Introduction
Cervical cancer has one of the highest incidences
among malignant tumors in women worldwide (1). A direct cause of cervical cancer is
persistent infection by high-risk human papillomavirus (HR-HPV).
HR-HPV infection may lead to integration of viral DNA into the host
cell DNA, resulting in abnormal proliferation, cell deformation
and, eventually, cervical intraepithelial neoplasia (CIN) (2). CIN may further progress into carcinoma
in situ and invasive cancer. It is believed that different
subtypes of HPV have a distinct virulence for the cervical
epithelium (3). Cervical cells may
become atypic following integration of the viral DNA into the host
cell DNA, eventually developing into cervical cancer.
The probability of genital HPV infection within a
woman's lifespan is >75%. Most infections are transient and
clear up without any intervention within a few months to 2 years
after the infection, while only a few are persistent (4). Due to the differences in host and
environmental factors, the natural progression of HPV infection
varies significantly among diverse populations. As a result, it is
difficult to identify which HPV-infected patients will eventually
develop cervical cancer. At present, follow-up or local physical
therapy (including laser ablation, cryosurgery and microwave
ablation) are applied to patients with CIN 1, while loop
electrosurgical excision procedure (LEEP) and cold knife conization
are applied to patients with CIN ≥2. However, these methods have
the disadvantages of incomplete treatment and recurrence. There is
also no proper treatment for patients with subclinical infection
who are persistently HPV-positive and for those who are
persistently HPV-positive following treatment for cervical CIN.
For the abovementioned reasons, we aimed to apply
the Chinese medicine paiteling to treat HR-HPV subclinical
infection and CIN, as paiteling has been reported to have
successfully treated genital warts (5). Paiteling selectively destroys cancer
cell membranes, including cytoplasmic and mitochondrial membranes,
thereby suppressing cancer cell proliferation by its cytotoxic
action, ultimately leading to tumor cell degeneration and necrosis;
it may also destroy intracellular parasitic virus, and inhibit and
clear HPV infection (6).
In the present study, patients with subclinical
HR-HPV infection or CIN, with or without LEEP therapy, were
selected to test the effects of paiteling on clearing HPV.
Patients and methods
Patient data
Patients confirmed to be HR-HPV-positive with the
Hybribio HPV Genotyping system (Hybribio Biotechnology Co. Ltd.,
Chaozhou, China) between January, 2011 and June, 2013 at the
Shanghai Pudong New Area People's Hospital (Shanghai, China) were
considered as eligible candidates. These patients were then
examined by liquid-based cervical cytology, colposcopy and
histopathological examination. Patients with one of the following
symptoms were finally selected: Normal or chronic cervicitis;
condyloma-like changes, with or without chronic cervicitis; CIN 1,
with or without condyloma-like changes; CIN ≥2, without invasive
carcinoma. The inclusion criteria were as follows: Cervical
pathology in line with the aforementioned conditions; women of
childbearing age; HR-HPV positive; no previous history of cervical
cancer or precancerous lesions; no previous history of cervical
physical therapy or surgery. The exclusion criteria were pregnancy
or lactation.
Prior written informed content was obtained from
each patient. This study was approved by the Ethics Committee of
the Shanghai Pudong New Area People's Hospital.
Grouping
A total of 321 patients were enrolled in this study.
The patients were divided into the LEEP (n=82) and non-LEEP (n=239)
groups, according to the CIN classification. Patients in each group
were randomly assigned into the drug and control subgroups. A total
of 239 cases were included in the non-LEEP group, with a mean age
of 36.07 years, with 109 cases in the drug and 130 cases in the
control subgroups.
The LEEP group included HR-HPV-positive patients
with a biopsy outcome of CIN 2 (equivalent to moderate cervical
dysplasia) or CIN 3 (equivalent to severe cervical dysplasia and
carcinoma in situ), as well as a few CIN 1 patients who
wished to undergo surgery. The non-LEEP group included
HR-HPV-positive patients with a biopsy outcome of CIN <2, as
well as a few CIN 2 patients who had not given birth and wished to
undergo conservative therapy. The pathological outcomes of the
patients were usually chronic cervicitis, condyloma-like changes
with or without chronic cervicitis, CIN 1 with or without
condyloma-like changes, CIN 1 and CIN 2.
Follow-up was performed for the control subgroup,
while the drug subgroup treated with paiteling was tested for
HR-HPV with the Thin-prep cytology test (TCT) every 3 months for 1
year. The HPV seroconversion rates and the regression rates of the
cervical lesions were compared between the control and drug
subgroups at different time points, and the natural seroconversion
rate of HPV was analyzed.
Hybribio HPV detection
The Hybribio HPV Genotyping system was used for
identifying 21 HPV subtypes of exfoliative cells within the
cervical canal. The detected HPV subtypes in this study included
HPV16, 18, 31, 33, 35, 45, 51, 52, 53, 56, 58, 59, 66, 68 and
CP8304. The presence of any of these HPV subtypes or multiple
infections was considered as HR-HPV infection.
