Introduction
Anastomosing haemangioma (AH) is a subtype of
capillary haemangioma that is rarely encountered in clinical
practice, particularly in the liver. AH is a recently described and
unusual variant of capillary hemangioma that appears to be unique
to the genitourinary system, with a particular proclivity for the
kidney. Previous studies provide strong evidence supporting the
benign behavior of AH, whereas others suggest that less aggressive
treatment may be preferable. Thus, the minimally invasive strategy,
which is diagnostic biopsy and no treatment, should be weighed
against the potential of significant tissue damage due to the
growing lesion over time (1,2). We herein present a rare case of
pathologically confirmed AH in the liver in a 57-year-old female
patient.
Case report
A 57-year-old Chinese woman with no significant
medical history was incidentally found to have a hepatic lesion on
ultrasound imaging during a routine health check-up on September
20, 2014. A mass was identified on color Doppler imaging, sized
~3.3×2.8 cm. The possibility of malignancy was high, and metastasis
was highly unlikely. The lesion was further evaluated with an
abdominal contrast-enhanced magnetic resonance imaging (MRI) scan.
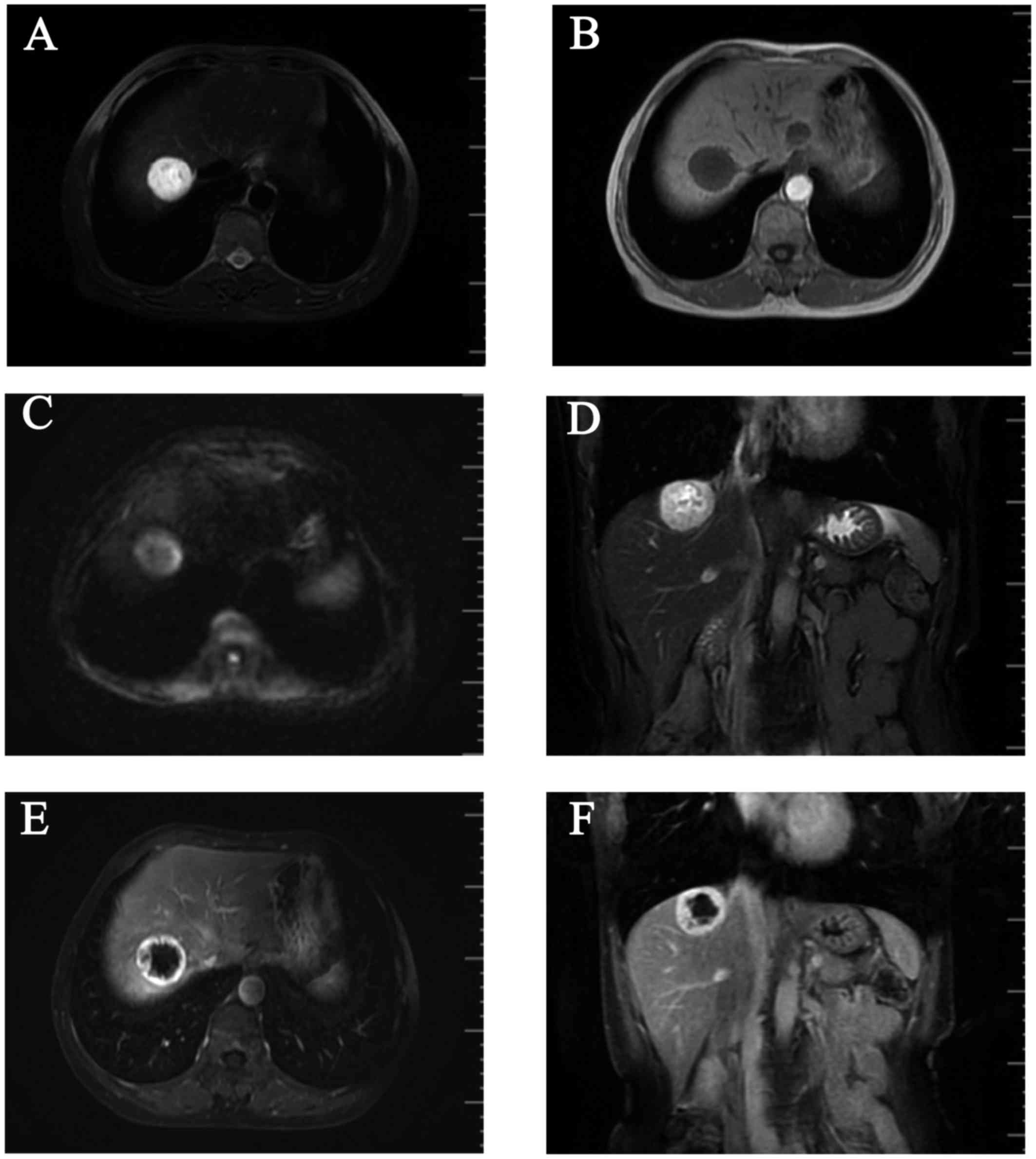
T1-weighted images (WI) on MRI revealed a round, well
circumscribed, homogeneously hypointense mass, sized ~3.3×3.0 cm,
in the right lobe of the liver (Fig.
1A), which was hyperintense on T2WI (Fig. 1B and D) and diffusion WI (DWI;
Fig. 1C). The lesion exhibited a
strong contrast enhancement in the periphery during the early phase
(Fig. 1E) and homogeneously
persistent enhancement with a well-circumscribed boundary during
the late phase (Fig. 1F). A positron
emission tomography-computed tomography (PET-CT) scan revealed a
low-density mass in the right hepatic lobe, with lower
glycometabolism compared with the normal liver. The patient
consented to receiving a hepatectomy in December 5, 2014, and she
remained alive and disease-free during the 12-month postoperative
follow-up (last follow-up, December 30, 2015). The patient
consented to the publication of the case details.
