Introduction
Lung cancer is a leading cause of morbidity and
mortality worldwide (1,2). The two main types of lung cancer are
non-small-cell lung cancer (NSCLC) and small-cell lung cancer
(SCLC), which account for 85 and 15% of all cases, respectively
(3). However, despite improvements
in the modalities for diagnosing and treating lung cancer, the
prognosis remains poor. Thus, novel and effective prognostic
factors, which may allow clinicians to use effective therapeutic
strategies, are urgently needed.
The traditional prognostic markers for lung cancer
prognosis include patient age (4),
sex (5), smoking (6), and TNM classification (7). There are also novel biomarkers that are
able to predict prognosis and guide clinical treatment, including
elevated levels of carcinoembryonic antigen, cytokeratin-19
fragments, squamous cell carcinoma antigen, progastrin-releasing
peptide, tumor M2-pyruvate kinase, and C-reactive protein (8). However, patients with the same TNM
stage may have a different prognosis (9), whereas some of the abovementioned
prognostic biomarkers are costly and, thus, not included in routine
tests for the majority of the patients.
During recent years, an increasing number of studies
have revealed an association between systemic inflammation and
tumorigenesis-related factors, including tumor angiogenesis,
progression, invasion and metastasis (10–13).
Accumulating evidence also indicates that tumor-associated
inflammation may be detected in the peripheral blood as
neutrophilia and/or lymphopenia (14). This finding suggests that the levels
of neutrophils and lymphocytes may function as a combined factor,
which may more accurately reflect the inflammatory response,
compared with a single factor. Thus, the neutrophil-to-lymphocyte
ratio (NLR) has been developed as a novel indicator of
inflammation, and an elevated NLR may be associated with a poor
prognosis (15). Moreover, routine
laboratory and blood tests are performed during the pre-treatment
work-up for all patients, and these results may be used to evaluate
the patient's NLR. Therefore, NLR is a minimally invasive and
inexpensive biomarker that may be used to predict prognosis among
patients with lung cancer. However, there is controversy regarding
whether NLR is a convincing or effective clinical indicator, and
the association between NLR and lung cancer remains unclear. The
aim of the present meta-analysis was to evaluate whether NLR is of
value for predicting the prognosis of lung cancer.
Data collection methods
Search strategy
A systematic search was performed through the
PubMed, Embase and Cochrane Library databases from inception up to
July 30, 2016. The search used the following terms:
Neutrophil-to-lymphocyte ratio, neutrophil to lymphocyte ratio,
neutrophil-lymphocyte ratio, neutrophil lymphocyte ratio, and MeSH
terms (lung neoplasms AND prognosis). The reference lists from the
identified reports were also reviewed, in order to retrieve other
potentially relevant studies.
Study selection criteria
Articles were considered eligible if they fulfilled
the following criteria: i) The patients were pathologically
diagnosed with lung cancer (NSCLC or SCLC); ii) the study
investigated the association between pretreatment NLR and various
outcomes, including overall survival (OS), progression-free
survival (PFS), disease-free survival, or recurrence-free survival;
iii) reported hazard ratios (HRs) and 95% confidence intervals
(CIs) or provided sufficient information to estimate the HRs and
95% CIs; and iv) the full text was accessible and written in
English.
Data extraction
All the retrieved articles were independently
reviewed by two investigators (Yu Yu and Lei Qian). The extracted
data included the first author's name, year of publication, study
duration, country, ethnicity, sample size, sex, age, stage, tumor
type, follow-up period, treatment, study design, and cut-off value
for elevated NLR with the HRs and/or 95% CIs. Disagreements were
discussed and resolved through consensus.
Statistical analysis
Based on the methods of Tierney et al
(16), the HRs and 95% CIs were
estimated or extracted to evaluate the significance of NLR
according to OS and PFS. A poorer prognosis was defined as an
elevated NLR being associated with an HR of >1. Heterogeneity of
the pooled results was tested using Cochran's Q test and Higgins'
I-squared statistic, with an I2 of >50% representing
significant heterogeneity. The pooled HRs and 95% CIs were
calculated using a random-effects model (Der Simonian-Laird method)
or a fixed-effects model (Mantel-Haenszel method), as appropriate
The random-effects model was defined as the preferred method when
heterogeneity was detected. Inter-study heterogeneity was also
investigated using subgroup analysis and meta-regression analysis.
Sensitivity analysis was also performed to evaluate the stability
and credibility of the results. Publication bias was assessed using
Egger's funnel plot. All statistical tests were two-sided and
P-values <0.05 were considered to indicate statistically
significant differences. All statistical analyses were performed
using Stata software, version 13.1 (StataCorp LP, College Station,
TX, USA).
Results
Study characteristics
The study selection flow chart is shown in Fig. 1. The initial search through the
PubMed, Embase and Cochrane Library databases identified 125
studies. After excluding duplicate reports, irrelevant reports,
reviews and conference abstracts, aa total of 37 full-text reports
were included in the evaluation. Subsequently, 19 studies were
excluded for the following reasons: 7 studies failed to provide
sufficient data for the analyses, 6 studies did not report the NLR
cut-off value, 4 studies did not report specific NLR data according
to OS, and 2 studies had data duplication. Thus, the final analyses
included 18 studies (19 cohorts) (17–34) with
7,219 patients, published between 2009–2016. The patients in the
report by Botta et al (22)
were split into two independent cohorts (Botta 1 and Botta 2) due
to the cohort design of the article.
The characteristics of the 18 studies are summarized
in Table I. A total of 7 studies
were conducted in Western countries, including the UK (17,26,34), USA
(28), Spain (20) and Italy (22,34), 1
study was performed in Turkey (23),
and 10 studies were performed in East Asian countries, including
Japan (18,19,31,32),
Korea (21,25) and China (24,27,30,33). The
study published by Mitchell et al (29) included patient data from 33
countries. The included studies evaluated 16 populations of
patients with NSCLC (17–24,26–29,31–34) and
2 populations of patients with SCLC (25,30). The
2 studies on SCLC reported tumor staging information as limited and
extensive disease, so only the staging information from the studies
regarding NSCLC were considered Only 1 study by Sarraf et al
(17) evaluated all tumor stages, 8
studies evaluated early-stage tumors (23,26–29,31–33), and
6 studies evaluated late-stage tumors (18,20–22,24,34). The
study by Tomita et al (19)
evaluated stages IA/III/IV. The cut-off values for elevated NLR
ranged from 2.5 to 5.
 | Table I.Characteristics of all the included
studies. |
Table I.