Cytological examination
TCT was used for cervical cytology. The specimens
were collected by a TCT special brush. Clinical diagnosis was based
on the 2001 revised descriptive diagnosis (The Bethesda System)
(7). Cellular morphology greater
than or equal to a result of atypical cells of undetermined
significance (ASC-US) was defined as an abnormality.
Colposcopy examination
For HR-HPV-positive patients, provided they
consented to further investigation, colposcopy was performed
regardless of the cytological status (normal or abnormal). A Leica
CH 9435 photoelectric vaginoscope (Leica Microsystems, Wetzlar,
Germany) was used for colposcopic examination. The cervical color,
blood vessels and white spots were observed. Following application
of a 5% acetic acid solution coat on the surface of the cervix,
dynamic changes of the lesion boundary, color and vascularity were
observed. The distribution and status of different iodine-stained
regions were observed following application of iodine solution to
the cervical surface.
Histopathological examination
The specimens collected under vaginoscopic guidance
were subjected to histopathological examination. The specimens were
routinely collected from 4 points, namely at the 3, 6, 9 and 12
o'clock positions of the transitional zone in cases without visible
abnormalities; if abnormalities were present, multiple points from
the abnormal area were biopsied.
LEEP
For the surgery, the iodine-colored area to an outer
zone of ~3–5 mm from the lesion was removed following coating with
Lugol's solution. Mixed cutting mode was applied, with an electrode
power of 50 W. If the iodine-negative area was close to the vaginal
vault and was difficult to access, a larger circumferential area
was resected. The removed lesions were subjected to pathological
examination.
Paiteling treatment
Paiteling (Beijing Paderborn Biological Technology
Co. Ltd., Beijing, China) was applied 3 days after the end of
menstruation. Starting on the 3rd day after the end of
menstruation, the drug was applied on days 1–4, 8–11 and 15–18,
with a total of 12 applications. At 12 days after the end of the
second menstruation, irrigation was performed with 1:50 diluted
liquid once a day, for 15 min per time. The treatment was
discontinued upon menstruation. Bathing and sexual intercourse were
prohibited during the treatment period.
Outcome evaluation
Negative conversion of HR-HPV was determined
according to the results of the Hybribio HPV Genotyping system,
regardless of the outcome of the cytological examination. In
addition, all the subtypes of multiple infections should be
converted to negative. Cervical lesion regression was determined by
the combination of cytological examination and pathological
diagnosis. If both cytological and histopathological results were
available, the regression outcome was determined according to the
histopathological results, while the cytopathological results were
considered as the standard if they were the only results available.
The histopathological criteria were as follows: Disease
progression, the pathological results were upgraded when compared
with those on enrollment; continuous lesions, the pathological
results remained unchanged; and lesion regression, the pathological
results were downgraded compared with those on enrollment. The
cytopathological criteria were as follows: Disease progression, the
TCT results were upgraded; continuous lesions, the TCT results
remained unchanged; and lesion regression, the TCT results were
normal. The results were evaluated at the 6-, 9- and 12-month time
point. For patients who remained HPV-positive, another treatment
course was performed according to the wishes of patients, until HPV
was negative. If HPV remained positive after 12 months of paiteling
treatment and follow-up, the treatment was considered
ineffective.
Statistical analysis
All the statistical analyses were performed using
SPSS software for Windows, version 10.0 (SPSS Inc., Chicago, IL,
USA) and P-values <0.05 were considered to indicate
statistically significant differences. The possibility of the
natural seroconversion rate of HR-HPV was expressed as a
percentage. The time to seroconversion indicated mean usage times.
The seroconversion rates of HR-HPV and the regression rates of the
cervical lesions were compared between subgroups using the
Chi-squared and exact tests.
