Introduction
Nasogastric and nutritional tubes are routinely used
for decompression and nutrient supply following total gastrectomy
in patients with gastric cancer. However, the traditional algorithm
was recently challenged by an increasing number of studies.
Enhanced Recovery After Surgery (ERAS), also
referred to as ‘fast-track’ (FT) perioperative care, initiated by
Bardram et al in 1995 (1),
represents a fundamental shift in perioperative care (2–4). ERAS
consists of multidisciplinary approaches, including no bowel
preparation (5,6), free intake of fluid (7) and no routine use of nasogastric tubes
or drains. Compared with traditional management, ERAS contributes
to shortening the time to passing of flatus and reducing
hospitalization costs (3), without
an increase in postoperative complications (8,9).
Apart from nausea, pharyngeal discomfort and
surgical stress, it has been reported that fever and pneumonia also
occur more frequently in patients with nasogastric intubation
compared with those without intubation (4,10),
whereas the incidence of gastro-esophageal reflux is increased and
bowel function is restored later in cases with nasogastric
intubation (4,11–13).
These data provide strong evidence that routine nasogastric
intubation should be avoided. However, although avoiding gastric
intubation is often reported in recent studies on abdominal
surgery, studies on avoiding nasogastric and nutritional intubation
in total gastric resection surgery are rare and evidence is
scarce.
According to ERAS, the majority of the patients may
drink and eat normal hospital food immediately following recovery
from anaesthesia (14–16). It has reported that early oral food
intake is safe and feasible following upper gastrointestinal and
hepatopancreaticobiliary surgery (17), and it may contribute to reducing the
rate of infection and anastomotic dehiscence (18,19).
However, the safety of early oral intake following total gastric
resection and esophagogastric anastomosis remains unclear.
The aim of the present study was to investigate the
safety of total gastric resection without nasogastric and
nutritional intubation and with earlier oral feeding.
Patients and methods
Patients
Between January 2010 and August 2015, 791
consecutive patients with gastric cancer underwent gastrectomy by
the same surgical team at the First Department of Digestive Surgery
of XiJing Hospital, Fourth Military Medical University (Xi'an,
China). For this retrospective cohort study, all patient data were
evaluated by two researchers and the patient inclusion criteria
were as follows: i) Adult patients (age >18 years); ii) patients
with gastric cancer who underwent total gastric resection and
esophagojejunal anastomosis; all surgeries performed under general
anaesthesia, and spleen-preserving D2 lymphadenectomy was
performed. An end-to-side Roux-Y anastomosis was created with a
26-mm diameter circular stapler.
This study was approved by the Ethics Committee of
the Fourth Military Medical University. All patients received
verbal and written information regarding the study and provided
informed consent prior to surgery.
Demographic and preoperative data
Demographic data, including sex, age, smoking
status, alcohol consumption and disease history were collected.
Preoperative data, including TNM clinical and pathological staging
classification, routine hematological and biochemical tests and
X-rays were collected to enable subsequent analysis of the
comparability of the groups.
Postoperative caring
The patients were divided into two groups: The
traditional group underwent insertion of nasogastric and
nutritional tubes perioperatively, and the nasogastric tube was
maintained until intestinal recovery, which was identified by the
first passage of flatus, and the nutritional tube was maintained
until ingestion of normal food was resumed. The FT group did not
undergo insertion of a nasogastric or nutritional tube.
Perioperative observations and data
collection
The surgical time was calculated from the first skin
incision to placement of the last suture. The recovery time of
gastrointestinal function, intraoperative blood loss, and the
highest postoperative temperature were recorded. The histological
subtype and pathological stage were determined using the Union for
International Cancer Control and TNM classification for gastric
cancer. Blood samples were collected and routine blood test, liver
and renal function tests were performed perioperatively.
Postoperative complications, including anastomotic complication,
wound infection, wound rupture, lung infection, bleeding,
reoperation, duodenal leak and intestinal obstruction were observed
and evaluated. Anastomotic complication assessment was performed
using a water-soluble radiological contrast enema at 6–8 days
postoperatively. A clinical leak was defined as the appearance of
food material in the abdominal drains, or the development of
systemic sepsis associated with local peritoneal signs during the
postoperative period. Any extravasation of the contrast medium
detected on radiography was considered as a radiological leak.