Characteristics of all the included
studies.
| First author | Year | Duration | Country | Ethnicity | Median follow-up,
months (range) | Sample size | Sex (M/F) | Median/mean age,
years ± SD (range) | Stage | Type | Treatment | Cut-off value | Survival
analysis | Study design | Method | (Refs.) |
|---|
| Sarraf | 2009 | 1999–2005 | UK | Caucasian | 29 (8–56) | 177 | 104/73 | 63±10 | I–IV | NSCLC | S | 3.81 | OS | R | MV | (17) |
| Teramukai | 2009 | 2001–2005 | Japan | Asian | 18.9 (2.3–57) | 388 | 276/112 | 65 (33–81) | IIIB/IV | NSCLC | C | 4.74 | OS, PFS | P | MV | (18) |
| Tomita | 2011 | 2000–2005 | Japan | Asian | 60.7–131.7 | 284 | 178/106 | 67 (26–85) | IA/III/IV | NSCLC | S | 2.5 | OS | R | MV/UV | (19) |
| Cedrés | 2012 | 2004–2009 | Spain | Caucasian | 9.1 (1–70.37) | 171 | 143/28 | 63 (30–81) | IV | NSCLC | C | 5 | OS | R | MV/UV | (20) |
| Lee | 2012 | 2005–2007 | Korea | Asian | 36 (33.6–37.9) | 199 | 17/182 | 57 (19–74) | IIIB/IV | NSCLC | C or T | 3.25 | OS, PFS | P | MV/UV | (21) |
| Botta | 2013 | 2008–2011 | Italy | Caucasian | 15 | 73 | 55/18 |
58.57±10.54a | IIIB/IV | NSCLC | C | 4 | PFS | R | UV | (22) |
| Botta | 2013 | 2008–2011 | Italy | Caucasian | 15 | 39 | 26/13 |
67.85±9.67a | IIIB/IV | NSCLC | C+T | 4 | PFS | R | UV | (22) |
| Unal | 2013 | NR | Turkey | Caucasian | NR | 94 | 88/6 | 58.1±8.6
(30–78)a | IIA-IIIB | NSCLC | C+R | 3.44 | OS, DFS | R | MV/UV | (23) |
| Yao | 2013 | 2007–2010 | China | Asian | NR | 182 | 119/63 | 59 (28–79) | III–IV | NSCLC | C | 2.63 | OS, PFS | R | MV/UV | (24) |
| Kang | 2014 | 2006–2013 | Korea | Asian | 40.28
(2.60–89.26) | 187 | 162/25 | 68 (43–84) | L+E | SCLC | C | 4 | OS, PFS | R | MV | (25) |
| Pinato | 2014 | 2004–2011 | UK | Caucasian | 13 (1–87) | 220 | 110/110 | 65 | IA-IIIA | NSCLC | NR | 5 | OS | P | MV/UV | (26) |
| Zhang | 2014 | 2006–2009 | China | Asian | 46 (1–78) | 400 | 272/128 | 60 (24–82) | I–II | NSCLC | S | 3.3 | OS, DFS | R | MV/UV | (27) |
| Choi | 2015 | 2004–2010 | USA | NR | 102 | 1,139 | 602/537 | 64.73a | I–III | NSCLC | S | 5 | OS, RFS | R | MV/UV | (28) |
| Mitchell | 2015 | 2007–2011 | 33 countries | NR | 58.7 | 1,239 | 846/393 | 61 (19–89) | III | NSCLC | C+R+T+I | 5 | OS | P | UV | (29) |
| Shao | 2015 | 2000–2009 | China | Asian | 68.5 | 112 | 98/14 | 62 (45–82) | L+E | SCLC | C+R | 4.15 | OS, PFS | R | MV/UV | (30) |
| Shimizu | 2015 | 2007–2012 | Japan | Asian | 32.0 (3–72) | 334 | 219/115 | 69.3
(46–88)a | I–III | NSCLC | S | 2.5 | OS, DFS | R | MV/UV | (31) |
| Takahashi | 2015 | 2000–2008 | Japan | Asian | 73.5 (15–159) | 342 | 167/175 | 68 (25–87) | I | NSCLC | S | 2.50 | OS, RFS | R | MV/UV | (32) |
| Zhang | 2015 | 2005–2009 | China | Asian | 45.0 (2–96) | 1,238 | 812/426 | 60 (24–82) | I–IIIA | NSCLC | S | 2.3 | OS, DFS | R | MV/UV | (33) |
| Berardi | 2016 | 2009–2014 | Italy, UK | Caucasian | 19.6
(16.5–28.6) | 401 | 275/126 | 68 (25–86) | III–IV | NSCLC | C or T | 3.7 | OS, PFS | R | MV/UV | (34) |
NLR and OS in lung cancer
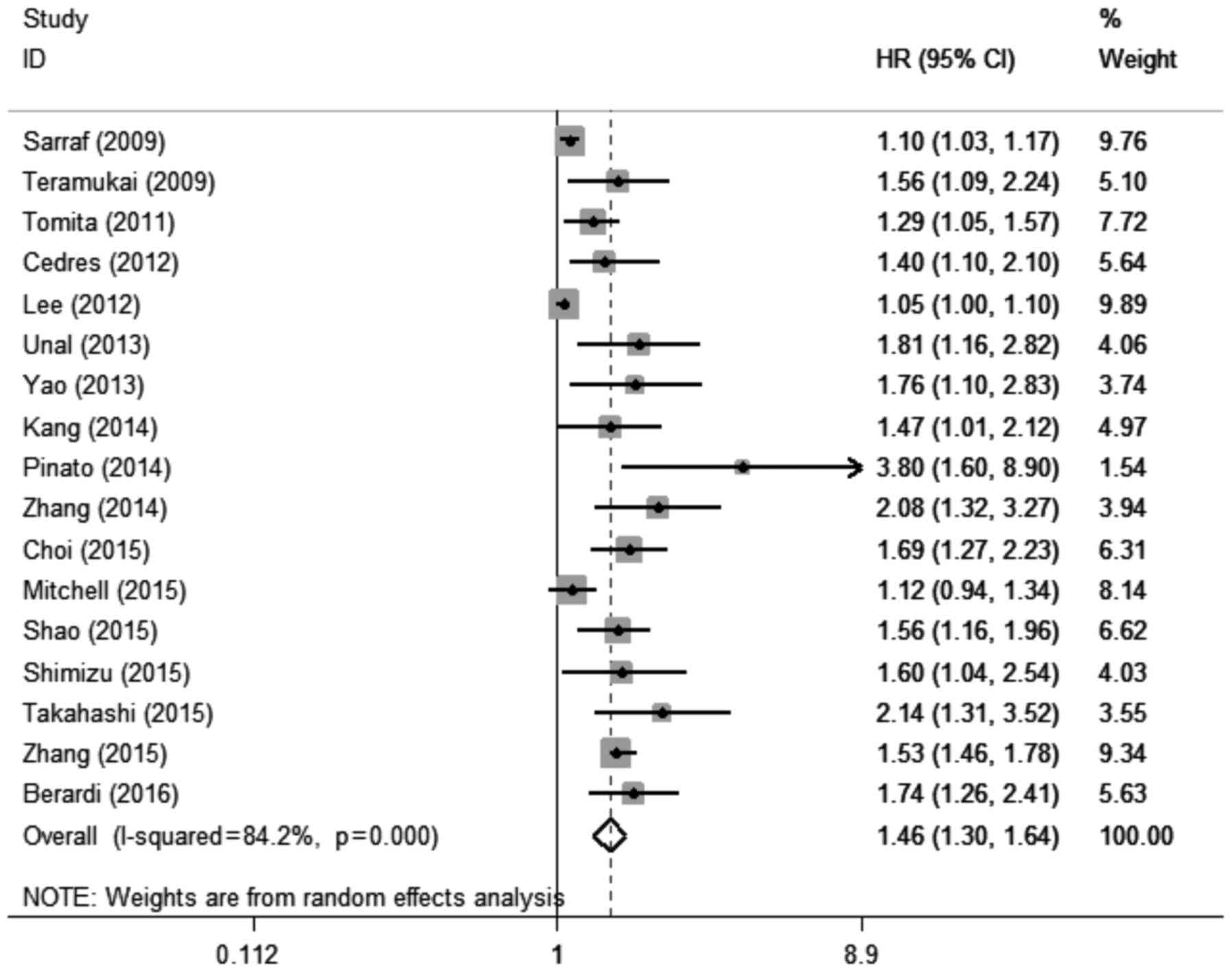
A total of 17 studies with 17 cohorts (17–21,23–34)
including 7,107 patients evaluated the association between elevated
pretreatment NLR and OS among patients with lung cancer. The
random-effects model was used for this analysis, as significant
heterogeneity was detected (I2=84.2%,
Pheterogeneity<0.001). The pooled HR was 1.46 (95%
CI: 1.30–1.64, P<0.001; Fig. 2),
which suggested that elevated pretreatment NLR predicted poor OS
after treatment for lung cancer.