Results
Effects of paiteling in the non-LEEP
group
To investigate the effects of paiteling in the
non-LEEP group, HPV negative conversion and cervical lesion
regression at different time points were compared. The cytological
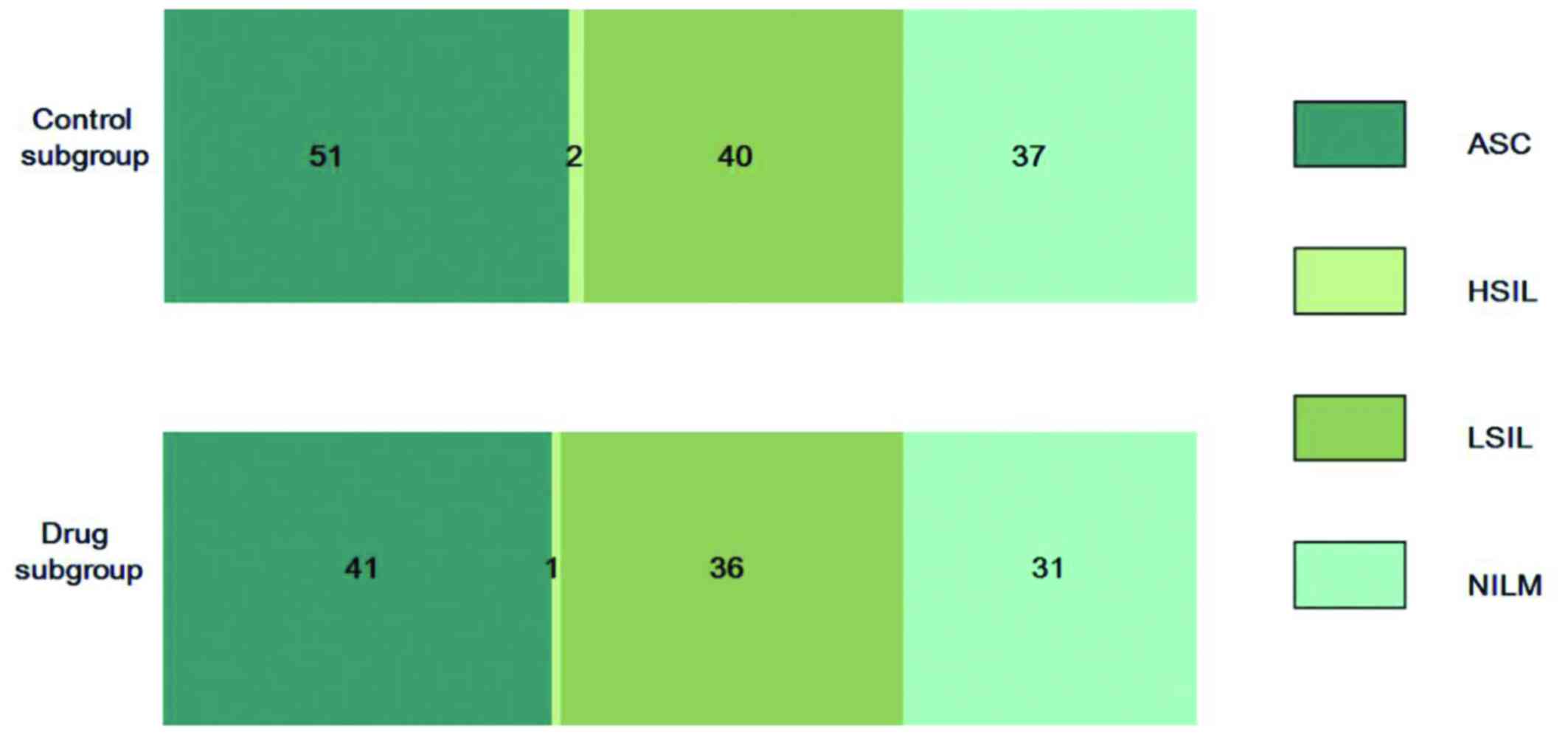
and pathological results of both groups are shown in Table I. There were no significant
differences in the cytological results and pathological diagnosis
between the drug and the control subgroups (Fig. 1). In the drug subgroup, 1 patient
discontinued treatment due to vaginitis, 3 cases were lost and 2
cases proceeded to undergo LEEP due to an HPV-positive status at 9
months. In the control subgroup, 4 cases were lost at 6 months, 10
cases were lost at 9 months and 15 cases were lost at 12
months.
 | Table I.Cytological and pathological results
in the drug and control subgroups of the non-LEEP group. |
Table I.
Cytological and pathological results
in the drug and control subgroups of the non-LEEP group.
|
| Subgroups |
| Subgroups |
|---|
|
|
|
|
|
|---|
| Cytological
results | Control | Drug | Pathological
results | Control | Drug |
|---|
| ASC (n=92) | 51 | 41 | Chronic cervicitis
(n=52) | 26 | 26 |
| HSIL (n=3) | 2 | 1 | Condyloma-like
changes with chronic cervicitis (n=60) | 30 | 30 |
| LSIL (n=76) | 40 | 36 | Condyloma-like
changes (n=52) | 26 | 26 |
| NILM (n=68) | 37 | 31 | CIN 1 with
condylomalike changes (n=26) | 13 | 13 |
| Total (n=266) | 148 | 118 | CIN 1 and CIN 1–2
(n=14) | 7 | 7 |
|
|
|
| CIN 2 (n=14) | 7 | 7 |
|
|
|
| Total (n=218) | 109 | 109 |
The natural seroconversion rate of HPV was analyzed
by comparing the seroconversion rate of HR-HPV at different time
points with that of the control subgroup. As shown in Table II, the natural seroconversion rates
at 6, 9 and 12 months were 27.8, 38.3 and 71.3%, respectively.
 | Table II.Comparison of seroconversion rates in
the non-LEEP group. |
Table II.
Comparison of seroconversion rates in
the non-LEEP group.
|
| Paiteling group | Control group |
|
|
|---|
|
|
|
|
|
|
|---|
| Follow-up time,
months | Total cases | Negative cases | Negative conversion
(%) | Total cases | Negative cases | Negative conversion
(%) | χ2 | P-value |
|---|
| 6 | 105 | 88 | 83.9 | 126 | 35 | 27.8 | 66.99 | <0.01 |
| 9 | 105 | 94 | 89.5 | 120 | 46 | 38.3 | 60.27 | <0.01 |
| 12 | 103 | 98 | 95.2 | 115 | 82 | 71.3 | 19.83 | <0.01 |
As shown in Table
III, the seroconversion rates were significantly higher
compared with those in the control subgroup (P<0.01). The
regression rates of the cervical lesions in the drug subgroup at 6,
9 and 12 months were 84.8, 85.7 and 91.3%, respectively, which were
also significantly higher (P<0.01) compared with those in the
control subgroup (30.2, 46.7 and 55.7%, respectively).
 | Table III.Comparison of regression rates in the
non-LEEP group. |
Table III.