The tumour was located in the VIII segment of the
liver in close proximity to the right hepatic vein. There were no
lymph node metastases and no tumour thrombi were identified in the
liver. On macroscopic inspection, the resected specimen was a
well-circumscribed, homogeneous, encapsulated nodular mass, sized
3.5×3×3 cm and the cut surface of the tumour was fleshy and
red-gray. On microscopic examination, various numbers of blood
cells were observed in the anastomosing vessels and differently
sized vascular compartments. The immunohistochemical staining for
CD31 was positive. The histopathological appearance together with
the immunophenotypic characteristics of this tumour were indicative
of AH of the liver.
Discussion
Hemangiomas are more commonly located in the skin
and subcutaneous tissues. Visceral hemangiomas are generally not
that common and occur mostly in the liver. Histologically,
hemangiomas have been broadly classified as cavernous and capillary
(3,4). Hepatic hemangioma (HH) is the most
common benign tumour of the liver during infancy (5). On the basis of its distribution in the
liver, hemangiomas may be classified as focal, multifocal, or
diffuse (6,7). The majority of hepatic hemangiomas are
of the cavernous type, followed by the capillary type. AH is a rare
subtype of hemangioma. AHs are distinguished from their cavernous
counterparts by the anastomosing sinusoidal-like pattern of tightly
packed capillary channels. Montgomery and Epstein described 6 cases
of new variants of benign vascular tumours involving the kidneys,
perinephric adipose tissue and testes, which the authors classified
as AHs in 2009 (6). Subsequently,
more cases of this vascular tumour have been reported in the
adrenal gland (7), breast (8), liver and gastrointestinal tract
(9). The mean size of this tumour in
the liver is reportedly ~3.35 cm, which is larger compared with
that in other organs (10). The
incidence in male patients suggests a slight male predilection,
with a reported male:female ratio of 1.8:1 (3). The association between end-stage renal
disease and malignant renal epithelial neoplasms is well-documented
(11).