Statistical analysis
Statistical analysis was performed using SPSS 11.5
software (SPSS Inc., Chicago, IL, USA). Differences in expression
rate among groups were analyzed by Pearson's Chi-squared
(χ2) test. The Fisher's exact test was used to assess
the difference of positive rate when the number of total cases was
<40. All statistical tests were two-sided; a P-value of <0.05
was considered statistically significant. Hierarchical logistic
regression was used to determine the association between tube
insertion and complications, and between eating time and
complications.
Results
Characteristics of patients
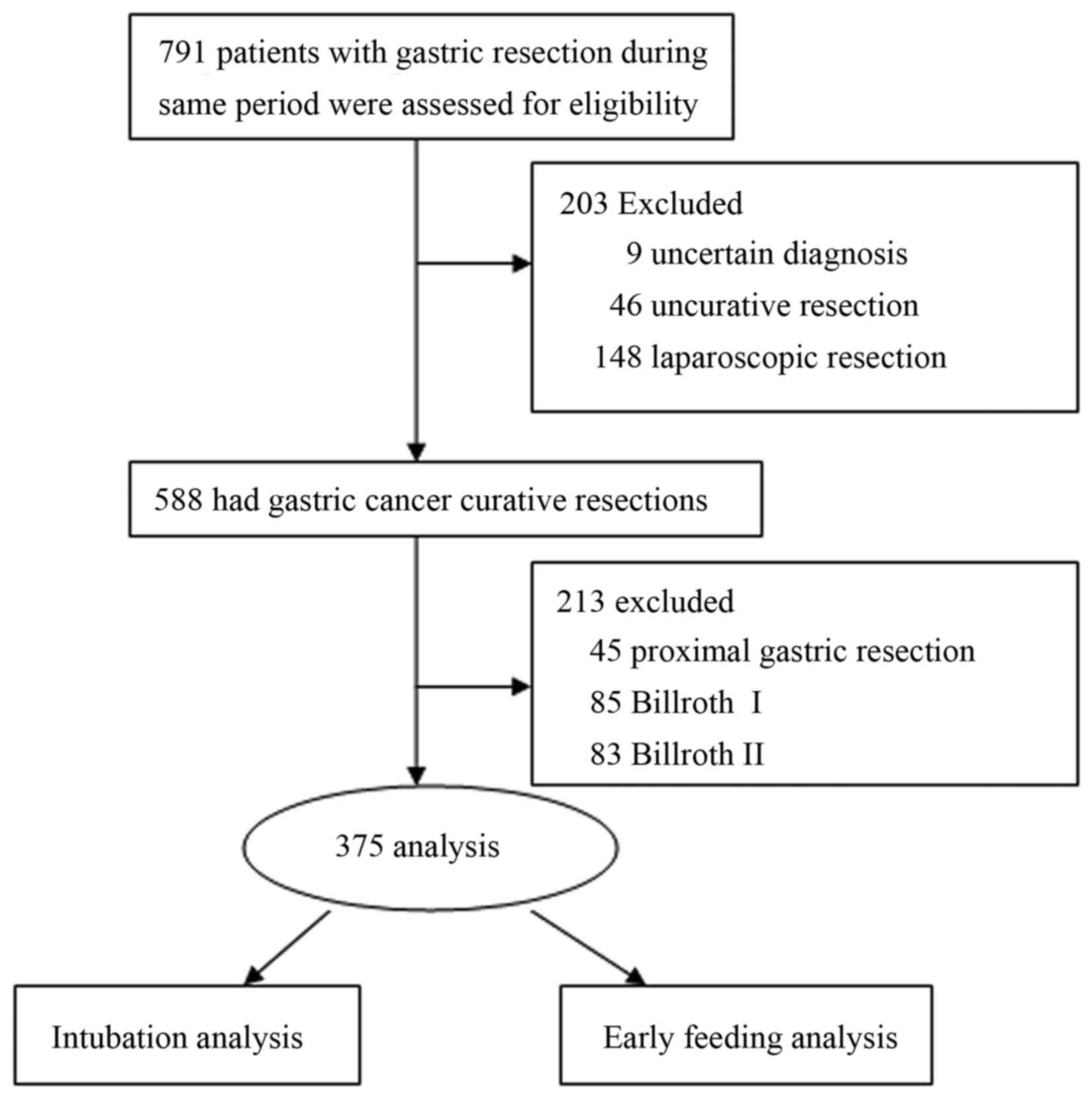
A total of 375 cases met the inclusion criteria and
were analyzed in this cohort study (Fig.
