Introduction
Bevacizumab, an anti-vascular endothelial growth
factor-A (VEGF-A) antibody, is one of the most commonly available
reagents for molecular-targeted therapy (1). It was reported that the therapeutic
mechanisms of bevacizumab are fundamentally based on its inhibitory
effects on neovascularization. In several fields, this drug is used
for less-invasive therapy against malignant diseases, expecting to
reduce the blood supply of cancer tissues without reducing the drug
delivery efficiency of the concurrently administered anticancer
drugs. Recently, bevacizumab was also applied for
platinum-resistant recurrent ovarian cancers in combination with
second-line anticancer drugs. According to the AURELIA (Avastin Use
in Platinum-resistant Epithelial Ovarian Cancer) open-label
randomized phase III trial, progression-free survival was extended
2-fold compared with anticancer drug use alone (2,3).
Although bevacizumab does not induce severe acute
side effects (4), its usage with
anticancer drugs is associated with non-negligible pathological
conditions such as gastrointestinal perforation (5). Recently, we encountered a case of
intestinal perforation at the ileum after the fifth course of
second-line chemotherapy using nogitecan and bevacizumab for
platinum-resistant recurrent ovarian cancer (2,3). The
perforated sites were surrounded by broad and longitudinal ulcer
lesions without apparent features showing direct invasion or
apoptosis of cancer cells beneath the mucosal layer. Microscopic
examination of the lesion sites around perforation revealed diffuse
invasion of VEGF-C-positive cancer cells in the subserous regions
along with marked edema and an increase of podoplanin-positive
cells. Since bevacizumab can inhibit VEGF-A, but not VEGF-C, and
could induce compensatory increase in VEGF-C production (6), the findings suggested that the
subserous involvement of VEGF-C-producing ovarian cancer cells is a
new risk factor for the development of intestinal ulceration and
subsequent perforation during combined therapy with an anticancer
drug and bevacizumab. Consequently, we provide precise clinical and
pathological information concerning this case.
Materials and methods
Tissue samples and patients
The surgical specimens were fixed with 20% formalin
and embedded in paraffin. The tissue sections (4 µm) were stained
by routine histopathological techniques for diagnosis.
Representative tissue sections of each specimen were subjected to
immunohistochemical examination in this study. Informed consent for
the use of these tissues in this study was obtained from the
patient.
Immunohistochemistry
Tissue localization of VEGF-C, podoplanin, and CD34
was immunohistochemically determined by the
avidin-biotin-peroxidase complex (VECTASTAIN ABC Kit, Vector
Laboratories, Burlingame, CA, USA) method according to the
manufacturer's protocol. Briefly, the sections of representative
blocks were deparaffinized in xylene, rehydrated in ethanol, and
antigen retrieval was subsequently performed in 0.01 M citrate
buffer, pH 6.0. The slides were immersed in 3% hydrogen peroxide
for 10 min to block endogenous peroxidase activity and then washed
in 0.05 mol/l phosphate-buffered saline (PBS), pH 7.4. The slides
were incubated with primary antibody, anti-human VEGF-C polyclonal
goat antibody (R&D Systems, MN, USA) at a concentration of 5
µg/ml, anti-human podoplanin monoclonal mouse antibody, clone D2-40
(Dako, Tokyo, Japan) at a concentration of 5 µg/ml, or anti-human
CD34 monoclonal mouse antibody, class II (Dako, Tokyo, Japan) at a
concentration of 5 µg/ml overnight at 4°C in a humidified chamber,
respectively. Control staining was obtained by replacing the
primary antibody by normal serum solution. After washing, the
sections were incubated for 30 min with biotin-labeled rabbit
anti-goat IgG for VEGF-C or horse anti-mouse IgG for podoplanin and
CD34 at room temperature, respectively. Then, they were treated
with the avidin-biotin complex at room temperature. Sites of
peroxidase activity were visualized with diaminobenzidine (Liquid
DAB+Substrate Chromogen System, Dako, Carpinteria, CA, USA), and
the sections were counterstained with hematoxylin.
