and immune
modulation via excretion of interferon-γ (25). These results indicated that
inhibition of the LMP1 oncogenic pathway and PD-1/PD-L1 checkpoints
may provide a clinical benefit during the treatment of
EBV-associated NPC.
In conclusion, PD-L1 is frequently upregulated in
NPC, and high PD-L1 expression was associated with poor prognosis.
These findings implicate PD-L1 in the development of NPC and
highlight the potential of PD-L1 as a therapeutic target for NPC.
Patients with NPC whose tumors express high levels of PD-L1 may be
optimal candidates for clinical trials of strategies aiming to
block PD-L1.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81460393), the Natural
Science Foundation of Jiangxi Province, China (grant nos.
20142BAB215039 and 20151BAB215020), the Project of Jiangxi Province
Science and Technology Plan (grant nos. 20141BBG70041 and
GJJ14059), the Youth Science Fund Project of the Second Affiliated
Hospital of Nanchang University (grant no. 2014YNQN12004 to Long
Huang), and in part by the Natural Science Foundation of China
(grant no. 81460449 to Ling-Min Liao).
References
|
1
|
Licitra L, Bernier J, Cvitkovic E, Grandi
C, Spinazzé S, Bruzzi P, Gatta G and Molinari R: Cancer of the
nasopharynx. Crit Rev Oncol Hematol. 45:199–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao SM, Simons MJ and Qian CN: The
prevalence and prevention of nasopharyngeal carcinoma in China.
Chin J Cancer. 30:114–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma BB, Hui EP and Chan AT: Systemic
approach to improving treatment outcome in nasopharyngeal
carcinoma: Current and future directions. Cancer Sci. 99:1311–1318.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao WW, Huang SM, Han F, Wu SX, Lu LX,
Lin CG, Deng XW, Lu TX, Cui NJ and Zhao C: Local control, survival,
and late toxicities of locally advanced nasopharyngeal carcinoma
treated by simultaneous modulated accelerated radiotherapy combined
with cisplatin concurrent chemotherapy: Long-term results of a
phase 2 study. Cancer. 117:1874–1883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Topalian SL, Drake CG and Pardoll DM:
Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor
immunity. Curr Opin Immunol. 24:207–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu J, Lee-Gabel L, Nadeau MC, Ferencz TM
and Soefje SA: Clinical evaluation of compounds targeting
PD-1/PD-L1 pathway for cancer immunotherapy. J Oncol Pharm Pract.
21:451–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zou W and Chen L: Inhibitory B7-family
molecules in the tumour microenvironment. Nat Rev Immunol.
8:467–477. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flies DB and Chen L: The new B7s: Playing
a pivotal role in tumor immunity. J Immunother. 30:251–260. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muenst S, Schaerli AR, Gao F, Däster S,
Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE,
et al: Expression of programmed death ligand 1 (PD-L1) is
associated with poor prognosis in human breast cancer. Breast
Cancer Res Treat. 146:15–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thompson RH, Kuntz SM, Leibovich BC, Dong
H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H,
et al: Tumor B7-H1 is associated with poor prognosis in renal cell
carcinoma patients with long-term follow-up. Cancer Res.
66:3381–3385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chua DT, Sham JS, Wei WI, Ho WK and Au GK:
The predictive value of the 1997 American joint committee on cancer
stage classification in determining failure patterns in
nasopharyngeal carcinoma. Cancer. 92:2845–2855. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dolan DE and Gupta S: PD-1 pathway
inhibitors: Changing he landscape of cancer immunotherapy. Cancer
Control. 21:231–237. 2014.PubMed/NCBI
|
|
15
|
Chen BJ, Chapuy B, Ouyang J, Sun HH,
Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA and Rodig
SJ: PD-L1 expression is characteristic of a subset of aggressive
B-cell lymphomas and virus-associated malignancies. Clin Cancer
Res. 19:3462–3473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin X, Gudgeon NH, Hui EP, Jia H, Qun X,
Taylor GS, Barnardo MC, Lin CK, Rickinson AB and Chan AT: CD4 and
CD8 T cell responses to tumour-associated Epstein-Barr virus
antigens in nasopharyngeal carcinoma patients. Cancer Immunol
Immunother. 57:963–975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li HP, Peng CC, Chung IC, Huang MY, Huang
ST, Chen CC, Chang KP, Hsu CL and Chang YS: Aberrantly
hypermethylated Homeobox A2 derepresses metallo proteinase-9
through TBP and promotes invasion in Nasopharyngeal carcinoma.
Oncotarget. 4:2154–2165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Teixido C, Karachaliou N, González-Cao M,
Morales-Espinosa D and Rosell R: Assays for predicting and
monitoring responses to lung cancer immunotherapy. Cancer Biol Med.
12:87–95. 2015.PubMed/NCBI
|
|
20
|
Patel SP and Kurzrock R: PD-L1 expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
D'Incecco A, Andreozzi M, Ludovini V,
Rossi E, Capodanno A, Landi L, Tibaldi C, Minuti G, Salvini J,
Coppi E, et al: PD-1 and PD-L1 expression in molecularly selected
non-small-cell lung cancer patients. Br J Cancer. 112:95–102. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang W, Zhang J, Hong S, Zhan J, Chen N,
Qin T, Tang Y, Zhang Y, Kang S, Zhou T, et al: EBV-driven LMP1 and
IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: Implications
for oncotargeted therapy. Oncotarget. 5:12189–12202. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thompson RH, Dong H and Kwon ED:
Implications of B7-H1 expression in clear cell carcinoma of the
kidney for prognostication and therapy. Clin Cancer Res.
13:709s–715s. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang
JY, Yang YP, Tien P and Wang FS: PD-1 and PD-L1 upregulation
promotes CD8(+) T-cell apoptosis and postoperative recurrence in
hepatocellular carcinoma patients. Int J Cancer. 128:887–896. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghebeh H, Tulbah A, Mohammed S, Elkum N,
Bin Amer SM, Al-Tweigeri T and Dermime S: Expression of B7-H1 in
breast cancer patients is strongly associated with high
proliferative Ki-67-expressing tumor cells. Int J Cancer.
121:751–758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muenst S, Soysal SD, Gao F, Obermann EC,
Oertli D and Gillanders WE: The presence of programmed death 1
(PD-1)-positive tumor-infiltrating lymphocytes is associated with
poor prognosis in human breast cancer. Breast Cancer Res Treat.
139:667–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parsa AT, Waldron JS, Panner A, Crane CA,
Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et
al: Loss of tumor suppressor PTEN function increases B7-H1
expression and immunoresistance in glioma. Nat Med. 13:84–88. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marzec M, Zhang Q, Goradia A, Raghunath
PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA and
Wasik MA: Oncogenic kinase NPM/ALK induces through STAT3 expression
of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad
Sci USA. 105:20852–20857. 2008. View Article : Google Scholar : PubMed/NCBI
|