Comparison of regression rates in the
non-LEEP group.
|
| Paiteling group | Control group |
|
|
|---|
|
|
|
|
|
|
|---|
| Follow-up time,
months | Total cases | Regression
number | Regression rate
(%) | Total cases | Regression
number | Regression rate
(%) | χ2 | P-value |
|---|
| 6 | 105 | 89 | 84.8 | 126 | 38 | 30.2 | 66.80 | <0.01 |
| 9 | 105 | 90 | 85.7 | 120 | 56 | 46.7 | 35.78 | <0.01 |
| 12 | 103 | 94 | 91.3 | 115 | 64 | 55.7 | 31.05 | <0.01 |
Therefore, the results suggested that paiteling was
effective against HR-HPV infection and it is possible that it
accelerated the regression of the lesions.
Effects of paiteling in the LEEP
group
To identify the effects of paiteling in the LEEP
group, the seroconversion rates and regression rates in the
subgroups were calculated. The cytological and pathological results
in both groups are summarized in Table
IV. The pathological diagnoses were similar between the two
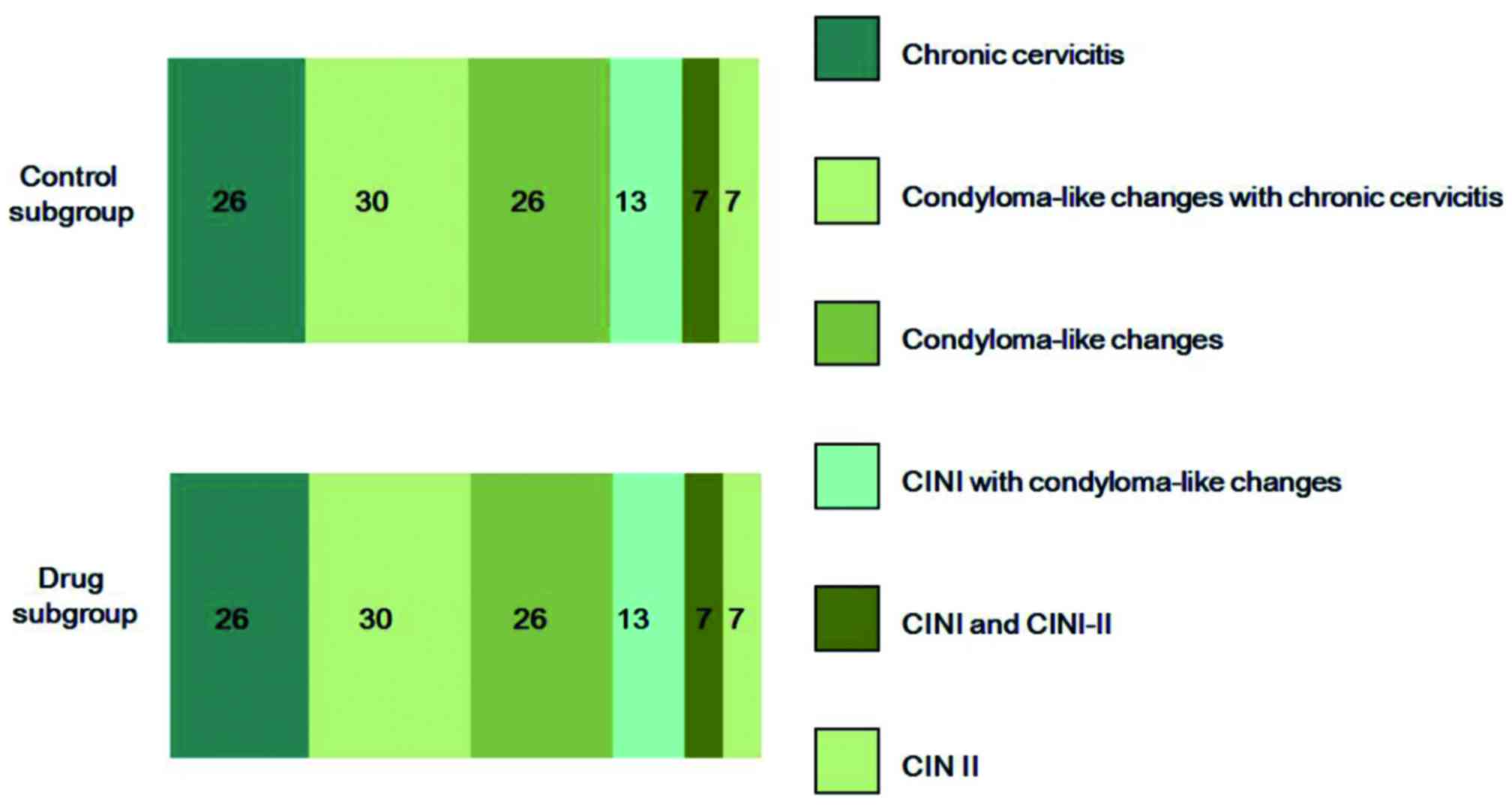
subgroups (Fig. 2). In the drug
subgroup, 2 cases were lost after being followed up for 6 months.
