Introduction
In head and neck cancer (HNSCC) and specifically in
tonsillar squamous cell carcinoma (TSCC) the mode of infection with
human papillomaviruses (HPV) seems to be significantly influenced
by the expression levels of two genes and their proteins, the
quantitative association of which is dependent on the smoking habit
of the patients. As shown previously (1–3), both
the secretory leucocyte protease inhibitor (SLPI; an
antiproteinase) and the membrane bound receptor Annexin A2 (AnxA2)
showed higher expression levels in smokers in comparison to
non-smokers. However, SLPI showed a clear surplus compared to AnxA2
in smokers vs. non-smokers whereas AnxA2 vs. SLPI showed a
significant surplus in cases tested HPV-positive when compared to
HPV-negative cases (1–3). Previous ex vivo data showed that
SLPI expression in clinically healthy mucosa of the lower
turbinates can be increased by incubation with nicotine (2). Additionally, in vitro studies
showed that the cell entry of HPV is hindered when AnxA2 is blocked
by SLPI in a cell culture model utilizing a cervix cancer cell line
(4).
The hypothesis presented below is plausible and, may
explain why non-smokers predominantly developing HPV-driven tumours
whereas smokers suffer from, in terms of survival, less favourable
HPV-negative tumours. Smoking induces a surplus of SLPI vs. AnxA2
in the mucosa of the head and neck, blocking AnxA2 as ligand for a
successful HPV infection. Non-smoking individuals show an even
amount of SLPI and AnxA2 or more likely even show a surplus of
AnxA2 vs. SLPI, thus, giving HPV access to the ligand AnxA2
consecutively leading to cell entry and successful infection.
Findings of previous studies have shown this correlation in 464
tissue specimens derived from 445 patients [HNSCC specimens, n=397;
clinically normal mucosa, n=57 (from HNSCC patients, n=19; from
healthy individuals, n=38); turbinates, n=10] (1–3). The
results, with only a few exceptions, were statistical significant.
In this context, however, it is noteworthy that it is assumed that
an HPV infection occurs prior to carcinogenesis, i.e., in
neoplasm-free mucosa of the upper aerodigestive tract. Since
specifically TSCC are driven by HPV-infections (5,6) with HPV
prevalence rates in this tumour entity of 30% to even 90% (7) of cases dependent on the geographical
region the patients reside in (6,8), the
neoplasm-free tissue of the tonsils is of interest to test the
described hypothesis.
Therefore, in the present study for the first time
worldwide to the best of our knowledge, tissue specimens derived
from 214 neoplasm-free palatine tonsils were tested for HPV- and
p16INK4A-status, expression of SLPI and AnxA2,
respectively, and the results were correlated with the smoking
habit of the patients.
Patients and methods
Patients and sample preparation
Tissue samples of 214 non-neoplastic tonsillar
tissue specimens with histopathologically confirmed chronic
tonsillitis [CT; n= 118 (55.1%)] or tonsillar hyperplasia [H; n=96
(44.9%)] were collected between 2013 and 2014. Of these, samples
from 64 patients were obtained prospectively during surgery between
2013 and 2014 [CT; n=43 (67.2%); H; n=21 (32.8%)] at the Department
of Otorhinolaryngology, Head and Neck Surgery,
Christian-Albrechts-University (Kiel, Germany), after informed
consent was obtained from the patients. The study was approved by
the local Εthics Committee of the Christian-Albrechts-University
(D509/13).
The tissue specimens were cut in halves, one part
was snap-frozen for gene expression analysis and the other was
FFPE-embedded for immunohistochemistry. The remaining 150 tissue
specimens, all FFPE samples (75 CT and 75 H), were retrospectively
retrieved form the archives of the Institute of Pathology,
University Hospital Schleswig Holstein, Campus Lübeck. The original
samples were obtained between 2009 and 2014 during surgery at the
Department of Otolaryngology, Head and Neck Surgery, University
Medical Center Schleswig-Holstein, University of Lübeck, Germany.
Gene expression analysis was therefore performed on FFPE and fresh
frozen material. Results were presented for the entire 214 patients
and for the FFPE and fresh frozen samples, separately, to
demonstrate homogeneity of results even when analyzing different
sample materials.
Nucleic acid extraction,
HPV-detection, cDNA synthesis and qPCR
From the retrospective FFPE material (n=150) DNA and
RNA samples were simultaneously extracted from 4–6 consecutive 10
µm sections using an ExpressArt Mag FFPE RNA+DNA ready kit (AmpTec
GmbH, Hamburg, Germany) according to the manufacturer's protocol.
For DNA- and RNA extraction of the prospectively collected
fresh-frozen material (n=64) 25 mg tissue was homogenized using a
Precellys homogenizer (PeqLab, Erlangen, Germany) and the 1.4/2.8
mm Ceramic kits in the presence of the AllPrep puffer (Qiagen,
Hilden, Germany). DNA and RNA were then simultaneously isolated
using the Allprep kit according to the manufacturer's protocol.
Nucleic acid quantity and quality was analyzed using the Nanodrop
1000 (PeqLab) and the Tapestation 2200 (Agilent, Böblingen,
Germany), respectively. HPV-DNA detection was performed by PCR
using the primers GP5+/GP6+, as described previously (9). RNA (200 ng) was transcribed into cDNA
using the TR cDNA synthesis kit (AmpTec GmbH). qPCR was performed
as described previously (10).