1), of whom 74 cases without gastric and nutritional intubation
were classified into the FT group, and the 301 cases with
intubations were classified into the traditional group for
comparison. The comparison of baseline data between the FT and
traditional groups is described in Table
I. There were no significant differences between the two groups
regarding preoperative variables, such as age, sex, tumor
differentiation, pathological stage, histological subtype, TNM
stage, laboratory test and basic health status.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| Nutritional tube,
n |
|
|---|
|
|
|
|
|---|
| Variables | No | Yes | P-value |
|---|
| Sex |
|
|
|
| Male | 59 | 245 | 0.743 |
|
Female | 15 | 56 |
|
| Age, years (mean ±
SD) | 56.32±11.53 | 58.33±10.43 | 0.075 |
| Gastric tube |
|
|
|
| No | 6 | 42 | 0.178 |
| Yes | 68 | 259 |
|
| Hypertension |
|
|
|
| No | 67 | 248 | 0.087 |
| Yes | 7 | 53 |
|
| Heart disease |
|
|
|
| No | 73 | 290 | 0.429 |
| Yes | 1 | 11 |
|
| Diabetes |
|
|
|
| No | 72 | 274 | 0.106 |
| Yes | 2 | 24 |
|
| Lung disease |
|
|
|
| No | 73 | 294 | 0.995 |
|
Yes | 1 | 4 |
|
| Laboratory tests
(mean ± SD) |
|
|
|
| WBC
count (×109/l) | 5.97±1.91 | 6.08±1.93 | 0.664 |
| RBC
count (×1012/l) | 4.42±0.60 | 4.35±0.54 | 0.375 |
| Hb
level (g/l) | 132.16±26.09 | 127.86±20.89 | 0.133 |
| TP
(g/l) | 69.20±6.09 | 68.28±5.18 | 0.188 |
| Pathological
type |
|
|
|
|
Papillary adenocarcinoma | 0 | 2 | 0.442 |
| High
differentiation | 13 | 44 |
|
|
Intermediate
differentiation | 6 | 45 |
|
| Poor
differentiation | 40 | 162 |
|
|
Mucous | 9 | 34 |
|
|
Others | 5 | 8 |
|
| TNM stage |
|
|
|
|
Tis | 1 | 1 | 0.884 |
| Ia | 23 | 112 |
|
| Ib | 2 | 9 |
|
| II | 5 | 17 |
|
|
IIIa | 6 | 19 |
|
|
IIIb | 5 | 17 |
|
| IV | 32 | 126 |
|
Comparison of intraoperative and
postoperative data
The mean surgical time in the FT group was
significantly shortened compared with the traditional group
(190.97±38.18 vs. 216.12±59.52 min, respectively; P=0.026). The
activity was resumed earlier (1.16±0.47 vs. 1.36±0.84 days,
respectively; P=0.009) and time to oral intake was also shorter
(4.28±1.79 vs. 5.71±2.66 days, respectively; P=0.001). Moreover,
fewer patients developed a fever in the FT compared with the
traditional group (12.16 vs. 29.23%, respectively; P=0.003). There
was no significant difference in the amount of intraoperative blood
loss, highest postoperative temperature, time to first flatus and
postoperative hospital stay between the two groups (Table II).
 | Table II.Perioperative observation. |
Table II.
Perioperative observation.