NLR and PFS in lung cancer
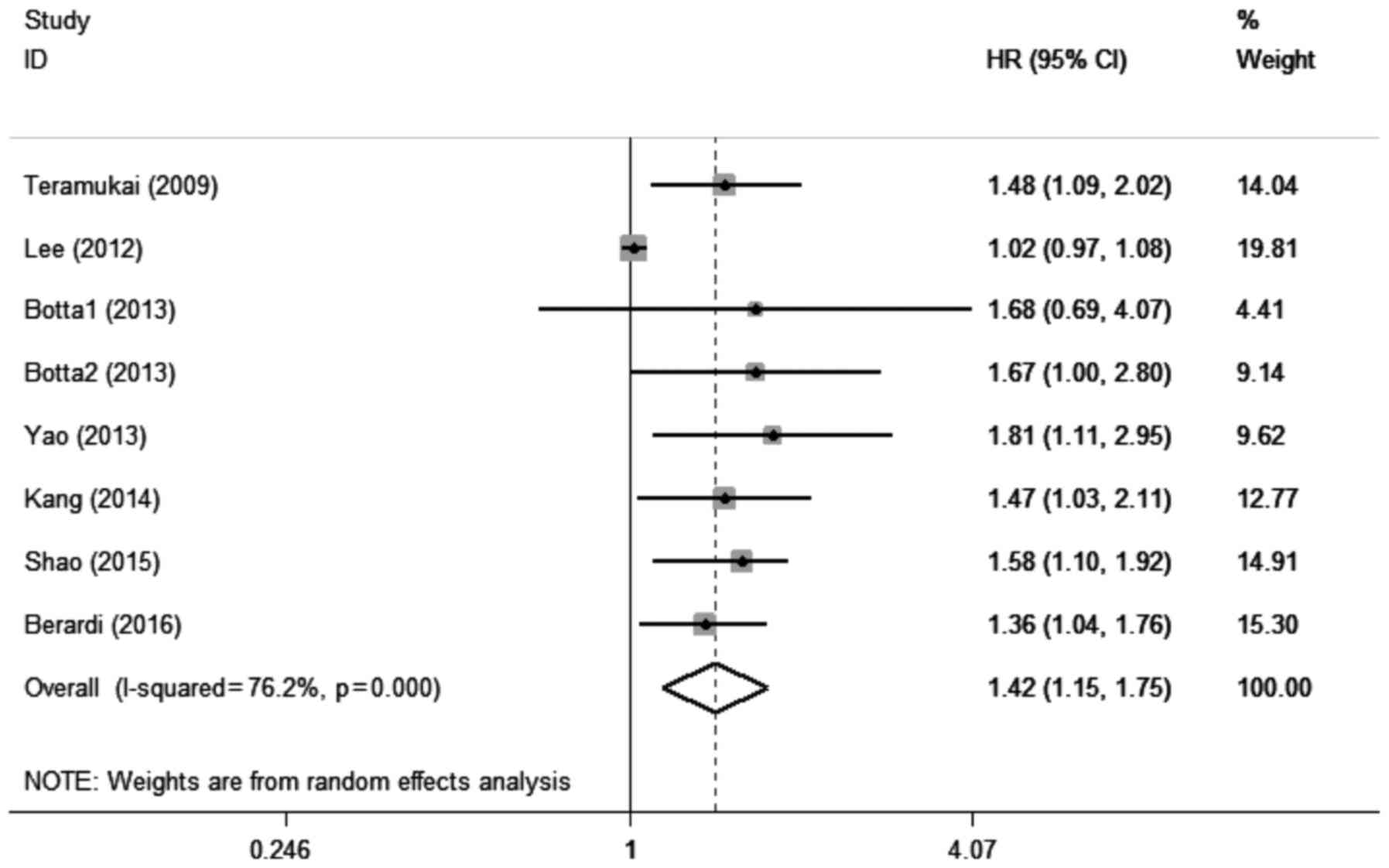
A total of 7 studies with 8 cohorts (18,21,22,24,25,30,34)
including 1,581 patients evaluated the association between elevated
pretreatment NLR and PFS among patients with lung cancer.
Significant heterogeneity was also detected among these studies
(I2=76.2%, Pheterogeneity<0.001). A pooled
HR of 1.42 (95% CI: 1.15–1.75, P=0.001; Fig. 3) suggested that elevated pretreatment
NLR predicted shorter PFS after treatment for lung cancer.
Subgroup analysis
A subgroup analysis was performed to identify the
possible reason(s) for the significant heterogeneity in the
meta-analysis (Table II). The
OS-related subgroup analysis included 7 subgroups: Treatment
(surgery and non-surgery), ethnicity (Caucasian and Asian), tumor
stage (late stage: IIIB-IV; and early stage: I–IIIA), sample size
(<200 and ≥200), NLR cut-off value (<4 and ≥4), tumor type
(NSCLC and SCLC), and analysis method (multivariate and
univariate). The results consistently demonstrated that elevated
pretreatment NLR predicted poor OS after treatment for lung cancer.