Radiologically, as the number of reported cases of
AH of the liver is limited, imaging information on the
characteristics of hepatic AHs is also limited. Tao et al
reported that the unenhanced axial CT scan showed a mass with a
round, well-circumscribed outline, appearing to be heterogeneous.
The boundary of the lesion exhibited an obviously annular and
nodular enhancement in the arterial phase of the contrast-enhanced
CT scan. In the venous phase, the lesion exhibited further intense
enhancement. The lesion displayed homogeneously persistent
enhancement and a well-circumscribed boundary on delayed images
(10). A contrast-enhanced CT of the
abdomen and pelvis confirmed a suspicious 3.4-cm renal mass,
located between the left and middle part of the liver. A
contrast-enhanced MRI scan revealed a round, well-demarcated mass
in the upper pole of the right kidney, homogeneously hyperintense,
measuring 2.3×2.1 cm (12).
In the present case, a contrast-enhanced abdominal
MRI scan revealed a round, well-circumscribed mass sized ~3.3×3.0
cm in the right hepatic lobe, homogeneously hypointense on T1WI and
hyperintense on T2WI and DWI. The lesion exhibited a strong
contrast enhancement in the periphery during the early phase, with
homogeneously persistent enhancement and a well-circumscribed
boundary during the late phase.
Macroscopically, AHs are reportedly 0.1-6 cm in
diameter and are well-demarcated, always unencapsulated, with a
mahogany brown spongy appearance, without grossly evident necrosis
or vascular invasion (3,4,8,9,13,14). On
pathological examination, the characteristic cytoarchitectural
features of AH include numerous thin-walled vascular channels
exhibiting a complex anastomosing growth pattern, but no evident
endothelial atypia or multilayering (14). Immunohistochemical studies
demonstrated that the tumour cells were diffusely positive for CD34
and CD31, the stromal cells were positive for smooth muscle actin,
and the Ki-67 indicates a low proliferative activity of the tumour
cells (15). In the present case,
the tumour was macroscopically fleshy and red-gray. The tumour
cells were abundant and formed anastomosing vascular channels. The
immunohistochemical staining for CD31 was positive.
The differential diagnosis of AH includes
hepatocellular carcinoma (HCC), primary hepatic angiosarcoma (PHA)
and hepatic angiomyolipoma (AML). Generally, HCC patients often
have a history of chronic liver disease or cirrhosis and high serum
α-fetoprotein (AFP) levels. The lesion exhibited strong contrast
enhancement in the early phase and weakened enhancement in the late
phase. Angiosarcoma, a subtype of soft tissue sarcoma, is an
aggressive malignant disease derived from the endothelium of
lymphatics or blood vessels (16).
The typical imaging manifestation of PHA is moderate to marked
enhancement at the peripheral and central area of the tumour during
the arterial phase, which persists during the portal and delayed
phases; in addition, in the majority of the cases, the central area
of the lesion cannot be completely filled with the contrast agent.
PHA is often accompanied by bleeding and is enclosed in a capsule.
The majority of the PHA cases are accompanied by obvious clinical
symptoms and compromised liver function. AML is a unique
mesenchymal neoplasm consisting of blood vessels, smooth muscle and
adipose cells (17). When the liver
lesions exhibit early enhancement, peripheral enhancement, no
capsule, the AFP levels are normal and there is no cirrhosis, the
diagnosis of hepatic AH should be considered (18).