In the control subgroup, 4 cases further underwent cold knife
conization due to positive surgical margins in the pathological
report following LEEP, and abnormal TCT and/or HPV-positive status
at the 6- and 9-month follow-up. One patient, who had negative
surgical margins but was HPV-positive, atypical squamous
cells-cannot exclude high-grade squamous intraepithelial lesion
(ASC-H) on TCT and emotional stress, also underwent cold knife
conization. The abovementioned 5 cases were excluded from further
analysis.
 | Table IV.Cytological results in the drug and
control subgroups of the LEEP group. |
Table IV.
Cytological results in the drug and
control subgroups of the LEEP group.
|
| Subgroups |
|---|
|
|
|
|---|
| Cytological
results | Control | Drug | Total |
|---|
| CIN 1 |
3 |
4 | 92 |
| CIN 2 | 17 | 16 |
3 |
| CIN 3 | 20 | 22 | 76 |
| Total | 148 | 118 | 266 |
The seroconversion rates of the drug subgroup at 6,
9 and 12 months were 83.3, 90.0 and 95.0%, respectively, which were
significantly higher (P<0.01) compared with those of the control
subgroup (60.0, 71.4 and 80.0%, respectively; Table IV). This result indicated that LEEP
surgery removed the majority of the cervical lesions at the time of
HPV clearance, and paiteling accelerated the clearance of residual
HPV virus.
As shown in Table
IV, the regression rates of the cervical lesions in the drug
subgroup at 6, 9 and 12 months were 92.2, 92.5 and 92.5%,
respectively, while they were 77.5, 85.7 and 88.6%, respectively,
in the control subgroup. The regression rate differed significantly
between the two subgroups at 6 months. Among the patients, 2 cases,
including one ASC-H and one ASCUS case, were converted to
HPV-negative and normal TCT at 6 months, but exhibited HPV
positivity at 9 months. Therefore, the possibility of re-infection
and the emergence of disease cannot be ruled out. In conclusion,
the results demonstrated that paiteling may significantly increase
the regression rate.
Correlation between HPV subtypes and
the effects of paiteling
In order to determine whether the effects of
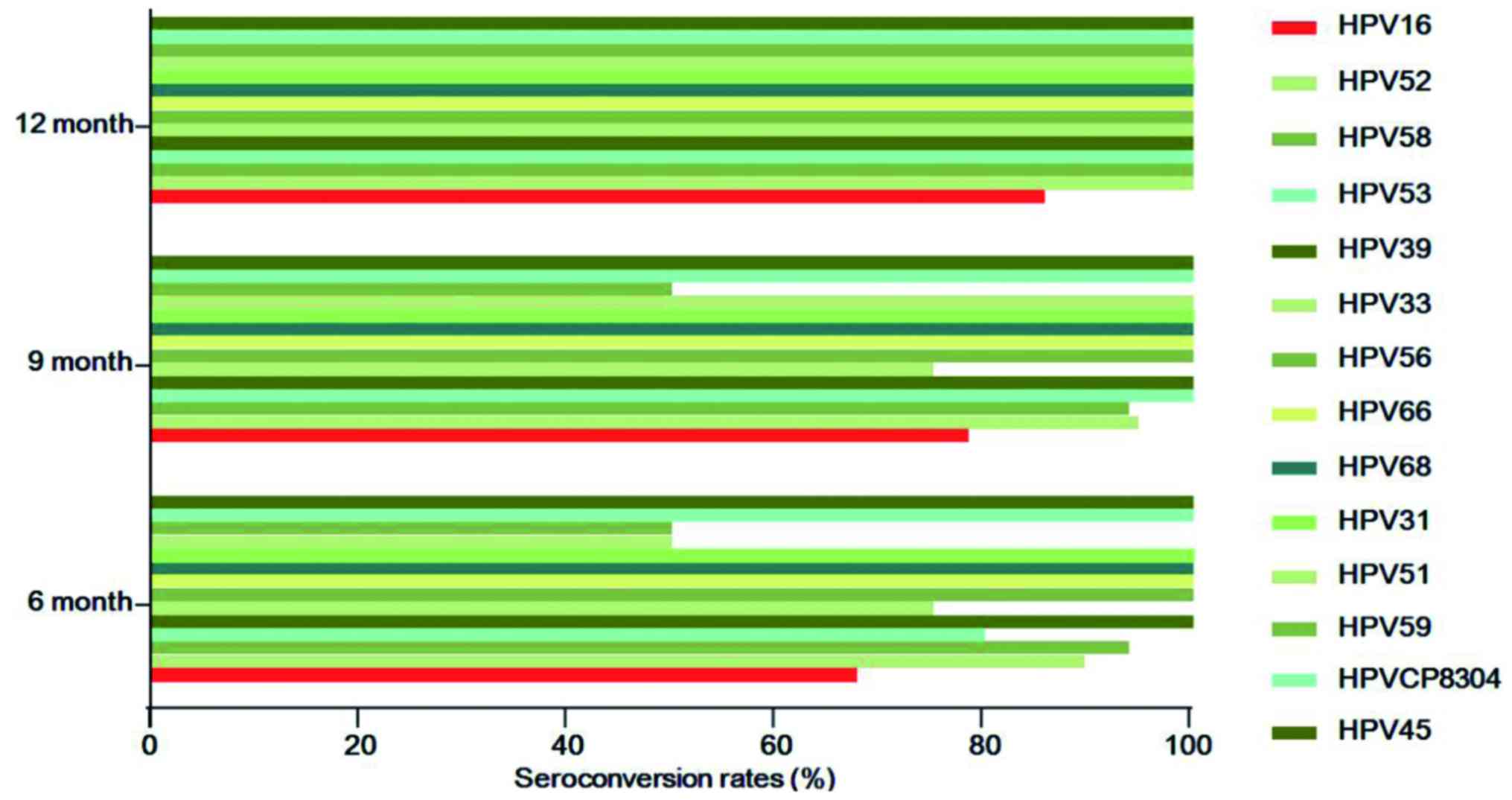
paiteling are associated with HPV subtype, the seroconversion rates
were compared among the drug subgroups. The top 5 HPV subtypes were
HPV16 (n=38), HPV52 (n=20), HPV58 (n=16), HPV53 (n=10) and HPV39
(n=9). The numbers of seroconversion cases (rates) of HPV16 at 6, 9
and 12 months were 25 (67.7%), 29 (78.4%) and 30 (85.7%),
respectively. In contrast to HPV16, the remaining subtypes all
reached 100% at 12 months, while there were cases remaining
HPV-positive at 6 and 9 months. However, 5 cases were lost and it
was possible that the lost cases did not convert to negative
(Fig. 3). During the follow-up,