The PFS-related subgroup analysis included 5 subgroups (ethnicity,
sample size, cut-off value, tumor type and analysis method), and
the combined results were similar to those for OS. Interestingly,
an NLR cut-off of ≥4 was found to be associated with significantly
lower heterogeneity (OS: I2=14.3%,
Pheterogeneity=0.323; PFS: I2=57.2%,
Pheterogeneity=0.029), suggesting that an NLR of ≥4 was
a useful prognostic indicator for both OS and PFS.
 | Table II.Summary of the meta-analysis
results. |
Table II.
Summary of the meta-analysis
results.
| A, Overall
survival |
|---|
|
|---|
|
|
| Random-effects
model | Fixed-effects
model | Heterogeneity |
|---|
|
|
|
|
|
|
|---|
| Subgroup | Number | HR (95% CI) | P-value | HR (95% CI) | P-value | I2
(%) | Ph |
|---|
| Treatment |
|
|
|
|
|
|
|
|
Surgery | 7 | 1.57
(1.21–1.95) | <0.001 | 1.25
(1.19–1.31) | <0.001 | 88.3 | <0.001 |
|
Non-surgery | 10 | 1.42
(1.21–1.68) | <0.001 | 1.11
(1.06–1.16) | <0.001 | 75.9 | <0.001 |
| Ethnicity |
|
|
|
|
|
|
|
|
Caucasian | 5 | 1.58
(1.15–2.17) |
0.005 | 1.14
(1.08–1.22) | <0.001 | 80.9 | <0.001 |
|
Asian | 10 | 1.51
(1.26–1.81) | <0.001 | 1.17
(1.12–1.21) | <0.001 | 87.7 | <0.001 |
| Tumor stage |
|
|
|
|
|
|
|
|
Late | 5 | 1.43
(1.09–1.87) | 0.1 | 1.08
(1.03–1.13) |
0.001 | 80.2 | <0.001 |
|
Early | 8 | 1.64
(1.37–1.97) | <0.001 | 1.50
(1.39–1.62) | <0.001 | 65.9 |
0.005 |
| Sample size, n |
|
|
|
|
|
|
|
|
<200 | 7 | 1.25
(1.11–1.41) | <0.001 | 1.09
(1.05–1.13) | <0.001 | 74.1 |
0.001 |
|
≥200 | 10 | 1.56
(1.35–1.79) | <0.001 | 1.48
(1.38–1.59) | <0.001 | 60.3 |
0.007 |
| Cut-off value |
|
|
|
|
|
|
|
|
<4 | 10 | 1.44
(1.24–1.66) | <0.001 | 1.14
(1.10–1.18) | <0.001 | 88.2 | <0.001 |
| ≥4 | 5 | 1.56
(1.31–1.85) | <0.001 | 1.55
(1.32–1.81) | <0.001 | 14.3 |
0.323 |
| Type |
|
|
|
|
|
|
|
|
NSCLC | 15 | 1.45
(1.28–1.64) | <0.001 | 1.16
(1.12–1.20) | <0.001 | 85.2 | <0.001 |
|
SCLC | 2 | 1.53
(1.23–1.89) | <0.001 | 1.53
(1.23–1.89) | <0.001 | 0 |
0.792 |
| Method |
|
|
|
|
|
|
|
|
Multivariate | 16 | 1.50
(1.32–1.70) | <0.001 | 1.17
(1.13–1.21) | <0.001 | 85.2 | <0.001 |
|
Univariate | 12 | 1.51
(1.28–1.78) | <0.001 | 1.17
(1.13–1.22) | <0.001 | 87.4 | <0.001 |
|
| B, Progression-free
survival |
|
| Ethnicity |
|
|
|
|
|
|
|
|
Caucasian | 3 | 1.43
(1.14–1.80) |
0.002 | 1.43
(1.14–1.80) |
0.002 | 0 |
0.735 |
|
Asian | 5 | 1.40
(1.07–1.83) |
0.015 | 1.06
(1.01–1.12) |
0.024 | 82.1 | <0.001 |
| Sample size, n |
|
|
|
|
|
|
|
|
<200 | 6 | 1.44
(1.09–1.90) | 0.1 | 1.06
(1.00–1.11) |
0.034 | 77.1 |
0.001 |
|
≥200 | 2 | 1.41
(1.15–1.72) |
0.001 | 1.41
(1.15–1.72) |
0.001 | 0 |
0.683 |
| Cut-off value |
|
|
|
|
|
|
|
|
<4 | 3 | 1.27
(0.93–1.72) |
0.128 | 1.04
(0.99–1.09) |
0.158 | 88.2 | <0.001 |
| ≥4 | 5 | 1.54
(1.13–1.82) | <0.001 | 1.54
(1.30–1.82) | <0.001 | 57.2 |
0.029 |
| Type |
|
|
|
|
|
|
|
|
NSCLC | 6 | 1.38
(1.08–1.75) | 0.1 | 1.06
(1.00–1.11) |
0.4 | 72.9 |
0.002 |
|
SCLC | 2 | 1.54
(1.24–1.92) | <0.001 | 1.54
(1.24–1.92) | <0.001 | 0 |
0.761 |
| Method |
|
|
|
|
|
|
|
|
Multivariate | 6 | 1.38
(1.10–1.74) |
0.027 | 1.07
(1.02–1.13) |
0.008 | 80.5 | <0.001 |
|
Univariate | 5 | 1.35
(1.03–1.75) |
0.002 | 1.05
(1.00–1.11) |
0.046 | 77 |
0.002 |
Heterogeneity
Meta-regression analysis was performed to explore
the potential source(s) of heterogeneity in the associations of NLR
with OS and PFS. The results revealed that heterogeneity in the OS
results was not significantly affected by treatment (P=0.725),
ethnicity (P=0.976), tumor stage (P=0.305), sample size (P=0.156),
cut-off value (P=0.807), or tumor type (P=0.884). The results also
revealed that heterogeneity in the PFS results was not
significantly affected by ethnicity (P=0.696), sample size
(P=0.942), cut-off value (P=0.137), or tumor type (P=0.844).