|
| Intubation |
|
|---|
|
|
|
|
|---|
| Variables | No | Yes | P-value |
|---|
| Operative time
(min) | 190.97±38.18 | 216.12±59.52 | 0.026 |
| Activity
(days) | 1.16±0.47 | 1.36±0.84 | 0.009 |
| Oral intake
(days) | 4.28±1.79 | 5.71±2.66 | 0.001 |
| Blood loss
(ml) | 240.27±199.32 | 295.15±229.70 | 0.060 |
| Fever (%) | 9 (12.16%) | 88 (29.23%) | 0.003 |
| Postoperative
hospital stay (days) | 9.00±4.2 | 9.84±4.12 | 0.119 |
| Intestinal function
recovery (days) | 4.13±1.37 | 4.31±1.51 | 0.374 |
Complications
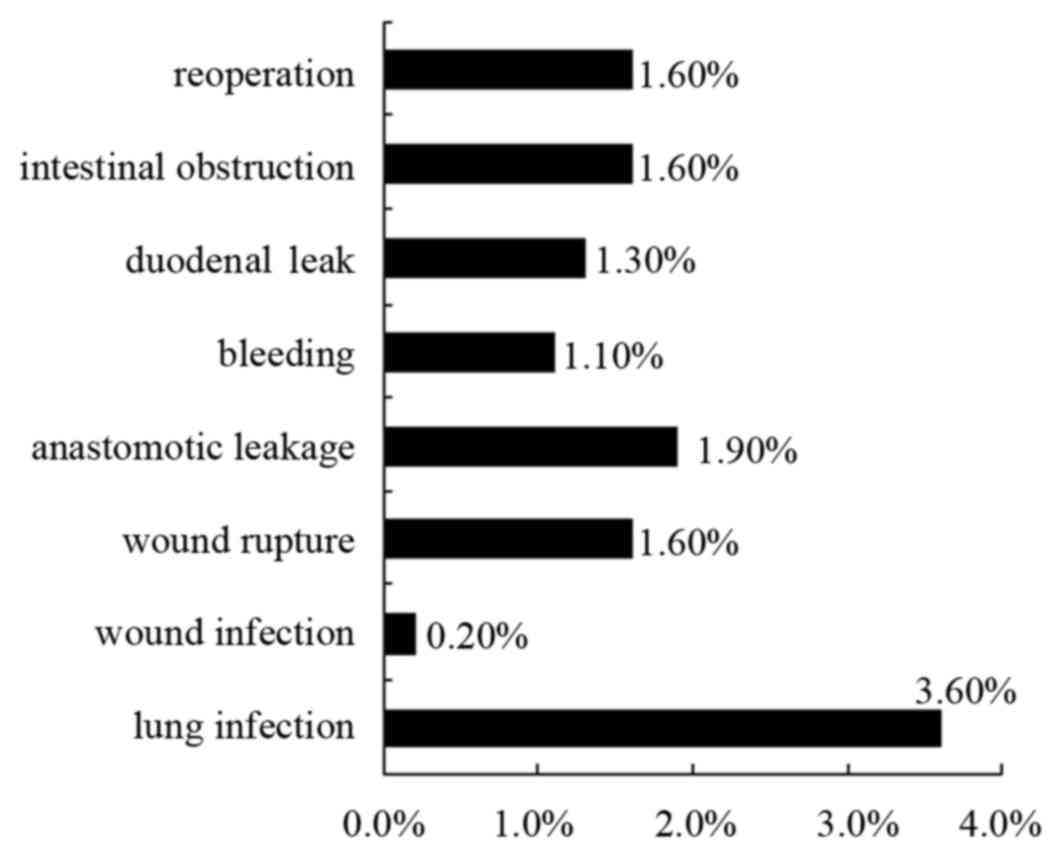
The distribution of complications in all the
patients is outlined in Fig. 2.
Postoperative complications included lung infection 3.60%, wound
infection 0.20%, wound rupture 1.60%, anastomotic leakage 1.90%,
bleeding 1.10%, duodenal leak 1.30%, intestinal obstruction 1.60%
and reoperation 1.60% in all the patients.
The incidence rate of total complications in the FT
group was not statistically significantly different from that in
the traditional group (9.45 vs. 6.31%, respectively; P=0.317). In
addition, there were no differences regarding lung infection, wound
infection, wound rupture, anastomotic leakage, bleeding, duodenal
leak, intestinal obstruction and reoperation rate according to the
univariate analysis (Table
III).
 | Table III.Comparison of complications in
gastric resection with and without intubation. |
Table III.