Case report
A 65-year-old woman (2 gravida, 2 para) visited
Kanazawa University Hospital with a clinical diagnosis of ovarian
cancer. After 3 cycles of neoadjuvant chemotherapy involving DC
therapy (docetaxel and carboplatin), the patient underwent
hysterectomy with bilateral salpingo-oophorectomy, omentectomy,
appendectomy, and reduction surgery of the peritoneally implanted
cancer masses. The pathological diagnosis was serous
adenocarcinoma. Six additional courses of chemotherapy with TC
therapy (paclitaxel and carboplatin) were administered. During the
clinical course, serum CA125 was reduced from 12, 770 to <35
IU/ml. However, 5 months later, intra-abdominal recurrence occurred
with the elevation of serum CA125 to 1,334 IU/ml. Accordingly,
second-line chemotherapy using nogitecan was performed in
combination with bevacizumab. Although serum CA125 was successfully
reduced within 2 courses, its serum value was maintained at
approximately 100 IU/ml without being reduced to the normal range
during this sequential regimen.
After the fifth course, the patient presented with
subileus and a mild fever. Several peritoneal recurrent masses were
detected by computed tomography (CT). The administration of
bevacizumab was immediately discontinued and supportive care,
including administration of antibiotics, was started. However, 17
days later, intestinal perforation occurred with acute abdominal
pain. An emergent operation demonstrated the presence of two nearby
bowel perforations in the ileum concomitant with diffuse peritoneal
recurrent lesions in the abdominal cavity. At the distal site of
perforations in the ileum, severe stenosis due to peritoneal
adhesion by cancer dissemination was observed. Partial small bowel
resection and ileostomy were performed. In the resected ileum,
longitudinal and segmental ulcerative lesions were observed in the
luminal face, and two perforation sites were located in the distal
area of the ulcerative lesions (Fig.
1A). Later, pathological examination revealed that there were a
number of cancer cell invasions in the subserous regions, which
were located on the reverse side of the ulcerative lesions at the
inner lumen site (Fig. 1B-D).
Although there were marked numbers of metastatic foci in the
intramesenteric regions, microscopic examinations could hardly