Primers for SLPI and AnxA2 were designed and used as described
elsewhere (2). Primers for the
housekeeping genes 18S rRNA, β-actin and b-2-microglobulin (B2M)
were purchased from Promolgene (Berlin, Germany) and used according
to the manufacturer's protocol. DNA integrity was analyzed using
genomic B2M primers (Promolgene) and used according to the
manufacturer's protocol.
Immunohistochemistry for SLPI and
p16INK4A
Paraffin-embedded tissue specimens were cut (4 µm)
and stained with hematoxylin and eosin to detect tonsillar crypts.
Areas containing such crypts were subjected to punch biopsies (2 mm
in diameter) and were further processed for tissue microarray
analysis (TMA) according to Kononen and co-workers (11). Immunohistochemical staining for SLPI
and p16INK4A expression was performed as described
previously (12,13). In brief: to assess SLPI and
p16INK4A protein levels the entire biopsies were
analyzed (magnification, ×2200). The percentage of positive
epithelial cells of the tonsillar crypts was determined and cases
were assigned to one of the categories: negative <5%, weak
5–30%, moderate 31–75% and strong >75% of the cells were
stained.
Statistical analysis
Immunohistochemical data were analyzed using
two-sided Fisher's exact test (SPSS 20.0 software; IBM SPSS,
Armonk, NY, USA). qPCR data were analyzed according to the ΔΔCq
method (14) using the mean Ct value
of the housekeeping genes. Fold changes of the expression levels
were calculated as described previously (14) and the obtained values were used for
statistical analysis (SPSS 20.0 software). Fisher's exact test was
performed relating SLPI and p16INK4A protein expression
to HPV-positivity and smoking habit. P<0.05 was considered
statistically significant for all tests performed.
Results
Patient characteristics
Patient characteristics, i.e., age, sex, diagnosis
and smoking habit, are given in Table
I. Patients diagnosed with hyperplasia were significantly
younger than those with chronic tonsillitis. In addition, the data
presented show a significant correlation between smoking habit and
diagnosis. Stratifying the data for an age cut-off of 18 years (the
legal age to consume tobacco in Germany) revealed that the
correlation between smoking habit and diagnosis was solely due to
the large amount of non-smoking patients diagnosed with hyperplasia
and who were <18 years (n=93 patients, representing 43.5% of the
study population and 56.0% of the non-smoking patients).
 | Table I.Patient characteristics, i.e., sex,
age and smoking habit, are shown for the entire cohort and divided
into retrospectively and prospectively collected samples. |
Table I.
Patient characteristics, i.e., sex,
age and smoking habit, are shown for the entire cohort and divided
into retrospectively and prospectively collected samples.
|
| Entire cohort
(n=214) | Retrospective
(n=150) | Prospective
(n=64) |
|---|
|
|
|
|
|
|---|
|
| Diagnosis |
| Diagnosis | P-value | Diagnosis |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Patient
characteristics | Chronic tonsillitis
118 (55.1%) | Hyperplasia 96
(44.9%) | P-value | Chronic tonsillitis
75 (50.0%) | Hyperplasia 75
(50.0%) |
| Chronic tonsillitis
43 (67.2%) | Hyperplasia 21
(32.8%) | P-value |
|---|
| Male | 58 (27.1) | 60 (28.0) | P>0.05 | 32 (21.3) | 49 (32.7) | P=0.009 | 26 (40.6) | 11 (17.2) | P>0.05 |
| Female | 60 (28.0) | 36 (16.8) |
| 43 (28.7) | 26 (17.3) |
| 17 (26.6) | 10 (15.6) |
|
| Median age
(range) | 23.5 (2.9–69.8) | 4.6 (0.6–57.1) | P<0.0001 | 23.6 (12.2–69.8) | 4.42 (0.6–12.9) | P<0.0001 | 23.1 (2.9–69.8) | 5.6 (2.4–57.1) | P<0.0001 |
|
<18 | 28 (13.1) | 93 (43.5) | P<0.0001 | 10 (6.7) | 65 (43.3) |
P<0.0001a | 18 (28.2) | 18 (28.2) | P=0.001 |
|
>18 | 90 (42.1) | 3 (1.4) |
| 75 (50.0) | 0 (0) |
| 25 (39.1) | 3 (4.7) |
|
|
Smoker | 47 (22.0) | 1 (0.5) | P<0.0001 | 36 (24.0) | 0 (0) |
P<0.0001a | 11 (17.2) | 1 (1.6) | P>0.05 |
|
Non-smoker | 71 (33.2) | 95 (44.4) |
| 39 (26.0) | 75 (50) |
| 32 (50.0) | 20 (31.3) |
|
| Smoker |
|
|
|
|
|
|
|
|
|
|
<18 | 6 (12.50) | 0 (0.0) |
P>0.05a | 5 (13.9) | 0 (0.0) |
P>0.05a | 1 (8.3) | 0 (0.0) |
P>0.05a |
|
>18 | 41 (85.4) | 1 (2.1) |
| 31 (86.1) | 0 (0.0) |
| 10 (83.3) | 1 (8.3) |
|
| Non-smoker |
|
|
|
|
|
|
|
|
|
|
<18 | 22 (13.3) | 93 (56.0) | P<0.0001 | 5 (4.4) | 75 (65.8) |
P<0.0001a | 17 (32.7) | 18 (34.6) | P=0.007 |
|
>18 | 49 (29.5) | 2 (1.2) |
| 34 (29.8) | 0 (0.0) |
| 15 (28.8) | 2 (3.8) |
|
Gene expression of SLPI and AnxA2
Patients with chronic tonsillitis had a
significantly higher SLPI gene expression levels than patients with
hyperplasia (2.6-fold higher in the entire population; 2.01-fold in
the fresh frozen and 2.6-fold in the FFPE samples). Similar results
were obtained when analyzing AnxA2 gene expression. In the entire
cohort, patients with chronic tonsillitis had 2.21-fold more AnxA2
than patients with hyperplasia. In the fresh-frozen material AnxA2
was 2.48-fold higher and in the FFPE samples 2.39-times more AnxA2
was measured.