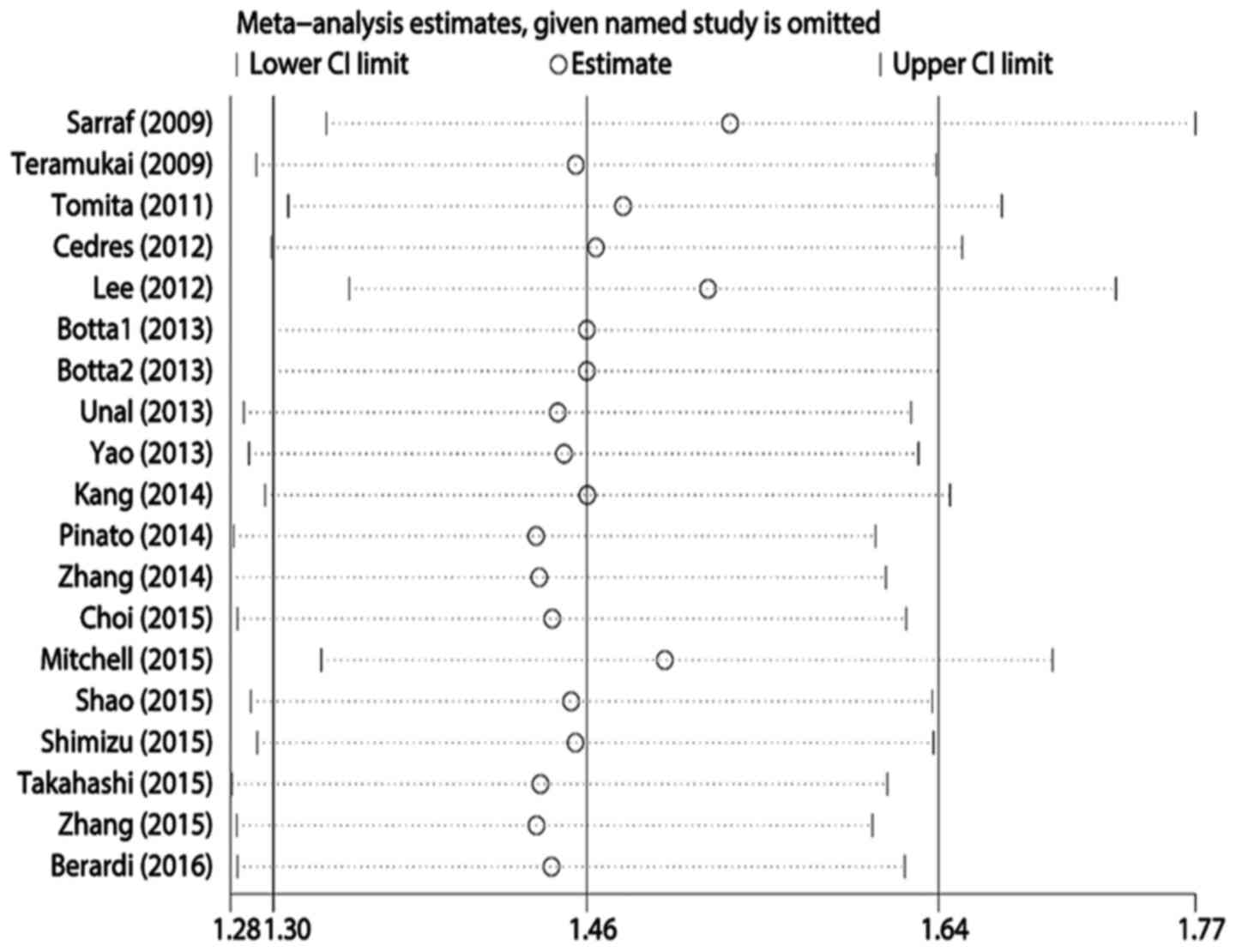
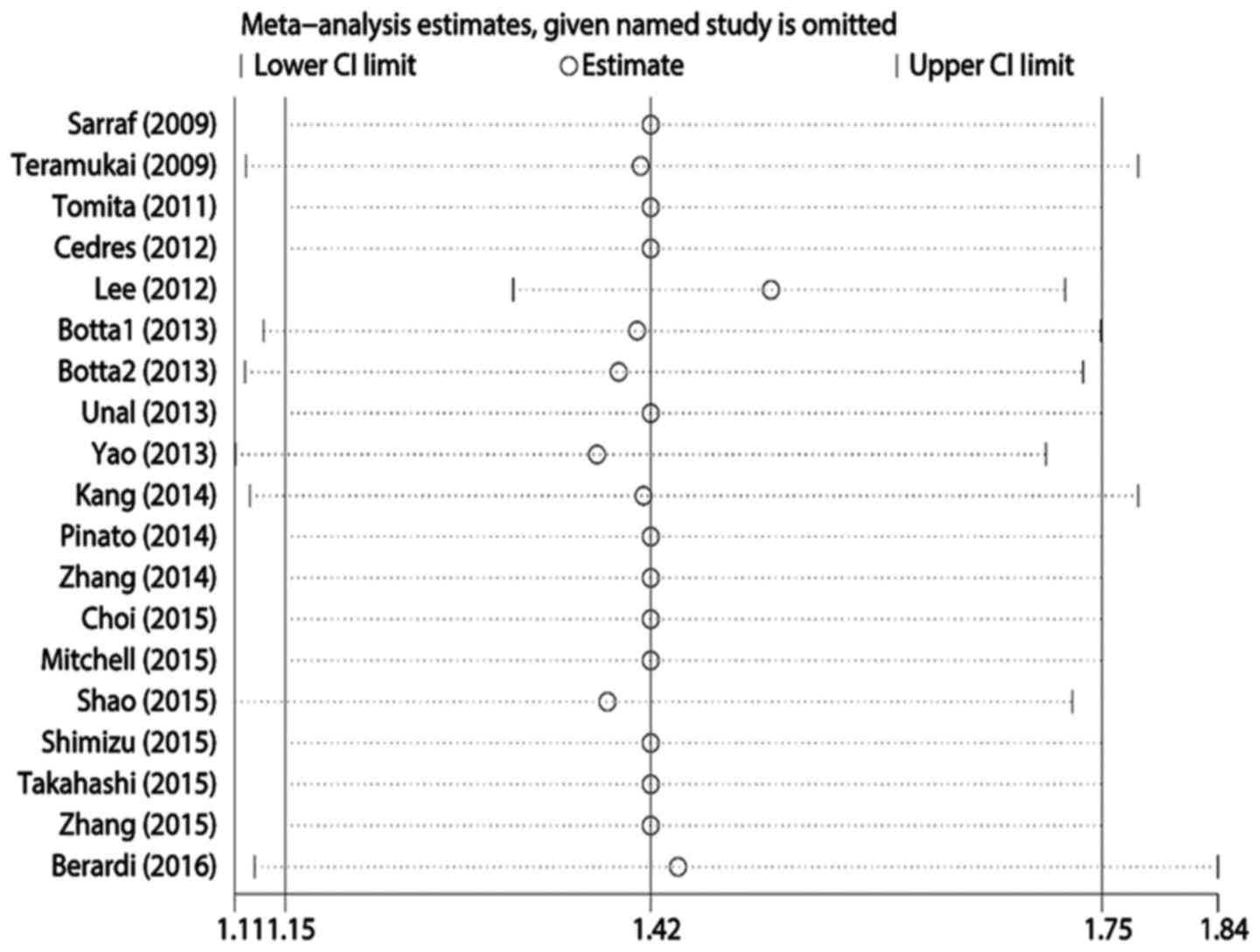
Publication bias and sensitivity
analysis
Egger's test revealed statistically significant
publication bias for both OS and PFS (both P<0.05). A
sensitivity analysis was performed by sequentially removing each
study, and evaluating whether any individual study significantly
affected the results. However, the pooled HRs and 95% CIs revealed
that no single study significantly affected OS or PFS (Figs. 4 and 5).