detect the metastatic lesions on the serous surface in the resected
ileum. On the other hand, cancer foci were abundant in the
subserous regions (Fig. 1B-D).
Notably, the expression of immunoreactive VEGF-C was observed on
the invading cancer cells in the subserous regions (Fig. 2A-B). In accordance with these
findings, marked edematous changes and an increase of
podoplanin-positive cells were frequently observed in the subserous
areas between the serous surface and muscle layer (Fig. 2C-D). However, the post-operative
control of intra-abdominal infection and intestinal ulceration was
difficult, and the patient died 2 months after the perforation.
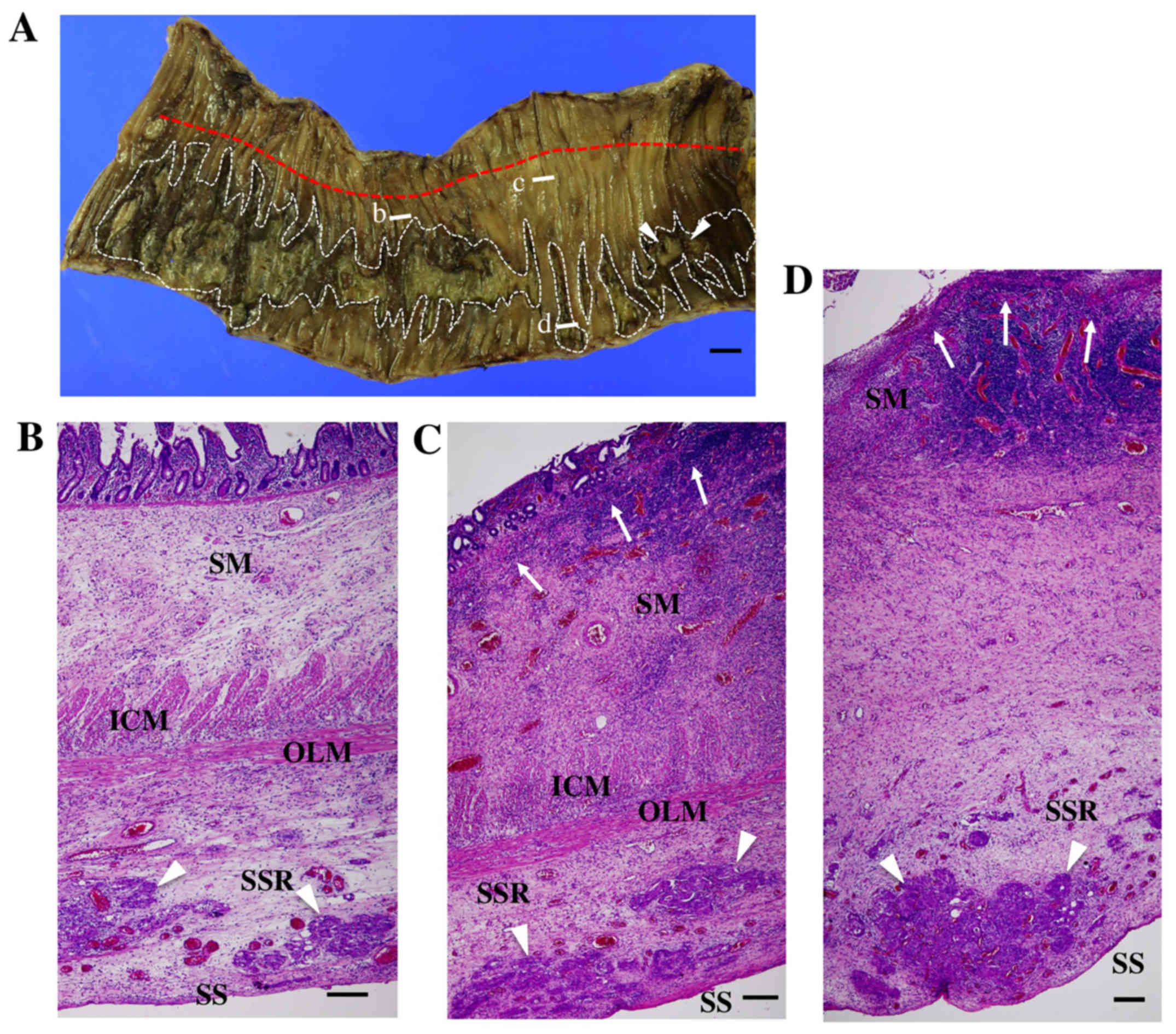 | Figure 1.Macroscopic and microscopic images of
the resected ileum. (A) a macroscopic image of the resected ileum
from the luminal aspect. Longitudinal and segmental ulcerative
lesions were observed in the luminal face (white dotted lines). Two
sites of perforation were detected in the distal region (white
arrowheads). The attachment line of the mesenterium at the opposite
site is shown by a red dotted line. White bars b, c, and d show the
sites of the following microscopic images of B-D, respectively.
(B-D) microscopic images of the resected ileum. (B) a
non-ulcerative lesion. Cancer cell invasion (white arrowheads) with
stromal edema was observed in the subserous region (SSR). Although
the submucous region (SM) was also edematous, the structures of the
mucosa, mucosal muscle, inner circular (ICM), and outer
longitudinal muscle layer (OLM) were maintained. C, an erosive
lesion progressing toward ulceration. Damage of the mucosal muscles
was observed (white arrows). (D) an ulcerative lesion. The mucosal
muscles were directly exposed to the luminal cavity (white arrows),
and the structures of the inner circular and outer longitudinal
muscle layer became obscure. On the other hand, the subserous
lesion showed marked edema with the cancer cell masses (white
arrowheads). SM, submucous region; ICM, inner circular muscle
layer; OLM, outer longitudinal muscle layer; SSR, subserous region;
SS, serous surface. Black bars show 1 cm (A) and 200 µm (B-D),
respectively. |
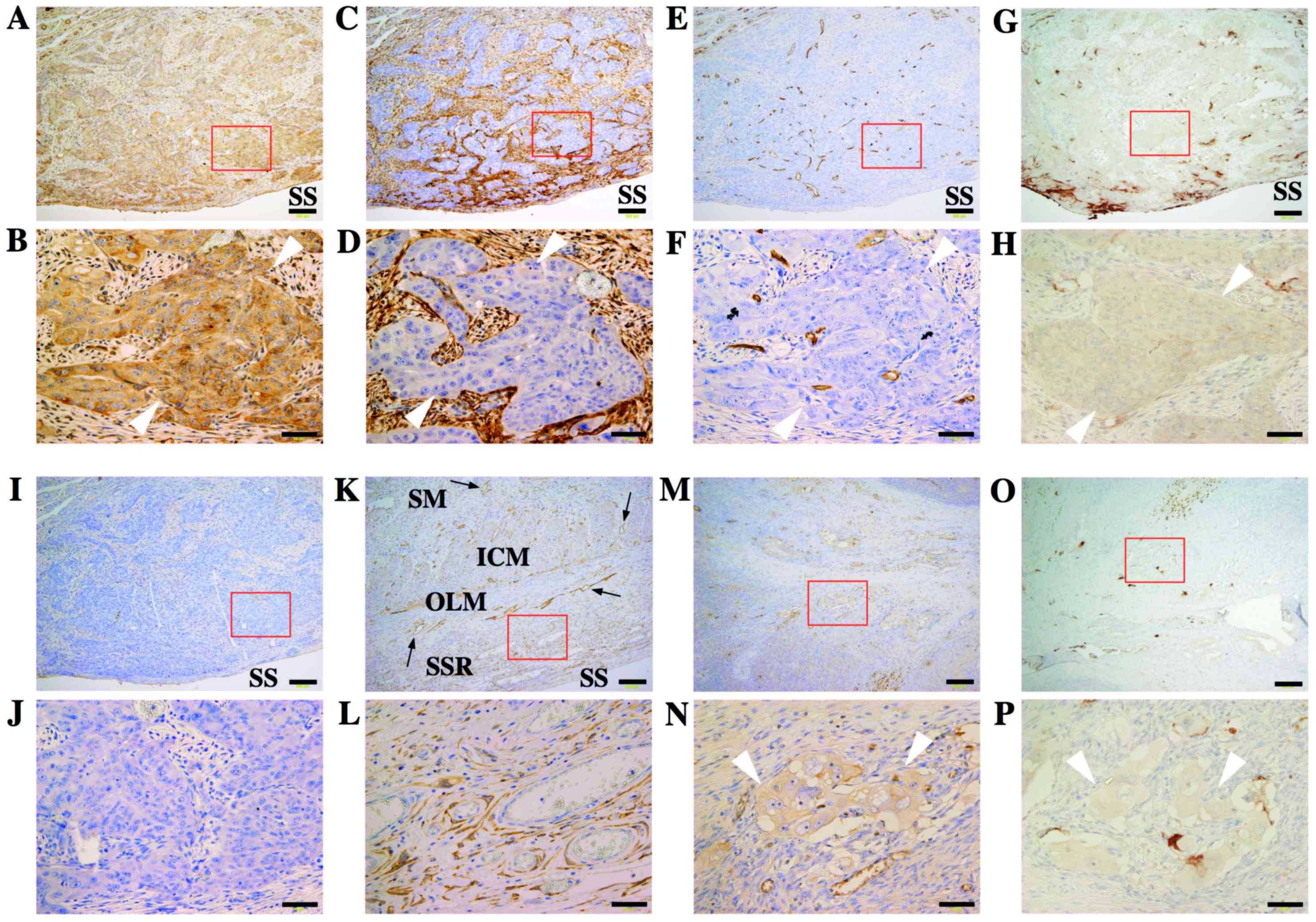 | Figure 2.Immunoreactive expression of VEGF-C,
podoplanin, and CD34 in the resected ileum. (A-J) a metastatic
