Introduction
Endometrial cancer is a common gynecological
malignancy in western countries, whereas its incidence has also
been increasing in Asia (1).
Endometrial cancer may be subdivided into types I and II. Type I
tumors, which are associated with obesity or exogenous estrogen
use, present at an early stage and have an excellent prognosis.
Histologically, these tumors are well- to moderately-differentiated
endometrioid adenocarcinomas, with typically minimal invasion and
infrequent nodal metastasis. Type II tumors are not classically
associated with obesity or exogenous estrogen use. Histologically,
these tumors are poorly differentiated endometrioid carcinomas,
with serous or clear-cell characteristics (2). The standard chemotherapy for
endometrial cancer worldwide currently consists of a combination of
doxorubicin and cisplatin (AP therapy). Combinations of taxanes and
platinum-containing drugs (TC therapy) have also been used as
adjuvant therapy and as chemotherapy for advanced or recurrent
endometrial cancer (3). However, the
prognosis of patients with metastatic disease remains
disappointing, with an overall survival of only 1 year commonly
reported, despite treatment efforts (4).
Currently, genetic abnormalities associated with the
onset and progression of cancer have been identified in the
endometrial cell membrane and signaling systems, and the
developmental mechanisms of endometrial cancer are gradually
becoming elucidated. The identification of the molecules associated
with these abnormalities has led to new potential treatment
regimens for endometrial cancer using molecularly-targeted agents
(3). For example, preclinical models
have demonstrated the efficacy of bevacizumab in combination with
chemotherapy against endometrial cell lines (4). In clinical practice, the GOG phase II
trial is the most recent completed trial of targeted therapy in
recurrent/persistent endometrial cancer (5). We hypothesized that bevacizumab may be
active in endometrial cancer. We herein present the case of a
patient with recurrent endometrial cancer who experienced a
prolonged response to combination treatment with bevacizumab +
chemotherapy.
Case report
A 78-year-old woman, gravida 3, para 2, was referred
to the Shimane University Hospital (Izumo, Japan) for examination
and treatment of a pelvic mass. The carbohydrate antigen (CA)-125
level was 32 ng/ml. No metastatic lesions were identified. The
patient underwent modified radical hysterectomy, bilateral
salpingo-oophorectomy, pelvic lymph node dissection and
omentectomy. Pathological examination revealed a stage IIA
(International Federation of Obstetrics and Gynecology 1988)
endometrioid adenocarcinoma, grade 2 endometrial cancer (myometrial
invasion 40%, ascitic fluid cytology-positive, estrogen
receptor-negative, progesterone receptor-negative and p53
mutation-positive). The immunohistochemistry results confirmed the
diagnosis of type II endometrial cancer.
As adjuvant therapy, the patient received
combination chemotherapy consisting of paclitaxel (135
mg/m2) and carboplatin [area under the curve (AUC)=4]
(TC therapy). Due to impaired hepatic function, chemotherapy was
discontinued after three cycles.
Eleven months after adjuvant chemotherapy, the
CA1-25 level started to increase; pleural metastasis, pelvic lymph
node metastasis, pleural effusion and ascites were observed on
computed tomography (CT) examination. The patient was diagnosed
with recurrence of endometrial cancer. After recovery of hepatic
function was confirmed, she received three cycles of TC therapy.