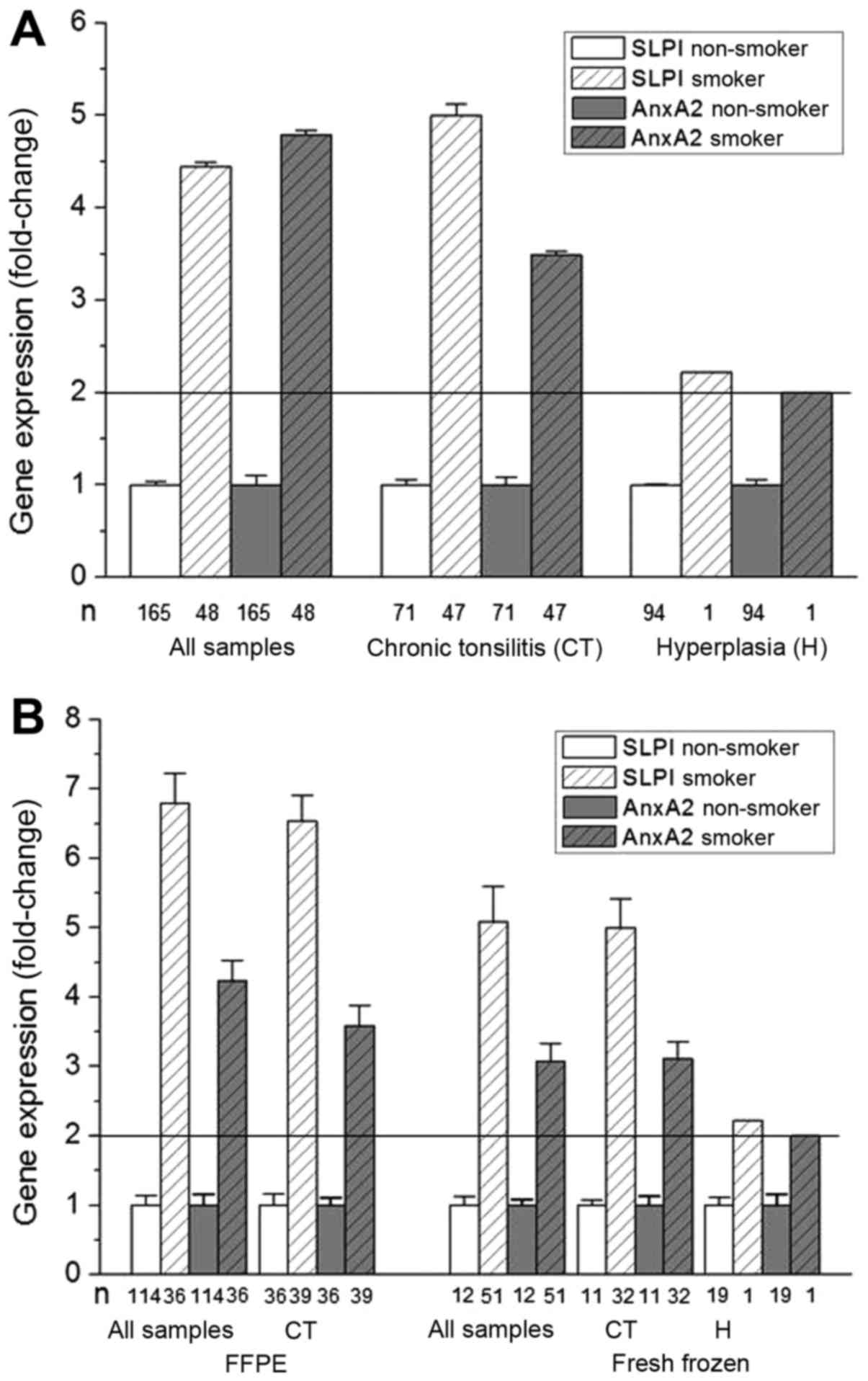
The effect of smoking on SLPI and AnxA2 gene
expression was then analyzed. As shown in Fig. 1A smoking resulted in significant
increases in SLPI and AnxA2 gene expression. This increase was
evident, not only when analyzing all samples but also when
stratifying for diagnosis (Fig. 1A)
and when stratifying for sample material and diagnosis (Fig. 1B). Of note, consistent results for
SLPI- and AnxA2 gene expression levels were obtained when analyzing
fresh frozen and FFPE material separately and stratified these for
diagnosis. The observed effects were more pronounced for SLPI than
for AnxA2 gene expression. However, due to the relatively small
amount of patients reporting a smoking habit, in particular in the
group of patients with hyperplasia (only 1 smoker), the results
need to be interpreted with caution.