Comparison of complications in
gastric resection with and without intubation.
|
| Intubation, n
(%) |
|
|---|
|
|
|
|
|---|
| Complications | No | Yes | P-value |
|---|
| Lung infection | 1 (1.35) | 8 (2.65) | 0.511 |
| Wound
infection | 1 (1.35) | 0 | 0.197 |
| Wound rupture | 2 (2.70) | 4 (1.32) | 0.338 |
| Reoperation | 1 (1.35) | 5 (1.66) | 0.849 |
| Bleeding | 0 | 5 (1.66) | 0.588 |
| Duodenal leak | 0 | 7 (2.32) | 0.353 |
| Anastomotic
leakage | 1 (1.35) | 2 (0.66) | 0.552 |
| Intestinal
obstruction | 2 (2.70) | 4 (1.32) | 0.338 |
| Total
complications | 7 (9.45) | 19 (6.31) | 0.317 |
Time to oral intake and
complications
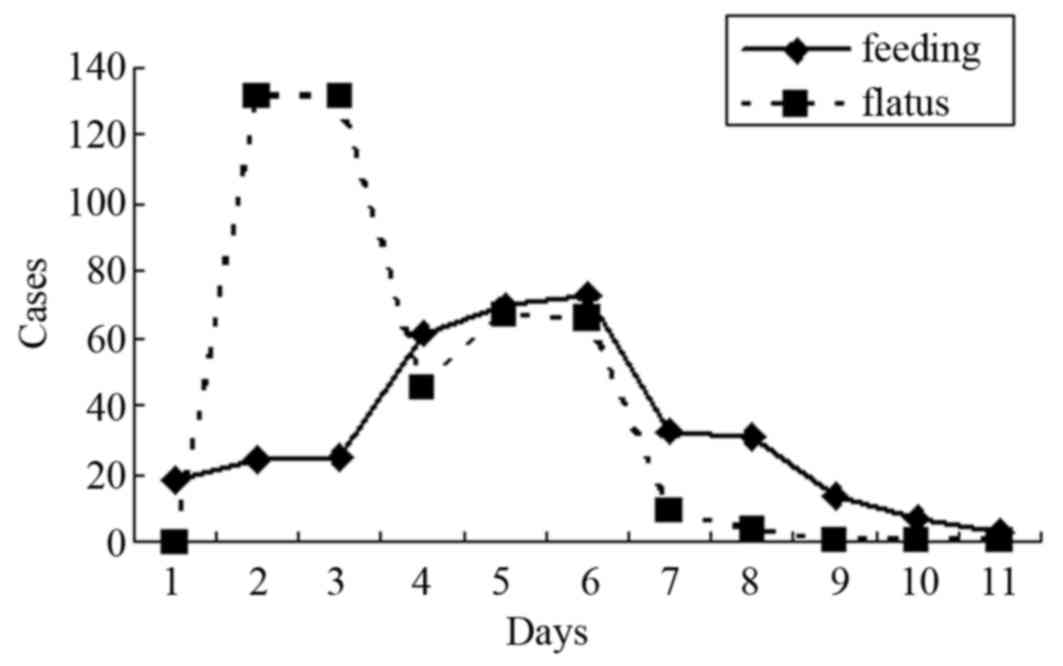
The distribution of time to oral intake and time to
first flatus of all patients is presented in Fig. 3, ranging from the 1st to the 13th
postoperative day. The mean time to the first oral intake time was
5.42±2.47 days, while 18 (4.9%), 24 (6.5%), 25 (6.8%) and 300
(81.7%) patients were fed on the 1st, 2nd, 3rd and after the 4th
postoperative day, respectively. The mean time to first flatus of
all patients was 4.28±1.48 days, while 0 (0.0%), 15 (4.5%), 132
(35.0%) and 320 (60%) patients passed flatus on the 1st, 2nd, 3rd
and after the 4th postoperative day.
The traditional standard for resuming oral feeding
is after the first flatus or 6 days later after expelling
anastomostic leakage by radiological tests. We determined 48 h as
the cut-off for earlier feeding, and found that 17 (23%) patients
were fed earlier in the FT group vs. 25 (8.3%) patients in the
traditional group (P<0.001). It was also observed that earlier
feeding did not significantly increase the rate of total
complications (7.21 vs. 4.76%, respectively, P=0.557; Table IV).
 | Table IV.Earlier oral feeding and complication
rate. |
Table IV.