Unfortunately, the pleural and pelvic lymph node metastasis
progressed, with detection of intraperitoneal implantations and
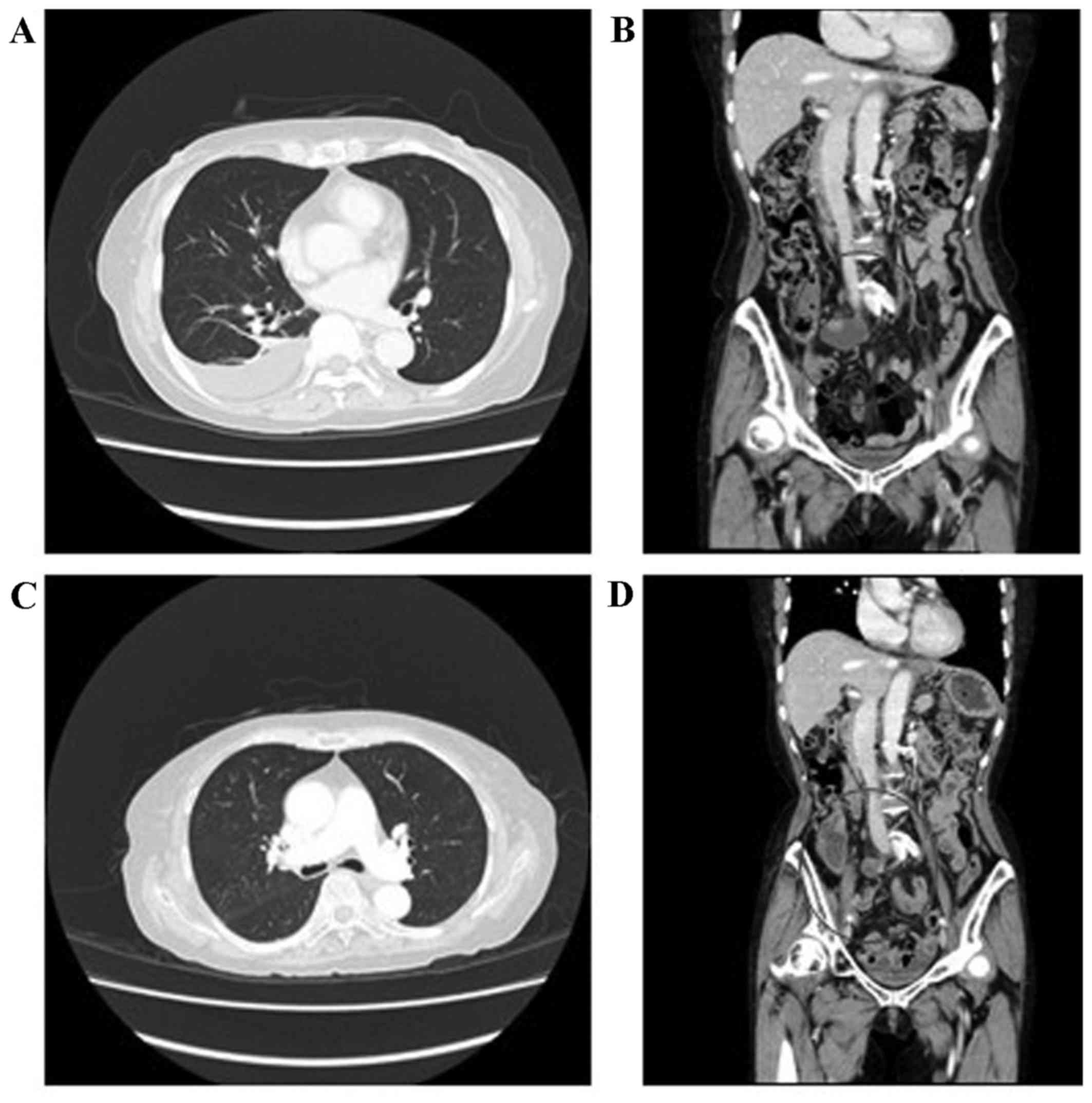
para-aortic lymph node metastasis (Fig.
1A and B). The patient was then initiated on combination
chemotherapy consisting of adriamycin (60 mg/m2) and
cisplatin (50 mg/m2) (AP therapy). After two cycles, an
increase in the amount of pleural effusion was observed. Although
both the TC and AP regimens were used, the CA-125 levels continued
to increase and the CT images suggested progressive disease. The
Ethics Committee of the hospital granted approval for the use of
bevacizumab to treat the recurrent and persistent endometrial
cancer, and treatment was initiated after obtaining written
informed consent from the patient.