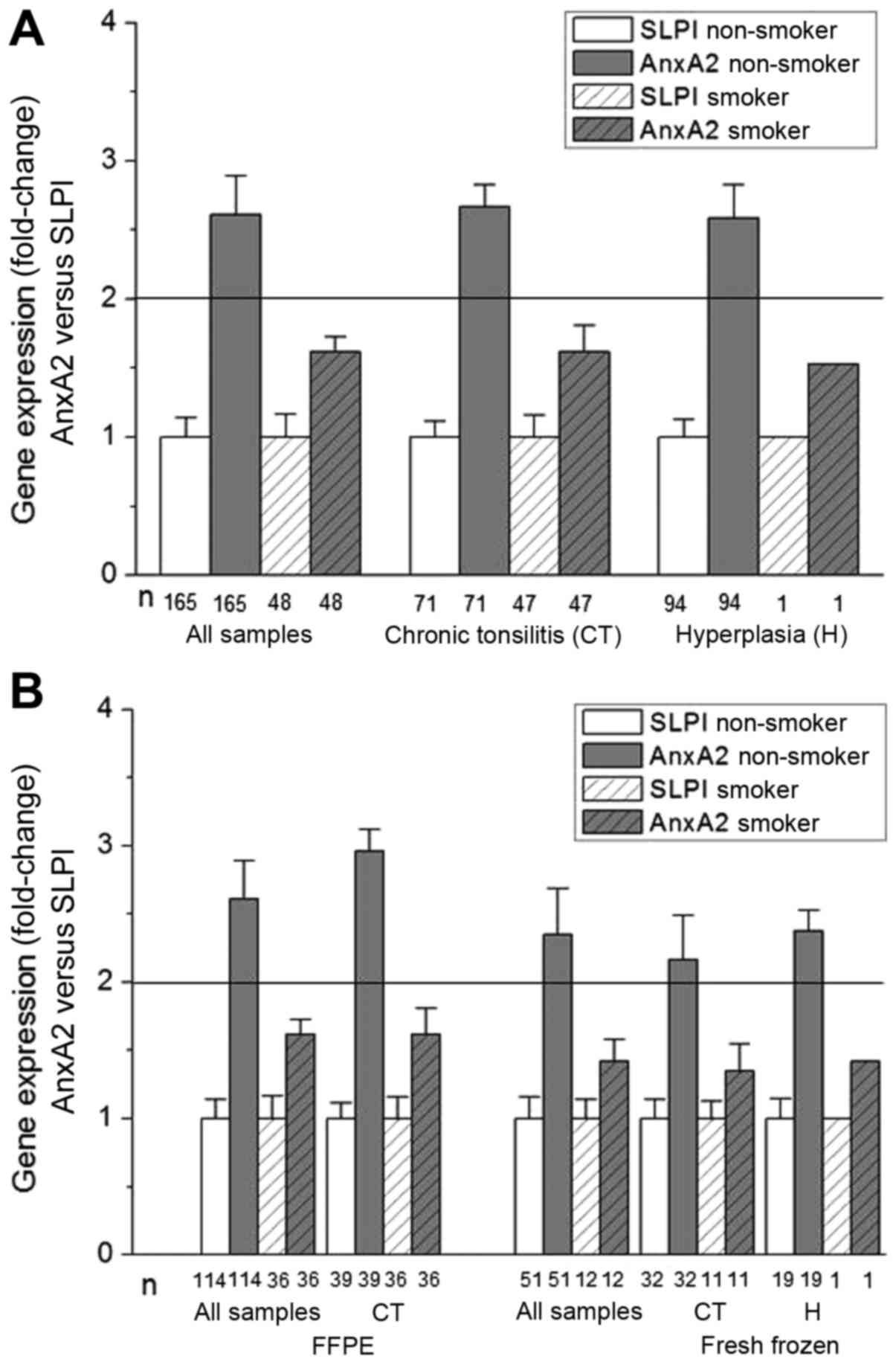
To determine the association between SLPI and AnxA2
gene expression, the fold change of AnxA2 gene expression in
relation to SLPI gene expression was calculated (Fig. 2). Again consistent results were
obtained analyzing either all samples, all samples stratified for
chronic tonsillitis or hyperplasia or analyzing the fresh-frozen
and FFPE material separately and these were stratified for
diagnosis. In all non-smoking patients AnxA2 gene expression was
significantly higher than the SLPI gene expression. This was not
the case in the group of patients reporting a smoking habit, where
AnxA2 and SLPI gene expression were nearly equal (fold change
levels <2).
Protein expression of SLPI
To corroborate SLPI gene expression by protein level
expression of SLPI protein was measured by means of
immunohistochemistry. The data were correlated with diagnosis and
smoking habit of the patients. Overall none of the samples showed
strong SLPI-expression (>75% of the epithelial cells of the
tonsillar crypts being SLPI-positive) and the majority of the
samples were classified as SLPI-negative (<5% of the epithelial
cells of the tonsillar crypts being SLPI-positive). Nonetheless,
the number of tissue specimens classified as weak or moderate was
higher in the group of patients diagnosed with chronic tonsillitis
than in the group of patients diagnosed with hyperplasia. This was
true when analyzing the entire cohort and the retrospectively and
prospectively samples separately, with the exception that, possible
due to the smaller sample size, no significant differences were
seen in the prospective samples. Correlation of SLPI protein
expression with smoking habit showed in all groups that SLPI
negativity was correlated with a negative smoking behavior.
However, again due to the extremely small number of patients
reporting a smoking habit, especially in the group of patients with
hyperplasia (n=1) these data need to be interpreted with caution
(Table II).
 | Table II.SLPI protein expression is shown for
the entire cohort and divided into retrospectively and
prospectively collected samples and correlated with diagnosis and
patient's smoking habits. |
Table II.