Discussion
The present meta-analysis evaluated the prognostic
value of elevated pretreatment NLR in 18 studies and 7,219 patients
with lung cancer. To the best of our knowledge, this is the largest
meta-analysis regarding this topic to date. The pooled HRs suggest
that elevated pretreatment NLR was associated with poor OS
(HR=1.46, 95% CI: 1.30–1.64) and poor PFS (HR=1.42, 95% CI:
1.15–1.75). Furthermore, the subgroup analysis revealed that an
elevated pretreatment NLR of ≥4 effectively predicted poor OS and
PFS after treatment for lung cancer, regardless of the analytical
method. One previous study had used an NLR cut-off of 4 (35), and another study used a cut-off of 5
(35). Our results confirmed that an
NLR of 4 is a more stable threshold for predicting prognosis, as
our subgroup analysis with an NLR of ≥4 revealed significantly
lower heterogeneity. We suggest that the setting of the reliable
threshold of the NLR for predicting prognosis of lung cancer may be
very helpful for clinical use. Of note, NLR may also better predict
a poor OS for patients who undergo surgery (HR=1.50, 95% CI:
1.21–1.84) or patients with early-stage tumors (HR=1.64, 95% CI:
1.37–1.97), suggesting that NLR may be used as an independent
prognostic indicator to monitor the postoperative outcome of
patients with early-stage lung cancer. In cases with SCLC, NLR
provided significantly improved prognostic value, without any
heterogeneity in the OS and PFS analysis. The use of NLR may be
promising in evaluating the prognosis of SCLC patients. However,
additional studies are required to validate this association, as
only 2 studies evaluating SCLC cases were identified.
Accumulating evidence suggests that a dysregulated
inflammatory response plays a vital role in cancer (36). Infiltration by immune cells is
increasingly accepted as an important part of the tumor
microenvironment, which may lead to cancer-related inflammation
(37). In this context, NLR has
recently been introduced as a simple index of the systemic
inflammatory response, as inflammation leads to more neutrophils
and fewer lymphocytes in the peripheral blood. Together, these
changes result in an elevated NLR. Neutrophils are the dominant
leukocytes in the blood, and are the first line of defense against
inflammation and infection (38).
Neutrophil infiltration is also observed in a number of tumor
types, and tumor-associated neutrophils in lung cancer are
associated with malignant potential and a poor prognosis (39). According to Proctor et al, NLR
may be a more sensitive composite index, compared with white blood
cell count (40). By contrast,
elevated levels of tumor-infiltrating lymphocytes are considered to
be associated with a better prognosis (41), and decreasing levels of
tumor-infiltrating lymphocytes are associated with a poor prognosis
in lung cancer (42). Thus, the
balance between the conflicting inflammatory responses in tumors is
likely an effective predictor of prognosis (43), and NLR appears to be a superior index
of the balance between the inflammatory response and tumor immune
status, compared with individual neutrophil or lymphocyte
counts.
The present study revealed significant heterogeneity
in the available data, which was not explained by the subgroup
analysis. Thus, we hypothesized that the heterogeneity may be
associated with confounding or unconsidered factors. In this
context, a number of traditional factors may be of value for
predicting prognosis in lung cancer cases, and some of these
factors may exert synergistic effects, particularly factors that
are associated with host status. However, the studies in the
present meta-analysis did not take into consideration factors in
combination with NLR, and there were insufficient data to perform
additional subgroup analysis. Thus, we considered other studies'
results to explore these potential factors and their prognostic
value in lung cancer. For example, young Japanese patients (aged
≤50 years) exhibit better survival after surgery for lung cancer,
compared with older patients, which may be associated with the
significantly better performance status among younger patients
(44). In addition, a study of two
cohorts in Australia and America revealed that male sex was
independently associated with poor prognosis in NSCLC (5). Furthermore, another meta-analysis
suggested that smoking cessation improves prognosis for patients
with early-stage lung cancer, and this result may also be
associated with a poor prognosis among male patients, as they are
relatively heavy smokers (45).
Moreover, smoking may promote the progression of both early- and
late-stage lung cancer through DNA alterations and modified protein
expression (6). According to Kanarek
et al (46), patients may
also achieve a rapid reduction in tumor burden if they have a short
referral interval and pre-surgery delay, which may be associated
with continued smoking. Thus, all these factors may be useful in
predicting the prognosis of lung cancer, with the exception of
histological classification after radiotherapy (47) or chemotherapy (48). In addition, there is a clear inverse
correlation between NLR and nutritional status (31), which indicates that NLR in the
inflammatory response may be a host-related prognostic factor.
Therefore, as NLR may be affected by various factors and their
combinations, large-scale studies are required to elucidate the
mechanism(s) underlying the association between elevated NLR and
prognosis after treatment for lung cancer.
Several recent meta-analyses have used NLR as an
important prognostic factor for various cancer types with different
but similar cut-off values, including esophageal cancer (2–5)
(49), breast cancer (3) (50),
gastric cancer (3) (51), hepatocellular carcinoma (3–4)
(52), pancreatic cancer (2.3–5)
(53), colorectal cancer (5) (54),
renal cell carcinoma (3) (55) and prostate cancer (3) (56). All
these studies have reported that NLR may be a promising prognostic
factor for that specific cancer. Moreover, NLR may be associated
with diseases other than cancer, such as diabetes mellitus and
cardiovascular disease (57). Thus,
NLR is likely of value for predicting prognosis in a wide range of
inflammation-associated diseases.
There are certain limitations regarding the present
meta-analysis that should be addressed. First, only 4 prospective
studies were identified, whereas 13 studies (14 cohorts) used a
retrospective design, which increases the risk of bias. Second,
substantial heterogeneity was observed in the various studies,
which was associated with various confounding factors, such as
ethnicity, sex, treatment method, follow-up period, age
distribution, and NLR cut-off value. However, this significant
heterogeneity was not attributable to a single factor in the
subgroup analysis, meta-regression, and sensitivity analysis,
suggesting that the heterogeneity may be associated with the
inter-related factors that were discussed in the previous
paragraphs. Third, only 2 studies on SCLC were identified, and
included a limited amount of data for the PFS-related analysis,
which increases the risk of bias in our findings. Fourth, the
association between the NLR and clinicopathological parameters
(e.g., lymph node metastasis) or pathological patterns was not
analyzed. Fifth, only 3 studies evaluated NLR using multivariate
analysis, and the remaining studies either performed univariate
analysis alone or a combination of multivariate and univariate
analysis. Sixth, significant publication bias was identified, which
was likely associated with the language restriction and the
increased likelihood that reports with positive results would be
published.