First, combination therapy consisting of paclitaxel
(175/m2), carboplatin (AUC=3.5) and bevacizumab (15
mg/kg) (TC + BEV) was initiated and administered every 3 weeks.
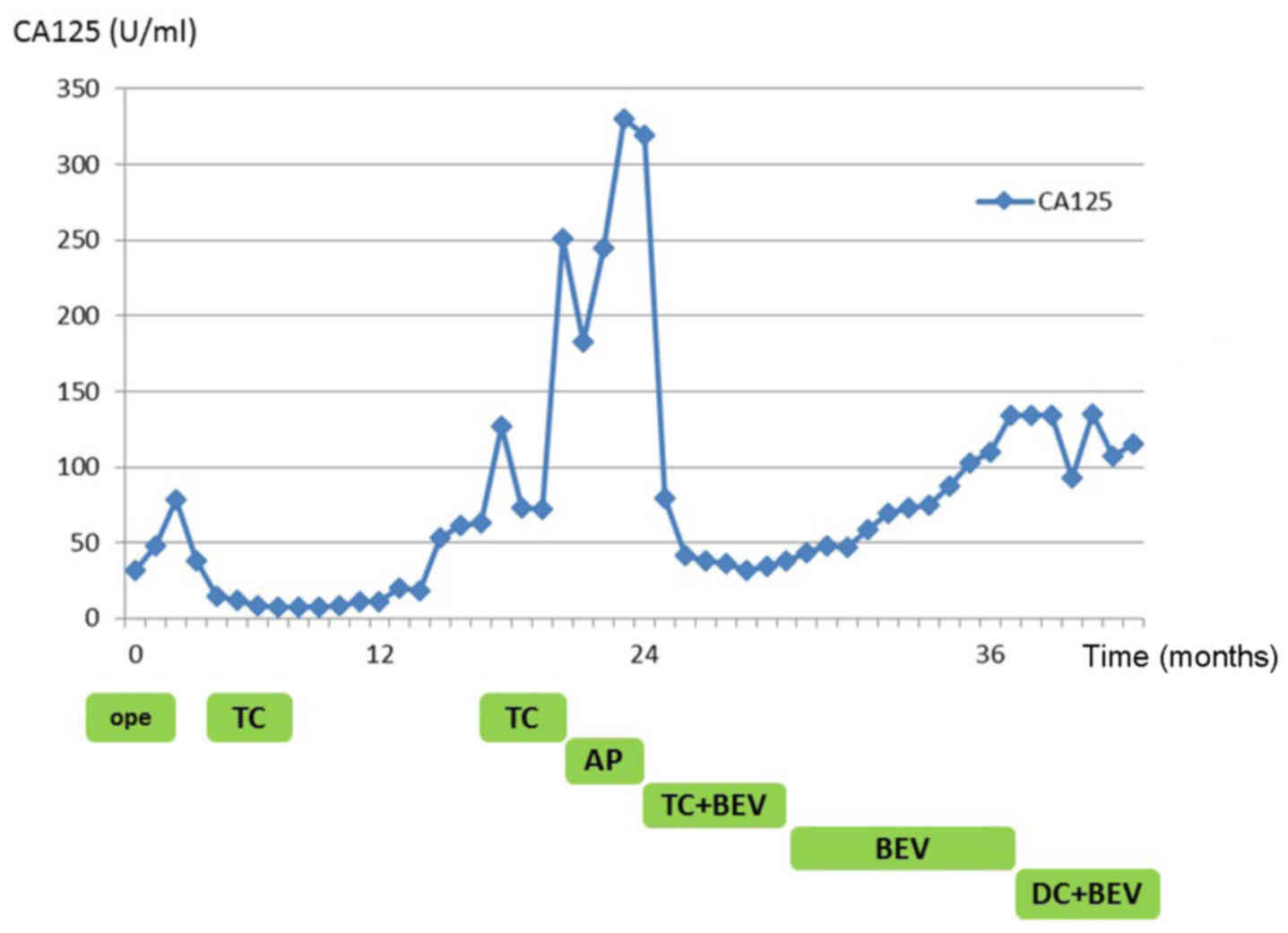
After two cycles of TC + BEV therapy, the CA-125 level started to
decrease. After six cycles of therapy, all the metastatic lesions
had shrunk, indicating partial response according to the Response
Evaluation Criteria in Solid Tumors (RECIST), version 1.1
(http://www.jcog.jp/doctor/tool/C_150_0010.pdf); the
pleural effusion and ascites also disappeared (Fig. 1C and D). Next, based on the regimen
used in ovarian cancer, the patient received single-agent
bevacizumab as maintenance therapy. After 12 cycles of bevacizumab
monotherapy (8 months after initiating TC + BEV), the CA-125 level
started to increase, and intraperitoneal implantations were once
again detected; thus, combination therapy consisting of doxorubicin
(70 mg/m2), carboplatin (AUC=3.5) and bevacizumab (15
mg/kg) (DC + BEV) was initiated. During bevacizumab therapy, the
patient developed grade 2 peripheral neuropathy and deep venous
thrombosis; no grade >2 toxicities were observed. Six cycles
after the initiation of DC + BEV therapy, the patient exhibited no
newly disseminated tumors, pleural effusion, or ascites. At 18
months after the initial bevacizumab treatment (last follow-up,
October 2014), the patient remained on combination chemotherapy,
without complaints or signs of tumor progression (Fig. 2).
Discussion
The prognosis of patients with recurrent/persistent
endometrial cancer remains disappointing, and an effective
treatment has not yet been established. Currently, bevacizumab is
reported to be effective against newly diagnosed as well as
recurrent epithelial ovarian cancer (6–8).
Furthermore, the addition of bevacizumab to combination
chemotherapy in patients with recurrent, persistent or metastatic
cervical cancer has been previously reported to improve the median
overall survival (9). However, for
recurrent/persistent endometrial cancer, the GOG phase II trial is
the most recently reported trial of bevacizumab monotherapy