SLPI protein expression is shown for
the entire cohort and divided into retrospectively and
prospectively collected samples and correlated with diagnosis and
patient's smoking habits.
| SLPI |
|
| Entire cohort
(n=214) (%) |
|
| Retrospective
(n=150) (%) |
|
| Prospective (n=64)
(%) |
|---|
| Negative |
|
| 163 (76.2) |
|
| 118 (78.7) |
|
| 45 (70.3) |
| Weak |
|
| 45 (21.0) |
|
| 31 (20.7) |
|
| 14 (21.9) |
| Moderate |
|
| 6 (2.8) |
|
| 1 (0.6) |
|
| 5 (7.8) |
|
|
| Diagnosis |
| Diagnosis |
| Diagnosis |
|
|
|
|
|
|
|
|
|
|
| Chronic tonsillitis
118 (55.1%) | Hyperplasia 96
(44.9%) | P-value | Chronic tonsillitis
75 (50.0%) | Hyperplasia 75
(50.0%) | P-value | Chronic tonsillitis
43 (67.2%) | Hyperplasia 21
(32.8%) | P-value |
|
| Negative | 79 (36.9) | 84 (39.3) | P<0.001 | 51 (34.0) | 67 (44.7) | P=0.002 | 28 (43.8) | 17 (26.6) | P>0.05 |
| Weak | 35 (16.4) | 10 (4.7) |
| 23 (15.3) | 8 (5.3) |
| 12 (18.8) | 2 (3.1) |
|
| Moderate | 4 (1.9) | 2 (0.9) |
| 1 (0.7) | 0 (0.0) |
| 3 (4.7) | 2 (3.1) |
|
|
|
| Entire cohort |
| Retrospective |
| Prospective |
|
|
|
|
|
|
|
|
|
| SLPI/smoke | Smoker 48
(22.4%) | Non-smoker 166
(77.6%) | P-value | Smoker 36
(24.0%) | Non-smoker 114
(76.0%) | P-value | Smoker 12
(18.8%) | Non-smoker 54
(81.2%) | P-value |
|
| Negative | 20 (9.3) | 143 (66.8) | P=0.001 | 18 (12.0) | 100 (66.7) | P=0.001 | 2 (3.1) | 43 (67.2) | P=0.001 |
| Weak | 23 (10.7) | 22 (10.3) |
| 17 (11.3) | 14 (9.3) |
| 6 (59.4) | 8 (12.5) |
|
| Moderate | 5 (2.3) | 1 (0.5) |
| 1 (0.7) | 0 (0.0) |
| 4 (6.3) | 1 (1.6) |
|
|
|
| Diagnosis: chronic
tonsillitis |
| Diagnosis: chronic
tonsillitis |
| Diagnosis: chronic
tonsillitis |
|
|
|
|
|
|
|
|
|
|
| Smoker 47
(39.8%) | Non-smoker 71
(60.2%) | P-value | Smoker 36
(48.0%) | Non-smoker 39
(52.0%) | P-value | Smoker 11
(25.6%) | Non-smoker 32
(74.4%) |
P-valuea |
|
| Negative | 20 (16.9) | 59 (50.0) |
P=0.001a | 18 (24.0) | 33 (44.0) |
P=0.003a | 2 (4.7) | 26 (60.3) | P=0.001 |
| Weak | 23 (19.5) | 12 (10.2) |
| 17 (22.7) | 6 (8.0) |
| 6 (14.0) | 6 (14.0) |
|
| Moderate | 4 (3.4) | 0 (0.0) |
| 1 (1.3) | 0 (0.0) |
| 3 (7.0) | 0 (0.0) |
|
|
|
| Diagnosis:
hyperplasia |
| Diagnosis:
hyperplasia |
| Diagnosis:
hyperplasia |
|
|
|
|
|
|
|
|
|
|
| Smoker 1
(1.0%) | Non-smoker 95
(99.0%) |
P-valueb | Smoker 0
(0.0%) | Non-smoker 75
(100%) |
P-valueb | Smoker 1
(4.8%) | Non-smoker 20
(95.2%) |
P-valueb |
|
| Negative | 0 (0.0) | 84 (87.5) |
| 0 (0.0) | 67 (89.3) |
| 0 (0.0) | 17 (81.0) |
| Weak | 0 (0.0) | 10 (10.5) |
| 0 (0.0) | 8 (10.7) |
| 0 (0.0) | 2 (9.4) |
|
| Moderate | 1 (1.0) | 1 (1.0) |
| 0 (0.0) | 0 (0.0) |
| 1 (4.8) | 1 (4.8) |
|
HPV-status
HPV-DNA analysis was performed in all 214 biopsies.
None of the samples showed any signal in the GP5+/GP6+PCR. However,
all samples were positive in the B2M-PCR, demonstrating DNA
integrity. In addition, a positive control (a synthetic
oligonucleotide of the HPV L1 gene, covered by the GP5+/GP6+
primers; Eurofins; Ebersberg Germany) was amplified in the
GP5+/GP6+PCRs and resulted in the expected signals.
p16INK4A
immunohistochemistry
Since p16INK4A overexpression is
frequently used as a surrogate marker for HPV positivity in HNSCC,
immunohistochemistry for p16INK4A was performed and the
results are shown in Table III.
p16INK4A-expression showed negative, weak, and moderate
reaction in a so-called patchy pattern in 20 (9.3%), 156 (72.9%),
and 38 (17.8%) cases. There was neither strong (>75% of cells
positive) nor continuous immunostaining of basal keratinocytes. In
addition, p16INK4A expression was correlated with
diagnosis (Table III). No
significant correlation between protein expression and diagnosis
was found.
 | Table III.p16INK4A protein
expression is shown for the entire cohort and divided into
retrospectively and prospectively collected samples and correlated
with diagnosis and smoking habit of the patients. |
Table III.