lesion in the subserous area. (B, D, F, H and J) are magnified
images of the areas within the red squares of (A, C, E, G and I,
respectively). (A and B) immunoreactive expression of VEGF-C was
observed on the invaded cancer cells (white arrowheads). (C and D)
an increase of podoplanin-positive cells was detected around
invading cancer cells (white arrowheads). (E and F) CD34-positive
vascular endothelial cells were recognized among invaded cancer
cells (white arrowheads). (G and H) weak VEGF-A expression were
observed in metastatic region. (I and J) negative controls. (K and
L) a non-metastatic lesion. (K) podoplanin-positive lymphatic
vessels were clearly observed in the submucous region, inner
circular muscle layer, and outer longitudinal muscle layer (black
arrows). (L) Although apparent cancer invasion was not detected,
podoplanin-positive fibroblast-like cells were observed in the
subserous region. (M and N) Primary ovarian lesion at the initial
operation after NAC therapy. (O and P) positive staining of VEGF-A
were also observed in primary tumor. The immunoreactive expression
of VEGF-C was observed on the surviving cancer cells (white
arrowheads). SM, submucous region; ICM, inner circular muscle
layer; OLM, outer longitudinal muscle layer; SSR, subserous region;
SS, serous surface. Black bars show 200 µm (A, C, E, G, I, and K)
and 50 µm (B, D, F, H, J, and L), respectively. |
Discussion
Gastrointestinal perforation is one of the most
severe complications of bevacizumab. Accordingly, in cases of
ovarian primary cancer, it has been reported that bowel obstruction
symptoms, rectosigmoid involvement, bowel involvement, and wall
thickening on CT, pretreatment with 3 or more regimens, and
platinum-resistant disease are risk factors for intestinal
perforation. It is also well-known that intestinal edematous
changes are frequently observed in cases with diarrhea and
mechanical obstruction. However, it is occasionally difficult to
predict the potential risk accurately and prevent the onset of
perforation. Indeed, in this case, although the administration of
bevacizumab was immediately discontinued after detecting the
subileus symptoms, the supportive care could not avoid bowel
perforation and the subsequent peritoneal leakage from the small
intestine.