(5).
This patient experienced a significant reduction of
both the disseminated tumors and ascites. Reduction of ascites may
be explained by inhibition of the vascular endothelial growth
factor pathway, as bevacizumab has the ability to suspend ascitic
fluid production resulting from peritoneal dissemination in solid
cancers. Of note, the disseminated tumors in the present case were
also reduced. By combining bevacizumab and chemotherapy, the
effects of each drug may have been enhanced.
The patient continued to receive bevacizumab, and
when disease progression was observed, bevacizumab + chemotherapy
was again initiated, after which time the patient experienced a
long-term response. It is possible that the anti-angiogenic effects
of bevacizumab require the continued presence of the drug to
effectively suppress the growth of minimal residual disease. Our
patient, who experienced a prolonged response during bevacizumab
therapy, may represent a group of patients that are particularly
sensitive to this agent, and who may respond to a bevacizumab
rechallenge at the time of relapse. Relapse following bevacizumab
withdrawal may not always represent resistance to this agent, but
rather the release of anti-angiogenic selection pressure that may
occasionally be restored by reinstituting the agent (6).
The cytotoxicity of current anticancer drugs also
applies to normal cells, and the achievement of therapeutic
efficacy is often associated with severe adverse events.
Chemotherapy must be performed while considering the balance
between therapeutic benefits and adverse effects. By contrast,
molecularly-targeted drugs act by specifically targeting the
mechanisms of cancer cell proliferation and metastasis at the
molecular level. The targeting of specific molecules in cancer
cells may achieve therapeutic efficacy with fewer side effects
(3). In fact, the patient in the
present case did not experience grade >2 toxicities throughout
the bevacizumab treatment.
The findings in this case suggest that bevacizumab
may be effective against recurrent/persistent endometrial cancer.