p16INK4A protein
expression is shown for the entire cohort and divided into
retrospectively and prospectively collected samples and correlated
with diagnosis and smoking habit of the patients.
|
p16INK4A |
|
| Entire cohort
(n=214) (%) |
|
| Retrospective
(n=150) (%) |
|
| Prospective (n=64)
SLPI (%) |
|---|
| Negative |
|
| 20 (9.3) |
|
| 16 (10.7) |
|
| 6 (6.2) |
| Weak |
|
| 156 (72.9) |
|
| 114 (76.9) |
|
| 42 (65.5) |
| Moderate |
|
| 38 (17.8) |
|
| 20 (13.3) |
|
| 18 (28.2) |
|
|
| Diagnosis |
| Diagnosis |
| Diagnosis |
|
|
|
|
|
|
|
|
|
|
| Chronic tonsillitis
118 (55.1%) | Hyperplasia 96
(44.9%) | P-value | Chronic tonsillitis
75 (50.0%) | Hyperplasia 75
(50.0%) | P-value | Chronic tonsillitis
43 (67.2%) | Hyperplasia 21
(32.8%) | P-value |
|
| Negative | 8 (3.7) | 12 (5.6) | P>0.05 | 5 (3.3) | 11 (7.3) | P>0.05 | 3 (4.7) | 1 (1.6) | P>0.05 |
| Weak | 92 (43.0) | 64 (29.9) |
| 64 (29.9) | 50 (33.3) |
| 28 (43.8) | 14 (21.9) |
|
| Moderate | 18 (8.4) | 20 (9.3) |
| 6 (4.0) | 14 (9.3) |
| 12 (18.8) | 6 (9.4) |
|
|
|
| Entire cohort |
| Retrospective |
| Prospective |
|
|
|
|
|
|
|
|
|
|
P16INK4A/smoker | Smoker 48
(22.4%) | Non-smoker 166
(77.6%) | P-value | Smoker 36
(24.0%) | Non-smoker 114
(76.0%) | P-value | Smoker 12
(18.8%) | Non-smoker 54
(81.2%) | P-value |
|
| Negative | 1 (0.4) | 19 (8.9) | P>0.05 | 1 (0.7) | 15 (10.0) | P=0.04 | 0 (0.0) | 4 (6.2) |
P>0.05a |
| Weak | 41 (19.2) | 115 (53.7) |
| 33 (21.9) | 81 (54.0) |
| 8 (12.5) | 34 (53.1) |
|
| Moderate | 6 (2.8) | 32 (15.0) |
| 2 (1.4) | 18 (12.0) |
| 4 (6.3) | 14 (21.9) |
|
|
|
| Diagnosis: chronic
tonsillitis |
| Diagnosis: chronic
tonsillitis |
| Diagnosis: chronic
tonsillitis |
|
|
|
|
|
|
|
|
|
|
| Smoker 47
(39.8%) | Non-smoker 71
(60.2%) | P-value | Smoker 36
(48.0%) | Non-smoker 39
(52.0%) | P-value | Smoker 11
(25.6%) | Non-smoker 32
(74.4%) | P-value |
|
| Negative | 1 (0.9) | 7 (5.9) | P>0.05 | 1 (1.3) | 4 (5.3) | P>0.05 | 0 (0.0) | 3 (7.0) |
P>0.05a |
| Weak | 41 (34.7) | 51 (43.2) |
| 33 (44.1) | 31 (41.4) |
| 8 (18.6) | 20 (46.5) |
|
| Moderate | 5 (4.2) | 13 (11.1) |
| 2 (2.6) | 4 (5.3) |
| 3 (7.0) | 9 (20.9) |
|
|
|
| Diagnosis:
hyperplasia |
| Diagnosis:
hyperplasia |
| Diagnosis:
hyperplasia |
|
|
|
|
|
|
|
|
|
|
| Smoker 1
(1.0%) | Non-smoker 95
(99.0%) |
P-valueb | Smoker 0
(0.0%) | Non-smoker 75
(100%) |
P-valueb | Smoker 1
(4.8%) | Non-smoker 20
(95.2%) |
P-valueb |
|
| Negative | 0 (0.0) | 12 (12.5) |
| 0 (0.0) | 11 (14.7) |
| 0 (0.0) | 1 (4.8) |
|
| Weak | 0 (0.0) | 64 (66.7) |
| 0 (0.0) | 50 (66.7) |
| 0 (0.0) | 14 (66.7) |
|
| Moderate | 1 (1.0) | 19 (19.8) |
| 0 (0.0) | 14 (18.6) |
| 1 (4.8) | 5 (23.7) |
|
Discussion
The results of this unique study investigating 214
tissue specimens derived from patients with tonsillar hyperplasia
(n=96) or chronic tonsillitis (n=118) reveal that also in
non-malignant tissue specimens of the tonsils there is a
significant correlation between the patients' smoking habit and the
expression levels of AnxA2 and SLPI, both showing significantly
higher expression levels in smokers in comparison to non-smokers.
These results were confirmed for SLPI on the protein level, showing
that SLPI negativity is associated with a negative smoking history
which holds true in all groups investigated. The additional
findings regarding gene expression levels demonstrate that within
the group of non-smokers AnxA2 shows a significant surplus when
compared to SLPI whereas this ratio is approximately even in
smokers. These findings corroborate the results shown for malignant
tissue specimens derived from head and neck cancers as published,
previously (1–3). Thus, the data of the present study are
in line with our hypothesis described earlier in the present study
as well as in previous studies (1–3), namely
that both, AnxA2 and SLPI, may play a role in the mode of infection
with human papillomaviruses in head and neck (HNSCC) and
specifically tonsillar squamous cell carcinomas (TSCC) (1–3).