Earlier oral feeding and complication
rate.
|
| Earlier feeding, n
(%) |
|
|---|
|
|
|
|
|---|
| Complications | No | Yes | P-value |
|---|
| Fever | 86 (25.82) | 11 (26.19) | 0.959 |
| Lung infection | 9 (2.70) | 0 | 0.606 |
| Wound
infection | 0 | 1 (2.38) | 0.112 |
| Wound rupture | 6 (1.80) | 2 (4.76) | 0.337 |
| Reoperation | 6 (1.80) | 0 | 0.381 |
| Bleeding | 5 (1.50) | 0 | 0.424 |
| Duodenal leak | 7 (2.10) | 0 | 0.343 |
| Anastomotic
leakage | 3 (0.90) | 0 | 0.537 |
| Intestinal
obstruction | 6 (1.80) | 0 | 0.381 |
| Total
complications | 24 (7.21) | 2 (4.76) | 0.557 |
Safety of non-intubation and earlier
feeding evaluated by multivariate analysis
To further assess the risk of postoperative
complications, a multivariate analysis was conducted using the
logistic regression model, including tumor stage, tumor grade, age,
sex, pathological type, underlying disease and surgical bleeding.
The analysis demonstrated that non-intubation contributed to a
decrease in the total complication rate [odds ratio (OR)=2.65,
P=0.063]. Moreover, by logistic regression analysis, earlier
feeding did not increase the risk of postoperative complications
(OR=1.63, P=0.543) (Table V).
 | Table V.Association between intubation and
morbidity of complications by logistic regression analysis. |
Table V.
Association between intubation and
morbidity of complications by logistic regression analysis.
| Variables | Regression
coefficient | Standard error | OR | P-value |
|---|
| Intubation | 0.97 | 0.52 | 2.65 | 0.063 |
| Operative time
(min) | 0.00 | 0.00 | 1.00 | 0.407 |
| Feeding time
(days) | 0.49 | 0.80 | 1.63 | 0.543 |
| Sex | −0.53 | 0.65 | 0.59 | 0.412 |
| Age | 0.05 | 0.02 | 1.05 | 0.025 |
| Basic disease | −0.39 | 0.42 | 0.68 | 0.345 |
| Blood loss
(ml) | 0.00 | 0.00 | 1.00 | 0.628 |
| Pathological
type | 0.15 | 0.54 | 1.16 | 0.785 |
| Degree of
differentiation | 0.39 | 0.25 | 1.48 | 0.123 |
| TNM stage | −0.10 | 0.10 | 0.90 | 0.276 |
| Constant | −8.47 | 2.39 | 0.01 | 0.001 |
Discussion
The aim of the present study was to investigate a
new management in total gastric resection, without insertion of
gastric or nutritional tubes. It was demonstrated that this
approach is feasible and appears to be as safe as the traditional
method. Compared with the traditional method, the FT management
contributed to reduced rate of fever, promoted intestinal function
recovery and decreased the rate of complications.
A previous meta-analysis reported a short interval
from surgery to the first passage of flatus and a short length of
hospital stay if nasogastric intubation was avoided (20,21). The
incidence of pulmonary complications, wound infection and
anastomotic leakage was also not significantly different in
colorectal surgery without intubation (22). In accordance with these previous
findings, we observed that gastric and nutritional intubation
prolonged operative time, increased the incidence of fever, and
increased the rate of total complications according to the logistic
regression analysis.
The safety of total gastric resection is closely
associated with anastomotic healing, which depends on adequate
blood supply and good nutritional status of anastomotic tissue
(23–25). It is generally accepted that the
strength of the anastomosed intestinal wall is largely derived from
collagen (26). During the first
postoperative days, wound strength is considered to solely depend
on the suture-holding capacity of the existing collagen fibrils
(27). From 3 h to 4 days after the
operation, the absolute and the relative collagen synthesis
steadily increases (26,28), providing evidence that an anastomosis
strength increases from the beginning of wound healing, and the
risk of anastomotic leakage decreases. The role of intubation in
the healing of anastomosis is controversial, as a previous study
demonstrated that an indwelling catheter traversing the anastomosis
slows down the healing process in that segment (29).
The fast-track approach theory emphasizes that
earlier food intake promotes intestinal function recovery (30,31), and
earlier food intake from the first postoperative day after
duodenopancreatectomy was proven to be safe (17,32).
Moreover, it was reported that early post-pyloric feeding offers no
advantage over early gastric feeding in terms of overall nutrition
received and complications (33),
which also reflects the safety of earlier oral intake in upper
digestive tract surgery. Our results also suggest that earlier oral
intake within 48 h is safe and feasible in patients undergoing
total gastric resection and esophagojejunal anastomosis.