In conclusion, the present meta-analysis
demonstrated that elevated pretreatment NLR was associated with
prognosis among patients with lung cancer. Thus, NLR may be an
easily accessible and effective prognostic biomarker in lung
cancer, as it may be evaluated during routine blood tests. However,
the specific mechanism underlying its prognostic value remains
unclear, as significant heterogeneity was observed in the present
meta-analysis. Therefore, additional well-designed large-scale
studies are required to clearly determine the prognostic role of
NLR in lung cancer.
Acknowledgements
The present study was supported by the Jilin
Provincial Science and Technology Department (grant no. 20150101176
to J.C.), the National Health and Family Planning Commission of the
People's Republic of China (grant no. ZX-07-C2016004), and the
National Key Research and Development Program of China (grant no.
2016YFC1303800).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: Epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tas F, Ciftci R, Kilic L and Karabulut S:
Age is a prognostic factor affecting survival in lung cancer
patients. Oncol Lett. 6:1507–1513. 2013.PubMed/NCBI
|
|
5
|
Wainer Z, Wright GM, Gough K, Daniels MG,
Choong P, Conron M, Russell PA, Alam NZ, Ball D and Solomon B:
Impact of sex on prognostic host factors in surgical patients with
lung cancer. ANZ J Surg. doi: 10.1111/ans.13728.
|
|
6
|
Yoshino I and Maehara Y: Impact of smoking
status on the biological behavior of lung cancer. Surg Today.
37:725–734. 2007. View Article : Google Scholar
|
|
7
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. 7th edition.
Wiley-Blackwell; 2009
|
|
8
|
Greenberg AK and Lee MS: Biomarkers for
lung cancer: Clinical uses. Curr Opin Pulm Med. 13:249–255. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoon HM, Ryu KW, Nam BH, Cho SJ, Park SR,
Lee JY, Lee JH, Kook MC, Choi IJ and Kim YW: Is the new seventh
AJCC/UICC staging system appropriate for patients with gastric
cancer? J Am Co Surg. 214:88–96. 2012. View Article : Google Scholar
|
|
10
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar
|
|
13
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar R, Geuna E, Michalarea V,
Guardascione M, Naumann U, Lorente D, Kaye SB and de Bono JS: The
neutrophil-lymphocyte ratio and its utilisation for the management
of cancer patients in early clinical trials. Br J Cancer.
112:1157–1165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guthrie GJ, Charles KA, Roxburgh CS,
Horgan PG, McMillan DC and Clarke SJ: The systemic
inflammation-based neutrophil-lymphocyte ratio: Experience in
patients with cancer. Crit Rev Oncol Hematol. 88:218–230. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sarraf KM, Belcher E, Raevsky E, Nicholson
AG, Goldstraw P and Lim E: Neutrophil/lymphocyte ratio and its
association with survival after complete resection in non-small
cell lung cancer. J Thorac Cardiovasc Surg. 137:425–428. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teramukai S, Kitano T, Kishida Y, Kawahara
M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, et al:
Pretreatment neutrophil count as an independent prognostic factor
in advanced non-small-cell lung cancer: An analysis of Japan
Multinational Trial Organisation LC00-03. Eur J Cancer.
45:1950–1958. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomita M, Shimizu T, Ayabe T, Yonei A and
Onitsuka T: Preoperative neutrophil to lymphocyte ratio as a
prognostic predictor after curative resection for non-small cell
lung cancer. Anticancer Res. 31:2995–2998. 2011.PubMed/NCBI
|
|
20
|
Cedrés S, Torrejon D, Martinez A, Martinez
P, Navarro A, Zamora E, Mulet-Margalef N and Felip E: Neutrophil to
lymphocyte ratio (NLR) as an indicator of poor prognosis in stage
IV non-small cell lung cancer. Clin Transl Oncol. 14:864–869. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee Y, Kim SH, Han JY, Kim HT, Yun T and
Lee JS: Early neutrophil-to-lymphocyte ratio reduction as a
surrogate marker of prognosis in never smokers with advanced lung
adenocarcinoma receiving gefitinib or standard chemotherapy as
first-line therapy. J Cancer Res Clin Oncol. 138:2009–2016. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Botta C, Barbieri V, Ciliberto D, Rossi A,
Rocco D, Addeo R, Staropoli N, Pastina P, Marvaso G, Martellucci I,
et al: Systemic inflammatory status at baseline predicts
bevacizumab benefit in advanced non-small cell lung cancer
patients. Cancer Bio Ther. 14:469–475. 2013. View Article : Google Scholar
|
|
23
|
Unal D, Eroglu C, Kurtul N, Oguz A and
Tasdemir A: Are neutrophil/lymphocyte and platelet/lymphocyte rates
in patients with non-small cell lung cancer associated with
treatment response and prognosis? Asian Pac J Cancer Prev.
14:5237–5242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao Y, Yuan D, Liu H, Gu X and Song Y:
Pretreatment neutrophil to lymphocyte ratio is associated with
response to therapy and prognosis of advanced non-small cell lung
cancer patients treated with first-line platinum-based
chemotherapy. Cancer Immunol Immunother. 62:471–479. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang MH, Go SI, Song HN, Lee A, Kim SH,
Kang JH, Jeong BK, Kang KM, Ling H and Lee GW: The prognostic
impact of the neutrophil-to-lymphocyte ratio in patients with
small-cell lung cancer. Br J Cancer. 111:452–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pinato DJ, Shiner RJ, Seckl MJ, Stebbing
J, Sharma R and Mauri FA: Prognostic performance of
inflammation-based prognostic indices in primary operable non-small
cell lung cancer. Br J Cancer. 110:1930–1935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang T, Jiang Y, Qu X, Shen H, Liu Q and
Du J: Evaluation of preoperative hematologic markers as prognostic
factors and establishment of novel risk stratification in resected
pN0 non-small-cell lung cancer. PLoS One. 9:e1114942014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi JE, Villarreal J, Lasala J,
Gottumukkala V, Mehran RJ, Rice D, Yu J, Feng L and Cata JP:
Perioperative neutrophil: Lymphocyte ratio and postoperative NSAID
use as predictors of survival after lung cancer surgery: A
retrospective study. Cancer Med. 4:825–833. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mitchell PL, Thatcher N, Socinski MA,
Wasilewska-Tesluk E, Horwood K, Szczesna A, Martín C, Ragulin Y,
Zukin M, Helwig C, et al: Tecemotide in unresectable stage III
non-small-cell lung cancer in the phase III START study: Updated
overall survival and biomarker analyses. Ann Oncol. 26:1134–1142.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shao N and Cai Q: High pretreatment
neutrophil-lymphocyte ratio predicts recurrence and poor prognosis
for combined small cell lung cancer. Clin Transl Oncol. 17:772–778.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shimizu K, Okita R, Saisho S, Maeda A,
Nojima Y and Nakata M: Preoperative neutrophil/lymphocyte ratio and
prognostic nutritional index predict survival in patients with
non-small cell lung cancer. World J Surg Oncol. 13:2912015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takahashi Y, Horio H, Hato T, Harada M,
Matsutani N, Morita S and Kawamura M: Prognostic significance of
preoperative Neutrophil-Lymphocyte ratios in patients with Stage I
non-small cell lung cancer after complete resection. Ann Surg
Oncol. 22 Suppl 3:S1324–S1331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang H, Zhang L, Zhu K, Shi B, Yin Y, Zhu
J, Yue D, Zhang B and Wang C: Prognostic significance of
combination of preoperative platelet count and
Neutrophil-Lymphocyte ratio (COP-NLR) in patients with non-small
cell lung cancer: Based on a large cohort study. PLoS One.