The described statistical significance regarding the
correlation of AnxA2, SLPI, and smoking habit can be seen only in
tests for trend for one single result when the entire study
population is analysed and, however, for some results in the
subgroup analysis (Tables I–III). The latter is due to the fact that
the study included tonsillectomy patients who were previously
diagnosed with tonsillar hyperplasia. As expected this specific
subgroup of patients with tonsillar hyperplasia predominantly
comprises children (median age, 4.6 years) with no smoking history
leading to a significant imbalance between the subgroups chronic
tonsillitis (median age, 23.5 years) and tonsillar hyperplasia when
data are correlated with smoking habit. The authors, however, were
aware of this fact ahead of the study and the reason for including
cases with tonsillar hyperplasia was that SLPI is also involved in
inflammation (15) present in
chronic tonsillitis. To rule out such an inflammation caused bias,
data of at least less inflamed tissue was needed, namely that of
patients (children) with tonsillar hyperplasia not showing
high-grade signs of inflammation. Against this backdrop and despite
the small case numbers among the various subgroups it is,
noteworthy that the majority of results specifically for gene
expression analysis very clearly show statistical significance and
this even in the subgroup of tonsillar hyperplasia, however, less
significant than in the group of chronic tonsillitis.
Somewhat unexpected is the absence of any HPV-DNA in
the tonsillar tissue analysed in the present study. Since in the
literature a very low but still present HPV detection rate was
described (16–18), it was assumed that at least two to
four of these 214 cases would carry HPV-DNA, enough to further
analyse the basic hypothesis of this study. In this context it is
notable that the rate of HPV-DNA-positivity in oral rinses is
reported to be approximately 7% (19) and it now remains to be clarified
which tissue harbours the HPV-DNA detected in oral gargle, when the
tonsillar tissue is not. Palmer and co-workers reported on 0%
HPV-positivity in non-malignant tonsillar tissue among a study
population of less than 3,300 patients in the UK (20). The latter and our data question as
already discussed by Franceschi and co-workers (18) whether or not the absence of HPV-DNA
in non-neoplastic tonsillar tissue justifies invasive detection
methods to detect HPV-infections in healthy individuals to identify
precancerous lesions and possibly prevent carcinogenesis.
The immunohistochemical results for
p16INK4A excluded any early HPV-induced (premalignant)
neoplasia. Table III shows that
the vast majority of cases show weak staining whereas negativity or
moderate staining occurs in approximately 10 and 20%, respectively.
The slightly higher expression pattern in chronic tonsillitis when
compared to tonsillar hyperplasia can most likely also be
attributed to inflammation processes in cases with chronic
tonsillitis. Another possible explanation for the slightly, albeit
not significant, higher p16INK4A expression pattern in
patients with chronic tonsillitis may be due to the smoking habit
of these patients since among the patients with chronic tonsillitis
the proportion of smokers was significantly higher than in the
group of patients with tonsillar hyperplasia. Since
p16INK4A is frequently used as a surrogate marker for
HPV infection this observation is in agreement with the finding
that HPV-induced HNSCC are often attributed to a negative smoking
history of the patients (21).
In conclusion, despite the lack of an HPV-positive
case the data shown in the present study are in agreement with
previous studies regarding the correlation between SLPI- and
AnxA2-expression and smoking habit of the patients. Smoking results
in higher SLPI as well as AnxA2 expression with an even
distribution of both parameters in smoking individuals whereas in
non-smoking patients there is a significant surplus of AnxA2 in
comparison to SLPI. Previous findings suggest that a surplus of
AnxA2 in the mucosa increases the susceptibility of HPV infections
while a surplus of SLPI seems to hinder successful HPV entry into
the cells since SLPI blocks AnxA2 for HPV interaction. Despite the
fact that HPV detection in non-malignant tonsillar tissue specimens
identifies only very small numbers of HPV-positive cases, the
results of the present study encourage further investigation of the
basic hypothesis regarding SLPI-, AnxA2-expression, smoking habit
and HPV status. Further knowledge regarding the mode of HPV cell
entry may, not only contribute to the understanding of HPV
infection and oncogenesis in head and neck cancer, but also
contribute to the understanding of other virus infections with
malignant potential such as EBV infections.
Acknowledgements
The authors would like to thank Gudrun Scherer and
Hilke Clasen (Institute of Immunology, Kiel, Germany) for skilful
technical assistance with immunohistochemistry and RNA-isolation,
cDNA synthesis, (q)PCR, respectively. Special thanks goes to Ralph
Pries and Maren Drenckhan (Department ORL, Head and Neck Surgery,
UKSH, Campus Lübeck, Germany) for performing the tissue
microarrays.
References
|
1
|
Hoffmann M, Quabius ES, Tribius S,
Hebebrand L, Görögh T, Halec G, Kahn T, Hedderich J, Röcken C, Haag
J, et al: Human papillomavirus infection in head and neck cancer:
The role of the secretory leukocyte protease inhibitor. Oncol Rep.
29:1962–1968. 2013.PubMed/NCBI
|
|
2
|
Quabius ES, Möller P, Haag J,
Pfannenschmidt S, Hedderich J, Görögh T, Röcken C and Hoffmann M:
The role of the antileukoprotease SLPI in smoking-induced human
papillomavirus-independent head and neck squamous cell carcinomas.