Although gastric and nutritional intubations were
suggested to be avoided in our study, this may not apply to all
patients. Under certain condition, such as anastomotic
complications, duodenal leakage and intestinal obstruction,
intubations are required to decompress and supply nutrition and,
particularly, intubation contributes to relieving delayed emptying
of the upper digestive tract and severe distention (4,32,34,35).
In conclusion, our results suggest that total
gastric resection without intubations and earlier feeding is a
novel, feasible and safe method, which may simplify the surgical
procedure and reduce overall complication rate and may applied to
selected patients.
Acknowledgements
The authors would like to thank Dr Guang-Hui Xu, Dr
Lei Zhang and Dr Lei Cai for their help in the design, analysis and
writing of this article.
References
|
1
|
Bardram L, Funch-Jensen P, Jensen P,
Crawford ME and Kehlet H: Recovery after laparoscopic colonic
surgery with epidural analgesia, and early oral nutrition and
mobilisation. Lancet. 345:763–764. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nygren J, Thacker J, Carli F, Fearon KC,
Norderval S, Lobo DN, Ljungqvist O, Soop M and Ramirez J: Enhanced
Recovery After Surgery Society: Guidelines for perioperative care
in elective rectal/pelvic surgery: Enhanced Recovery After Surgery
(ERAS®) Society recommendations. Clin Nutr. 31:801–816. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gustafsson UO, Scott MJ, Schwenk W,
Demartines N, Roulin D, Francis N, McNaught CE, Macfie J, Liberman
AS, Soop M, et al: Guidelines for perioperative care in elective
colonic surgery: Enhanced Recovery After Surgery (ERAS (®)) Society
recommendations. World J Surg. 37:259–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lassen K, Coolsen MM, Slim K, Carli F, de
Aguilar-Nascimento JE, Schäfer M, Parks RW, Fearon KC, Lobo DN,
Demartines N, et al: Guidelines for perioperative care for
pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®)
Society recommendations. World J Surg. 37:240–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holte K, Nielsen KG, Madsen JL and Kehlet
H: Physiologic effects of bowel preparation. Dis Colon Rectum.
47:1397–1402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung B, Lannerstad O, Påhlman L, Arodell
M, Unosson M and Nilsson E: Preoperative mechanical preparation of
the colon: The patient's experience. BMC Surg. 7:52007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ljungqvist O and Søreide E: Preoperative
fasting. Br J Surg. 90:400–406. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li YJ, Huo TT, Xing J, An JZ, Han ZY, Liu
XN and Zhao QC: Meta-analysis of efficacy and safety of fast-track
surgery in gastrectomy for gastric cancer. World J Surg.
38:3142–3151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng F, Ji G, Li JP, Li XH, Shi H, Zhao
ZW, Wu GS, Liu XN and Zhao QC: Fast-track surgery could improve
postoperative recovery in radical total gastrectomy patients. World
J Gastroenterol. 19:3642–3648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheatham ML, Chapman WC, Key SP and
Sawyers JL: A meta-analysis of selective versus routine nasogastric
decompression after elective laparotomy. Ann Surg. 221:469–478.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Manning BJ, Winter DC, McGreal G, Kirwan
WO and Redmond HP: Nasogastric intubation causes gastroesophageal
reflux in patients undergoing elective laparotomy. Surgery.
130:788–791. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fisher WE, Hodges SE, Cruz G, Artinyan A,
Silberfein EJ, Ahern CH, Jo E and Brunicardi FC: Routine
nasogastric suction may be unnecessary after a pancreatic
resection. HPB (Oxford). 13:792–796. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roland CL, Mansour JC and Schwarz RE:
Routine nasogastric decompression is unnecessary after pancreatic
resections. Arch Surg. 147:287–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gustafsson UO, Thorell A, Soop M,
Ljungqvist O and Nygren J: Haemoglobin A1c as a predictor of
postoperative hyperglycaemia and complications after major
colorectal surgery. Br J Surg. 96:1358–1364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hendry PO, Hausel J, Nygren J, Lassen K,
Dejong CH, Ljungqvist O and Faron KC: Enhanced Recovery After
Surgery Study Group: Determinants of outcome after colorectal
resection within an enhanced recovery programme. Br J Surg.