~5.3–25% cases infected with HPV52, 58, 33 or 39 had not converted
to negative at 6 and 9 months, whereas they had all become negative
at 12 months. The seroconversion rates of HPV39, 56, 66, 68, 31,
CP8304 and 45 were all 100% when reviewed the first time after
applying the drug, and the HPV-negative status was maintained until
the end of follow-up (Table VII).
Therefore, the effects of paiteling on different HPV subtypes
varied significantly.
 | Table VII.Seroconversion rates of HPV subtypes
at different time points following application of paiteling. |
Table VII.
Seroconversion rates of HPV subtypes
at different time points following application of paiteling.
|
|
| Seroconversion
cases, n (%) |
|
|---|
|
|
|
|
|
|---|
| HPV subtype | Total cases | 6 months | 9 months | 12 months | Note |
|---|
| 16 | 38 | 25 (67.7) | 29 (78.4) | 30 (85.7) | One lost; two lost
at 12 months |
| 52 | 20 | 17 (89.5) | 18 (94.7) | 19 (100.0) | One lost |
| 58 | 16 | 15 (93.8) | 15 (93.8) | 16 (100.0) |
|
| 53 | 10 | 8 (80.0) | 10 (100.0) | 10 (100.0) |
|
| 39 | 9 | 9 (100.0) | 9 (100.0) | 9 (100.0) |
|
| 33 | 6 | 3 (75.0) | 3 (75.0) | 4 (100.0) | One lost; one with
positive HPV merged with HPV59 |
| 56 | 5 | 5 (100.0) | 5 (100.0) | 5 (100.0) |
|
| 66 | 5 | 5 (100.0) | 5 (100.0) | 5 (100.0) |
|
| 68 | 5 | 5 (100.0) | 5 (100.0) | 5 (100.0) | Two merged with
HPV16 and remained positive; one lost, while all others were
negative |
| 31 | 2 | 2 (100.0) | 2 (100.0) | 2 (100.0) |
|
| 51 | 2 | 1 (50.0) | 2 (100.0) | 2 (100.0) | One with positive
HPV at 6 months merged with HPV52 |
| 59 | 2 | 1 (50.0) | 1 (50.0) | 2 (100.0) |
|
| CP8304 | 2 | 2 (100.0) | 2 (100.0) | 2 (100.0) |
|
| 45 | 1 | 1 (100.0) | 1 (100.0) | 1 (100.0) |
|
Discussion
The majority CIN 1 or HPV-infected cases may be
reversed naturally without any intervention; however, some will
progress to advanced grades (8). In
a previous study, 817 HR-HPV-positive Korean patients were followed
up for 24 months (9). It was found
that HPV was cleared in 648/817 (79.3%) cases within 24 months. Of
the remaining 169 cases, 66 (39.1%) progressed to CIN ≥2. Bae et
al (10) reported that the
possibility of cytological abnormalities, CIN and high-grade CIN in
patients with persistent HR-HPV infection for 24 months was 38.2,
21.7 and 8.5%, respectively. In the present study, 130
HR-HPV-infected patients were followed up and the seroconversion
rates, without any intervention, at 6, 9 and 12 months were 27.8,
38.3 and 71.3%, respectively. This finding was similar to the
previous study (9), suggesting that
28.7% of the cases were HPV-positive at 12 months. These results
prompted us to investigate the reasons for the different outcomes
following HPV infection.