Notably, antibiotic is also a reported risk factor
for bowel perforation in the patient heavily treated with
bevacizumab by unknown mechanisms (7). In the present case, although we have
treated patient with antibiotics for subileus symptom with
mild-fever and elevated WBC counts, the bevacizumab was only
administrated for 5 courses, that the contribution of antibiotics
to intestinal perforation is unclear.
Macroscopic and microscopic pathological
examinations of the resected intestine revealed the marked
subserous involvement of ovarian cancer cells. The metastatic
masses that directly invaded from the serosa to mucosa were not
observed around the two perforated sites. In addition, although
there were longitudinal and segmental ulcerations, we could hardly
observe cancer cell invasion in the mucosal layer. Multiple
ulcerations and bowel perforation by bevacizumab were reported to
develope during chemotherapy for breast and lung cancers even under
cancer-free conditions in the abdomen. Accordingly, it should be
noted that the combination therapy of an anticancer drug with
bevacizumab adversely works to maintain the physiological
homeostasis of the intestinal mucosal layer, where certain
additional exacerbating factors can induce mucosal disorders such
as ulcerations and perforation.
In the present case, severe edema was widely
observed in the subserous regions along with invading cancer cell
foci in the resected ileum, suggesting poor lymphatic return and
drainage around these areas. In general, since the epithelium of
the small intestinal has high proliferative properties, the direct
attack of epithelial cells by anticancer drugs frequently causes
mucosal ulceration. Under these conditions, the inhibitory effects
of bevacizumab on neovascularization can exacerbate the ulceration
by interfering with the repair process. In addition to the above
mechanisms, it should be noted that lymphatic dysfunction was
reported to affect ulcerative disorders such as Crohn's disease.
Anatomically, the small intestine has two distinct lymphatic
networks: A submucosal network including the lacteals, and that of
the muscle layer (8). Although these
two lymphatic networks have no direct connections, both systems
drain into common collecting lymphatics in the subserous regions
near the mesenteric border of the intestine (9). It was reported that lymphangitis was
one of the earliest pathological findings in Crohn's disease
(10), and that the sclerosis of
regional intestinal lymphatics can induce the onset of Crohn's
disease in pigs (11).
Lymphangiogenesis and lymphangiectasia were frequently associated
with ileal and colonic Crohn's disease (12). Consequently, it is theoretically
possible that edematous changes caused by poor lymphatic return and
drainage in the subserous regions may subsequently lead to a
disturbance of the distant lymphatic microenvironment of the
mucosal layer, causing chronic ulcerative lesions.
When the intestine has chronic ulcerative lesions,
it is reasonable to speculate that a continuous mechanical stress
at the proximal site of the narrowing region would trigger
intestinal perforation. This mechanical stress may also contribute
to further edematous changes in the ileum. However, considering the
coexistence of edematous changes with cancer cell invasion in the
subserous regions, it is more appropriate to suggest that cancer
cell invasion would be mainly responsible for the subserous edema.
In this case, the metastatic pathway along the subserous regions
remains unknown. Although a large number of cancer cell foci in the
intramesenteric regions can be a candidate for a local origin, this
study provides no evidence for retrograde lymphatic progression
toward the subserous from intramesenteric regions.
It is well-known that VEGF families also play
important roles in lymphangiogenesis. The activation of VEGF
receptor-2 (VEGFR-2) and VEGFR-3 by VEGF-C and -D was reported to
initiate lymphangiogenesis by promoting lymphatic proliferation and
migration and by organizing new lymphatic endothelial cells into
functional capillaries. Accordingly, the coneutralization of
VEGFR-2 and VEGFR-3 completely inhibited lymphatic organization
(13). Importantly, although
bevacizumab is an anti-VEGF-A antibody, this agent was demonstrated
to induce the compensatory expression of VEGF-C and -D in glioma
cells, suggesting that VEGF-C and -D facilitate escape from
bevacizumab therapy (6). In this
case, immunoreactive VEGF-C, but not VEGF-D (data not shown), was
observed in the invading cancer cells. The intensity of VEGF-C
expression in the subserous metastatic region was considered to be
higher than that of the primary lesion (Fig. 2K and L). VEGF-D expression were
negative both in primary and metastatic regions (data not shown).