96:197–205. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nygren J, Soop M, Thorell A, Hausel J and
Ljungqvist O: ERAS Group: An enhanced-recovery protocol improves
outcome after colorectal resection already during the first year: A
single-center experience in 168 consecutive patients. Dis Colon
Rectum. 52:978–985. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lassen K, Kjaeve J, Fetveit T, Tranø G,
Sigurdsson HK, Horn A and Revhaug A: Allowing normal food at will
after major upper gastrointestinal surgery does not increase
morbidity: A randomized multicenter trial. Ann Surg. 247:721–729.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lewis SJ, Egger M, Sylvester PA and Thomas
S: Early enteral feeding versus ‘nil by mouth’ after
gastrointestinal surgery: Systematic review and meta-analysis of
controlled trials. BMJ. 323:773–776. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han-Geurts IJ, Hop WC, Kok NF, Lim A,
Brouwer KJ and Jeekel J: Randomized clinical trial of the impact of
early enteral feeding on postoperative ileus and recovery. Br J
Surg. 94:555–561. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nelson R, Tse B and Edwards S: Systematic
review of prophylactic nasogastric decompression after abdominal
operations. Br J Surg. 92:673–680. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rao W, Zhang X, Zhang J, Yan R, Hu Z and
Wang Q: The role of nasogastric tube in decompression after
elective colon and rectum surgery: A meta-analysis. Int J
Colorectal Dis. 26:423–429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mack LA, Kaklamanos IG, Livingstone AS,
Levi JU, Robinson C, Sleeman D, Franceschi D and Bathe OF: Gastric
decompression and enteral feeding through a double-lumen
gastrojejunostomy tube improves outcomes after
pancreaticoduodenectomy. Ann Surg. 240:845–851. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chung RS: Blood flow in colonic
anastomoses. Effect of stapling and suturing. Ann Surg.
206:335–339. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fawcett A, Shembekar M, Church JS,
Vashisht R, Springall RG and Nott DM: Smoking, hypertension and
colonic anastomotic healing; A combined clinical and
histopathological study. Gut. 38:714–718. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Markar SR, Karthikesalingam A, Vyas S,
Hashemi M and Winslet M: Hand-sewn versus stapled oesophago-gastric
anastomosis: Systematic review and meta-analysis. J Gastrointest
Surg. 15:876–884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martens MF and Hendriks T: Postoperative
changes in collagen synthesis in intestinal anastomoses of the rat:
Differences between small and large bowel. Gut. 32:1482–1487. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Högström H and Haglund U: Postoperative
decrease in suture holding capacity in laparotomy wounds and
anastomoses. Acta Chir Scand. 151:533–535. 1985.PubMed/NCBI
|
|
28
|
de Waard JW, Wobbes T, de Man BM, van der
Linden CJ and Hendriks T: Post-operative levamisole may compromise
early healing of experimental intestinal anastomoses. Br J Cancer.
72:456–460. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Borgstrom S and Lundh B: Healing of
esophageal anastomosis; Animal experiments. Ann Surg. 150:142–148.
1959. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Halaszynski TM, Juda R and Silverman DG:
Optimizing postoperative outcomes with efficient preoperative
assessment and management. Crit Care Med. 32 4 Suppl:S76–S86. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Forster AJ, Clark HD, Menard A, Dupuis N,
Chernish R, Chandok N, Khan A, Letourneau M and van Walraven C:
Effect of a nurse team coordinator on outcomes for hospitalized
medicine patients. Am J Med. 118:1148–1153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Berberat PO, Ingold H, Gulbinas A, Kleeff
J, Müller MW, Gutt C, Weigand M, Friess H and Büchler MW: Fast
track-different implications in pancreatic surgery. J Gastrointest
Surg. 11:880–887. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
White H, Sosnowski K, Tran K, Reeves A and
Jones M: A randomised controlled comparison of early post-pyloric
versus early gastric feeding to meet nutritional targets in
ventilated intensive care patients. Crit Care. 13:R1872009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Balzano G, Zerbi A, Braga M, Rocchetti S,
Beneduce AA and Di Carlo V: Fast-track recovery programme after
pancreatico-duodenectomy reduces delayed gastric emptying. Br J
Surg. 95:1387–1393. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
di Sebastiano P, Festa L, De Bonis A,
Ciuffreda A, Valvano MR, Andriulli A and di Mola FF: A modified
fast-track program for pancreatic surgery: A prospective
single-center experience. Langenbecks Arch Surg. 396:345–351. 2011.
View Article : Google Scholar : PubMed/NCBI
|