It has been demonstrated that the clearance of HPV
mainly depends on HPV subtypes, and high-risk HPV is associated
with lower clearance rates. HR-HPV persistence is also affected by
other factors, such as the body's immunity, while HPV clearance has
been associated with ethnicity and the presence of chlamydia
infection. No significant correlation with marital status or the
number of sexual partners was identified (11). It has been reported that age, number
of sexual partners, HR-HPV viral load, integrated state and the
results of cytology and pathology may be used to predict the
progression and regression of HPV infection and CIN 1. However, the
results reported by different studies have been inconsistent, or
even opposing (12–16). Kim et al suggested cytological
results and viral load as useful indicators to predict viral
clearance and the progression of lesions (9).
There is currently no drug effectively blocking HPV
infection, or used specifically for the treatment of CIN 1–2. As
the pathogens associated with CIN and condylomata acuminata both
belong to the HPV family, it would be of interest to test the
effects of paiteling on HP-HPV infection and its consequences. It
was reported that paiteling has been successfully used for the
treatment of condylomata acuminata (17). In the present study, for patients in
the non-LEEP group, it was found that the seroconversion and
cervical lesion regression rates in the drug subgroup were
significantly higher compared with those in the control subgroup.
The results suggested that paiteling promoted the clearance of
HR-HPV infection. A previous study also reported that more HR-HPV
infection patients without LEEP converted to negative with
paiteling treatment (18).
For patients in the LEEP group, the relevant
literature reported that the seroconversion rates at 6 months after
LEEP were 62.58–87.3% (19,20). Alonso et al reported
HR-HPV-positive rates of 35.3 and 27.1% at 6 and 12 months,
respectively, without a statistically significant difference
(21). Kim et al found that
the rates of HR-HPV positivity at 3, 6, 9, 12, 18 and 24 months
were 45.6, 14.3, 6.3, 2.2, 1.5 and 1.1%, respectively, in patients
with a negative surgical margin following LEEP (22). These findings suggest that the
majority of the lesions and the HPV infection are eliminated after
LEEP; however, there may still remain residual HPV and cervical
lesions. More importantly, the clearance of HR-HPV after surgery
directly affects residual lesions or recurrence (23).
Nagai et al believe that HPV positivity is a
marker of residual or recurrent lesions (24). The residuality and/or recurrence of
lesions is closely associated with HPV infection at 1 month after
surgery and their likeliness increases with the prolongation of
follow-up. Thus, early follow-up is necessary; additionally, close
monitoring and treatment as required are recommended. We observed
that the seroconversion and regression rates of the cervical
lesions were higher in the drug subgroup compared with those in the
control subgroup of the LEEP group. This finding was particularly
significant at 6 months. Our results demonstrated that paiteling
may accelerate HPV clearance. Guoqin et al reported that the
improvement of the HPV load in the drug subgroup was significantly
higher compared with that in the control subgroup (25). These results suggested that HR-HPV
may be suppressed and cleared by paiteling, which provides a new
approach to the treatment of residual HPV or the recurrence of
subclinical infection following surgery in patients with advanced
CIN lesions. However, the persistence of the effects of paiteling
on HPV clearance requires an extended follow-up time and large
clinical trials.
Different HPV subtypes exhibit variable
pathogenicity for the cervical epithelium. HPV genotyping, as a
complement to the traditional cytological examination, has been
widely used, particularly for patients with indeterminate
cytological diagnosis or for those comprising the population with
critical and degenerative changes or persistent infection; it is
also a crucial tool for predicting the cancerous tendency of
cervical cells, enabling early detection, prevention and treatment
of early cervical cancer. The Center for Disease Control and tumor
prevention systems in the United States and Europe have strongly
recommended that HPV genotyping is included in the screening for
cervical cancer, and recommend multi-center cooperation to
establish an effective early warning system. The present study used
the Hybribio HPV Genotyping system, which has received the European
CE certification and has been widely applied in clinical and
scientific investigations. We observed that the seroconversion rate
of HPV16-infected patients at 12 months was 85.7%, while all other
subtypes reached 100%.
Different HPV subtypes exhibited distinct negative
conversion rates with paiteling treatment. The correlation of the
phenotype to the natural seroconversion rate has not yet been fully
elucidated. Studies suggest that different HPV subtypes require
different times to clear naturally. A meta-analysis based on 86
studies worldwide, including 100,000 women, demonstrated that the
persistence of the HPV infection exhibited regional variation and
subtype specificity. HPV16, 31, 33 and 52 are associated with high
rates of persistent infection. The median time for HPV clearance is
9.8 months, whereas HPV16 requires 12.4 months (26). Dong et al reported
seroconversion rates of 67.7, 67.4 and 43.5% for HPV16, 52 and 58,
respectively, and the differences between HPV58 and each of the
other two subtypes were found to be statistically significant
(27). These findings suggest that
the natural seroconversion rate should be considered when analyzing
the correlation between HPV subtypes and the effects of
paiteling.