Int J Cancer. 134:1323–1334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quabius ES, Görögh T, Fischer GS, Hoffmann
AS, Gebhard M, Evert M, Beule A, Maune S, Knecht R, Óvári A, et al:
The antileukoprotease secretory leukocyte protease inhibitor (SLPI)
and its role in the prevention of HPV-infections in head and neck
squamous cell carcinoma. Cancer Lett. 357:339–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Woodham AW, Da Silva DM, Skeate JG, Raff
AB, Ambroso MR, Brand HE, Isas JM, Langen R and Kast WM: The
S100A10 subunit of the annexin A2 heterotetramer facilitates
L2-mediated human papillomavirus infection. PLoS One. 7:e435192012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoffmann M, Ihloff AS, Görögh T, Weise JB,
Fazel A, Krams M, Rittgen W, Schwarz E and Kahn T: p16(INK4a)
overexpression predicts translational active human papillomavirus
infection in tonsillar cancer. Int J Cancer. 127:1595–1602. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quabius ES, Haag J, Kühnel A, Henry H,
Hoffmann AS, Görögh T, Hedderich J, Evert M, Beule AG, Maune S, et
al: Geographical and anatomical influences on human papillomavirus
prevalence diversity in head and neck squamous cell carcinoma in
Germany. Int J Oncol. 46:414–422. 2015.PubMed/NCBI
|
|
7
|
Mehanna H, Beech T, Nicholson T, El-Hariry
I, McConkey C, Paleri V and Roberts S: Prevalence of human
papillomavirus in oropharyngeal and nonoropharyngeal head and neck
cancer-systematic review and meta-analysis of trends by time and
region. Head Neck. 35:747–755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castellsagué X, Alemany L, Quer M, Halec
G, Quirós B, Tous S, Clavero O, Alòs L, Biegner T, Szafarowski T,
et al: HPV involvement in head and neck cancers: Comprehensive
assessment of biomarkers in 3680 patients. J Natl Cancer Inst.
108:djv4032016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Remmerbach TW, Brinckmann UG, Hemprich A,
Chekol M, Kühndel K and Liebert UG: PCR detection of human
papillomavirus of the mucosa: Comparison between MY09/11 and
GP5+/6+ primer sets. J Clin Virol. 30:302–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Quabius ES, Ossenkop L, Harder S and Kern
M: Dental implants stimulate expression of Interleukin-8 and its
receptor in human blood-an in vitro approach. J Biomed Mater Res B
Appl Biomater. 100:1283–1288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kononen J, Bubendorf L, Kallioniemi A,
Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G
and Kallioniemi OP: Tissue microarrays for high-throughput
molecular profiling of tumor specimens. Nat Med. 4:844–847. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cordes C, Häsler R, Werner C, Görögh T,
Röcken C, Hebebrand L, Kast WM, Hoffmann M, Schreiber S and
Ambrosch P: The level of secretory leukocyte protease inhibitor is
decreased in metastatic head and neck squamous cell carcinoma. Int
J Oncol. 39:185–191. 2011.PubMed/NCBI
|
|
13
|
Klaes R, Friedrich T, Spitkovsky D, Ridder
R, Rudy W, Petry U, Dallenbach-Hellweg G, Schmidt D and von Knebel
Doeberitz M: Overexpression of p16(INK4A) as a specific marker for
dysplastic and neoplastic epithelial cells of the cervix uteri. Int
J Cancer. 92:276–284. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Majchrzak-Gorecka M, Majewski P, Grygier
B, Murzyn K and Cichy J: Secretory leukocyte protease inhibitor
(SLPI), a multifunctional protein in the host defense response.
Cytokine Growth Factor Rev. 28:79–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klingenberg B, Hafkamp HC, Haesevoets A,
Manni JJ, Slootweg PJ, Weissenborn SJ, Klussmann JP and Speel EJ:
p16 INK4A overexpression is frequently detected in tumour-free
tonsil tissue without association with HPV. Histopathology.
56:957–967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rusan M, Klug TE, Henriksen JJ, Bonde JH,
Fuursted K and Ovesen T: Prevalence of tonsillar human
papillomavirus infections in Denmark. Eur Arch Otorhinolaryngol.
272:2505–2512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Franceschi S, Combes JD, Dalstein V,
Caudroy S, Clifford G, Gheit T, Tommasino M, Clavel C, St Guily J
Lacau and Birembaut P: Study of Natural History of Human
Papillomavirus Infection and Precancerous Lesions in the Tonsils
(SPLIT): Deep brush-based cytology in tonsils resected for benign
diseases. Int J Cancer. 137:2994–2999. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kreimer AR, Johansson M, Waterboer T,
Kaaks R, Chang-Claude J, Drogen D, Tjønneland A, Overvad K, Quirós
JR, González CA, et al: Evaluation of human papillomavirus
antibodies and risk of subsequent head and neck cancer. J Clin
Oncol. 31:2708–2715. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Palmer E, Newcombe RG, Green AC, Kelly C,
Noel Gill O, Hall G, Fiander AN, Pirotte E, Hibbitts SJ, Homer J
and Powell NG: Human papillomavirus infection is rare in
nonmalignant tonsil tissue in the UK: Implications for tonsil
cancer precursor lesions. Int J Cancer. 135:2437–2443. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gillison ML, D'Souza G, Westra W, Sugar E,
Xiao W, Begum S and Viscidi R: Distinct risk factor profiles for
human papillomavirus type 16-positive and human papillomavirus type
16-negative head and neck cancers. J Natl Cancer Inst. 100:407–420.
2008. View Article : Google Scholar : PubMed/NCBI
|