10:e01264962015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Berardi R, Rinaldi S, Santoni M,
Newsom-Davis T, Tiberi M, Morgese F, Caramanti M, Savini A, Ferrini
C, Torniai M, et al: Prognostic models to predict survival in
patients with advanced non-small cell lung cancer treated with
first-line chemo- or targeted therapy. Oncotarget. 7:26916–26924.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin Y, Wang J, Wang X, Gu L, Pei H, Kuai
S, Zhang Y and Shang Z: Prognostic value of the neutrophil to
lymphocyte ratio in lung cancer: A meta-analysis. Clinics (Sao
Paulo). 70:524–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sethi G, Shanmugam MK, Ramachandran L,
Kumar AP and Tergaonkar V: Multifaceted link between cancer and
inflammation. Biosci Rep. 32:1–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Candido J and Hagemann T: Cancer-related
inflammation. J Clin Immunol. 33 Suppl 1:S79–S84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kumar V and Sharma A: Neutrophils:
Cinderella of innate immune system. Int Immunopharmacol.
10:1325–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Borregaard N: Neutrophils, from marrow to
microbes. Immunity. 33:657–670. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, Fletcher CD, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC:
A comparison of inflammation-based prognostic scores in patients
with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer.
47:2633–2641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu P and Fu YX: Tumor-infiltrating T
lymphocytes: Friends or foes? Lab Invest. 86:231–245. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kinoshita T, Muramatsu R, Fujita T, Nagumo
H, Sakurai T, Noji S, Takahata E, Yaguchi T, Tsukamoto N,
Kudo-Saito C, et al: Prognostic value of tumor-infiltrating
lymphocytes differs depending on histological type and smoking
habit in completely resected non-small-cell lung cancer. Ann Oncol.
27:2117–2123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Inoue M, Okumura M, Sawabata N, Miyaoka E,
Asamura H, Yoshino I, Tada H, Fujii Y, Nakanishi Y, Eguchi K, et
al: Clinicopathological characteristics and surgical results of
lung cancer patients aged up to 50 years: The Japanese lung cancer
registry study 2004. Lung Cancer. 83:246–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Parsons A, Daley A, Begh R and Aveyard P:
Influence of smoking cessation after diagnosis of early stage lung
cancer on prognosis: Systematic review of observational studies
with meta-analysis. BMJ. 340:b55692010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kanarek NF, Hooker CM, Mathieu L, Tsai HL,
Rudin CM, Herman JG and Brock MV: Survival after community
diagnosis of early-stage non-small cell lung cancer. Am J Med.
127:443–449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mak RH, Hermann G, Lewis JH, Baldini EH,
Chen AB, Colson YL, Hacker FH, Kozono D, Wee JO, Chen YH, et al:
Outcomes by tumor histology and KRAS mutation status after lung
stereotactic body radiation therapy for early-stage non-small-cell
lung cancer. Clin Lung Cancer. 16:24–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Trani L, Myerson J, Ashley S, Young K,
Sheri A, Hubner R, Puglisi M, Popat S and O'Brien ME: Histology
classification is not a predictor of clinical outcomes in advanced
non-small cell lung cancer (NSCLC) treated with vinorelbine or
gemcitabine combinations. Lung Cancer. 70:200–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yodying H, Matsuda A, Miyashita M,
Matsumoto S, Sakurazawa N, Yamada M and Uchida E: Prognostic
significance of Neutrophil-to-Lymphocyte ratio and
platelet-to-Lymphocyte ratio in oncologic outcomes of esophageal
cancer: A systematic review and meta-analysis. Ann Surg Oncol.
23:646–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wei B, Yao M, Xing C, Wang W, Yao J, Hong
Y, Liu Y and Fu P: The neutrophil lymphocyte ratio is associated
with breast cancer prognosis: An updated systematic review and
meta-analysis. Onco Targets Ther. 9:5567–5575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sun J, Chen X, Gao P, Song Y, Huang X,
Yang Y, Zhao J, Ma B, Gao X and Wang Z: Can the neutrophil to
lymphocyte ratio be used to determine gastric cancer treatment
outcomes? A systematic review and meta-analysis. Dis Markers.
2016:78624692016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun XD, Shi XJ, Chen YG, Wang CL, Ma Q and
Lv GY: Elevated preoperative Neutrophil-Lymphocyte ratio is
associated with poor prognosis in hepatocellular carcinoma patients
treated with liver transplantation: A meta-analysis. Gastroenterol
Res Pract. 2016:47438082016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang JJ, Hu ZG, Shi WX, Deng T, He SQ and
Yuan SG: Prognostic significance of neutrophil to lymphocyte ratio
in pancreatic cancer: A meta-analysis. World J Gastroenterol.
21:2807–2815. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tsai PL, Su WJ, Leung WH, Lai CT and Liu
CK: Neutrophil-lymphocyte ratio and CEA level as prognostic and
predictive factors in colorectal cancer: A systematic review and
meta-analysis. J Cancer Res Ther. 12:582–589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hu K, Lou L, Ye J and Zhang S: Prognostic
role of the neutrophil-lymphocyte ratio in renal cell carcinoma: A
meta-analysis. BMJ Open. 5:e0064042015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tang L, Li X, Wang B, Luo G, Gu L, Chen L,
Liu K, Gao Y and Zhang X: Prognostic value of
Neutrophil-to-Lymphocyte ratio in localized and advanced prostate
cancer: A systematic review and meta-analysis. PLoS One.
11:e01539812016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Davis JL, Moutinho V Jr, Panageas KS and
Coit DG: A peripheral blood biomarker estimates probability of
survival: The neutrophil-lymphocyte ratio in noncancer patients.
Biomark Med. 10:953–957. 2016. View Article : Google Scholar : PubMed/NCBI
|