References
|
1
|
Brown JG, Folpe AL, Rao P, Lazar AJ, Paner
GP, Gupta R, Parakh R, Cheville JC and Amin MB: Primary vascular
tumors and tumor-like lesions of the kidney: A clinicopathologic
analysis of 25 cases. Am J Surg Pathol. 34:942–949. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wetherell DR, Skene A, Manya K, Manecksha
RP, Chan Y and Bolton DM: Anastomosing haemangioma of the kidney: A
rare morphological variant of haemangioma characteristic of
genitourinary tract location. Pathology. 45:193–196. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyers RL: Tumors of the liver in
children. Surg Oncol. 16:195–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Christison-Lagay ER, Burrows PE, Alomari
A, Dubois J, Kozakewich HP, Lane TS, Paltiel HJ, Klement G,
Mulliken JB and Fishman SJ: Hepatic hemangiomas: Subtype
classification and development of a clinical practice algorithm and
registry. J Pediatr Surg. 42:62–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kulungowski AM, Alomari AI, Chawla A,
Christison-Lagay ER and Fishman SJ: Lessons from a liver hemangioma
registry: Subtype classification. J Pediatr Surg. 47:165–170. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Montgomery E and Epstein JI: Anastomosing
hemangioma of the genitourinary tract: A lesion mimicking
angiosarcoma. Am J Surg Pathol. 33:1364–1369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ross M, Polcari A, Picken M, Sankary H and
Milner J: Anastomosing hemangioma arising from the adrenal gland.
Urology. 80:e27–e28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brehm B, Rauh C, Dankerl P and
Schulz-Wendtland R: Anastomosing hemangioma in the male breast-a
rarity). Rofo. 186:80–81. 2014.PubMed/NCBI
|
|
9
|
Lin J, Bigge J, Ulbright TM and Montgomery
E: Anastomosing hemangioma of the liver and gastrointestinal tract:
An unusual variant histologically mimicking angiosarcoma. Am J Surg
Pathol. 37:1761–1765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tao LL, Dai Y, Yin W and Chen J: A case
report of a renal anastomosing hemangioma and a literature review:
An unusual variant histologically mimicking angiosarcoma. Diagn
Pathol. 9:1592014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kryvenko ON, Haley SL, Smith SC, Shen SS,
Paluru S, Gupta NS, Jorda M, Epstein JI, Amin MB and Truong LD:
Haemangiomas in kidneys with end-stage renal disease: A novel
clinicopathological association. Histopathology. 65:309–318. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao M, Li C, Zheng J and Sun K:
Anastomosing hemangioma of the kidney: A case report of a rare
subtype of hemangioma mimicking angiosarcoma and review of the
literature. Int J Clin Exp Pathol. 6:757–765. 2013.PubMed/NCBI
|
|
13
|
Kryvenko ON, Gupta NS, Meier FA, Lee MW
and Epstein JI: Anastomosing hemangioma of the genitourinary
system: Eight cases in the kidney and ovary with
immunohistochemical and ultrastructural analysis. Am J Clin Pathol.
136:450–457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mehta V, Ananthanarayanan V, Antic T,
Krausz T, Milner J, Venkataraman G and Picken MM: Primary benign
vascular tumors and tumorlike lesions of the kidney: A
clinicopathologic analysis of 15 cases. Virchows Arch. 461:669–676.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Omiyale AO: Anastomosing hemangioma of the
kidney: A literature review of a rare morphological variant of
hemangioma. Ann Transl Med. 3:1512015.PubMed/NCBI
|
|
16
|
Heidegger I, Pichler R, Schäfer G, Zelger
B, Zelger B, Aigner F, Bektic J and Horninger W: Long-term follow
up of renal anastomosing hemangioma mimicking renal angiosarcoma.
Int J Urol. 21:836–838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai PQ, Wu YP, Xie CM, Zhang WD, Han R and
Wu PH: Hepatic angiomyolipoma: CT and MR imaging findings with
clinical-pathologic comparison. Abdom Imaging. 38:482–489. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li T, Wang L, Yu HH, Sun HC, Qin LX, Ye
QH, Fan J and Tang ZY: Hepatic angiomyolipoma: A retrospective
study of 25 cases. Surg Today. 38:529–535. 2008. View Article : Google Scholar : PubMed/NCBI
|