In particular, patients with type II endometrial cancer, which is
less sensitive to chemotherapy, may benefit from the use of
bevacizumab. Our patient experienced a partial response according
to RECIST, and a significant decrease of the CA-125 level as a
result of bevacizumab treatment. The patient repeatedly experienced
disease progression while on bevacizumab monotherapy, but then
responded following reinitiation of combination
bevacizumab/chemotherapy, suggesting that the anti-angiogenic
action of bevacizumab reduced tumor neovascularization, promoting
permeation of the tumor by the chemotherapy agent through the
remaining vessels. To the best of our knowledge, this case is the
first report of bevacizumab + chemotherapy administered following
disease progression in a patient with type II endometrial cancer
previously treated with bevacizumab + chemotherapy.
A recent phase II trial of a paclitaxel, carboplatin
and bevacizumab regimen that is effective and tolerable in advanced
and recurrent endometrial cancer was reported (10). The patient in the present case also
experienced a partial response to combination bevacizumab +
chemotherapy and an unusually long duration of response. Taken
together, the recent report and the present case suggest that a
taxane/platinum + bevacizumab regimen may be a viable therapeutic
option for the treatment of endometrial cancer.
Given the lack of effective therapies for patients
with recurrent/persistent endometrial cancer, this case suggests
that prospective randomized clinical trials of combination
bevacizumab + chemotherapy regimens in endometrial cancer may be
required. Furthermore, the present case also suggested that
bevacizumab + chemotherapy may be an effective treatment option for
patients with endometrial cancer who previously received
bevacizumab + standard chemotherapy.
Acknowledgements
The authors would like to thank the medical staff of
the Shimane University Hospital.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nogami Y, Banno K, Kisu I, Yanokura M,
Umene K, Masuda K, Kobayashi Y, Yamagami W, Nomura H, Tominaga E,
et al: Current status of molecular-targeted drugs for endometrial
cancer (Review). Mol Clin Oncol. 1:799–804. 2013.PubMed/NCBI
|
|
4
|
Zaqouri F, Bozas G, Kafantari E, Tsiatas
M, Nikitas N, Dimopoulos MA and Papadimitriou CA: Endometrial
cancer: What is new in adjuvant and molecularly targeted therapy?
Obstet Gynecol Int. 2010.7495792010.PubMed/NCBI
|
|
5
|
Aqhajanian C, Sill MW, Darcy KM, Greer B,
McMeekin DS, Rose PG, Rotmensch J, Barnes MN, Hanjani P and Leslie
KK: Phase II trial of bevacizumab in recurrent or persistent
endometrial cancer: A Gynecologic Oncology Group Study. J Clin
Oncol. 29:2259–2265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Konstantinopoulos PA, Berlin ST, Campos
SM, Matulonis UA and Cannistra SA: Bevacizumab rechallenge after
first line maintenance bevacizumab. Gynecol Oncol. 125:510–511.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistanct recurrent ovarian cancer: The AURELIA
open-label randomized phase III trial. J Clin Oncol. 32:1302–1308.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aqhajanian C, Blank SV, Goff BA, Judson
PL, Teneriello MG, Husain A, Sovak MA, Yi J and Nycum LR: OSEANS: A
randomized, double-blind, placebo-controlled phase III trial of
chemotherapy with or without bevacizumab in patients with
platinum-sensitive recurrent epithelial ovarian, primary
peritoneal, or fallopian tube cancer. J Clin Oncol. 30:2039–2045.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tewari KS, Sill MW, Long HJ III, Penson
RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao
MM, et al: Improved survival with bevacizumab in advanced cervical
cancer. N Engl J Med. 370:734–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simpkins F, Drake R, Escobar PF, Nutter B,
Rasool N and Rose PG: A phase II trial of paclitaxel, carboplatin,
and bevacizumab in advanced and recurrent endometrial carcinoma
(EMCA). Gynecol Oncol. 136:240–245. 2015. View Article : Google Scholar : PubMed/NCBI
|