In addition, the abundant proliferation of spindle shape cells that
were positive for a lymphatic endothelial cell marker, podoplanin,
was also observed in the metastatic regions. Therefore, it can be
speculated that bevacizumab directly interferes with the angiogenic
environment around the affected lesions, and then induces
compensatory production of VEGF-C by cancer cells, leading to a
local disturbance of the lymphatic environment by poorly controlled
lymphoangiogenesis.
In conclusion, this case suggests that local
disturbance of the lymphatic function in subserous regions
associated with cancer cell invasion can be an additional risk
factor to promote the intestinal ulceration and perforation during
bevacizumab therapy. Clinical signs of risk factors for
gastrointestinal perforation, such as subileus symptom and
thickness of the bowel wall, may represent progressive functional
disorders of intestinal muscles and edematous change derived from a
poor lymphatic return and drainage. This study also demonstrates
the possible involvement of VEGF-C-producing cancer cells in the
edematous changes. Further investigation of mechanisms leading to
the local disturbance of the lymphatic function in subserous
regions may contribute to developing new approaches to predict or
avoid gastrointestinal perforation during bevacizumab therapy.
Acknowledgements
The present study was supported in part by
Grants-in-Aid for Scientific Research (no. 26293358). The authors
appreciate Associate Professor Hiroko Ikeda at the Division of
Pathology, Kanazawa University Hospital for her valuable
advice.
References
|
1
|
Reinthaller A: Antiangiogenic therapies in
ovarian cancer. Memo. 9:139–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: The AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Husain A, Wang Y, Hanker LC, Ojeda B,
Anttila M, Breda E, Vuylsteke P and Pujade-Lauraine E: Independent
radiologic review of AURELIA, a phase 3 trial of bevacizumab plus
chemotherapy for platinum-resistant recurrent ovarian cancer.
Gynecol Oncol. 142:465–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin JY, Urban RR, Liao JB and Goff BA:
Bevacizumab toxicity in heavily pretreated recurrent epithelial
ovarian, fallopian tube, and primary peritoneal cancers. J Gynecol
Oncol. 27:e472016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keating GM: Bevacizumab: A review of its
use in advanced cancer. Drugs. 74:1891–1925. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grau S, Thorsteinsdottir J, von Baumgarten
L, Winkler F, Tonn JC and Schichor C: Bevacizumab can induce
reactivity to VEGF-C and -D in human brain and tumour derived
endothelial cells. J Neurooncol. 104:103–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogata K, Takamori H, Umezaki N, Yagi T,
Ogawa K, Ozaki N, Hayashi H, Tanaka H, Ikuta Y and Doi K:
Gastrointestinal perforation during regorafenib administration in a
case with hepatic metastases of colon cancer. J Chemother. 20:1–3.
2016.
|
|
8
|
Miller MJ, McDole JR and Newberry RD:
Microanatomy of the intestinal lymphatic system. Ann N Y Acad Sci.
1207 Suppl 1:E21–E28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Unthank JL and Bohlen HG: Lymphatic
pathways and role of valves in lymph propulsion from small
intestine. Am J Physiol. 254:G389–G398. 1988.PubMed/NCBI
|
|
10
|
von der Weid PY, Rehal S and Ferraz JG:
Role of the lymphatic system in the pathogenesis of Crohn's
disease. Curr Opin Gastroenterol. 27:335–341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalima TV, Saloniemi H and Rahko T:
Experimental regional enteritis in pigs. Scand J Gastroenterol.
11:353–362. 1976.PubMed/NCBI
|
|
12
|
Pedica F, Ligorio C, Tonelli P, Bartolini
S and Baccarini P: Lymphangiogenesis in Crohn's disease: An
immunohistochemical study using monoclonal antibody D2-40. Virchows
Arch. 452:57–63. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Holzheimer RG and Mannick JA: Surgical
Treatment: Evidence-Based and Problem-Oriented. Part II: Small
bowel obstruction. WZuckschwerdt Verlag GmbH. Munich: 2001
|
|
14
|
Goldman J, Rutkowski JM, Shields JD,
Pasquier MC, Cui Y, Schmökel HG, Willey S, Hicklin DJ, Pytowski B
and Swartz MA: Cooperative and redundant roles of VEGFR-2 and
VEGFR-3 signaling in adult lymphangiogenesis. FASEB J.
21:1003–1012. 2007. View Article : Google Scholar : PubMed/NCBI
|
