In the present study, the beneficial effects of
paiteling on patients with HR-HPV infection were demonstrated. Our
results also suggested that the effects of paiteling were
associated with HPV subtype, although the association cannot be
precisely reflected due to the limited sample size. The assessment
requires larger groups for analysis.
Acknowledgements
The present study was supported by the Key
Scientific Research Project of Shaoxing City of Pudong Health and
Family Planning Commission of Shanghai (grant no. PWZz2013-14), the
Leader Training Plan of the Health Department Pudong New District
of Shanghai (PWRd2013-09) and by grants from the Key Scientific
Research Project of Shaoxing City of Zhejiang Province
(2011A23017).
References
|
1
|
Ogunwale AN, Coleman MA, Sangi-Haghpeykar
H, Valverde I, Montealegre J, Jibaja-Weiss M and Anderson ML:
Assessment of factors impacting cervical cancer screening among
low-income women living with HIV-AIDS. AIDS Care. 28:1–4. 2016.
View Article : Google Scholar
|
|
2
|
Qiu AD, Wu EQ, Yu XH, Jiang CL, Jin YH, Wu
YG, Chen Y, Chen Y, Shan YM, Zhang GN, et al: HPV prevalence, E6
sequence variation and physical state of HPV16 isolates from
patients with cervical cancer in Sichuan, China. Gynecol Oncol.
104:77–85. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahmed HG, Bensumaidea SH and Ashankyty IM:
Frequency of human papilloma virus (HPV) subtypes 31,33,35,39 and
45 among yemeni women with cervical cancer. Infect Agent Cancer.
10:292015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ingles DJ, Pierce Campbell CM, Messina JA,
Stoler MH, Lin HY, Fulp WJ, Abrahamsen M, Sirak BA, O'Keefe MT,
Papenfuss M, et al: Human papillomavirus virus (HPV) genotype- and
age-specific analyses of external genital lesions among men in the
HPV infection in men (HIM) study. J Infect Dis. 211:1060–1067.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan Q, Han J, Cong W, Ge Y, Ma D, Dai Z,
Li Y and Bi X: Docetaxel-loaded solid lipid nanoparticles suppress
breast cancer cells growth with reduced myelosuppression toxicity.
Int J Nanomedicine. 9:4829–4846. 2014.PubMed/NCBI
|
|
6
|
Parris GE: Hypothesis links emergence of
chloroquine-resistant malaria and other intracellular pathogens and
suggests a new strategy for treatment of diseases caused by
intracellular parasites. Med Hypotheses. 62:354–357. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kostova P and Zlatkov V: The Bethesda
system: New revision of the terminology from 2001 year. Akush
Ginekol (Sofiia). 43:52–55. 2004.(In Bulgarian). PubMed/NCBI
|
|
8
|
Bansal N, Wright JD, Cohen CJ and Herzog
TJ: Natural history of established low grade cervical
intraepithelial (CIN 1) lesions. Anticancer Res. 28:1763–1766.
2008.PubMed/NCBI
|
|
9
|
Kim JW, Song SH, Jin CH, Lee JK, Lee NW
and Lee KW: Factors affecting the clearance of high-risk human
papillomavirus infection and the progression of cervical
intraepithelial neoplasia. J Int Med Res. 40:486–496. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bae J, Seo SS, Park YS, Dong SM, Kang S,
Myung SK and Park SY: Natural history of persistent high-risk human
papillomavirus infections in Korean women. Gynecol Oncol.
115:75–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sycuro LK, Xi LF, Hughes JP, Feng Q, Winer
RL, Lee SK, O'Reilly S, Kiviat NB and Koutsky LA: Persistence of
genital human papillomavirus infection in a long-term follow-up
study of female university students. J Infect Dis. 198:971–978.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ye J, Cheng X, Chen X, Ye F, Lu W and Xie
X: Short-term type-specific HPV persistence and its predictors in
an asymptomatic general female population in Zhejiang, China. Int J
Gynaecol Obstet. 110:217–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fukuchi E, Sawaya GF, Chirenje M, Magure
T, Tuveson J, Ma Y, Shiboski S, Da Costa M, Palefsky J, Moscicki
AB, et al: Cervical human papillomavirus incidence and persistence
in a cohort of HIV-negative women in Zimbabwe. Sex Transm Dis.
36:305–311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Munoz N, Hernandez-Suarez G, Mendez F,
Molano M, Posso H, Moreno V, Murillo R, Ronderos M, Meijer C and
Muñoz A: Instituto Nacional de Cancerología HPV Study Group:
Persistence of HPV infection and risk of high-grade cervical
intraepithelial neoplasia in a cohort of Colombian women. Br J
Cancer. 100:1184–1190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo YL, You K, Qiao J, Zhao YM, Liu CR and
Geng L: Natural history of infections with high-risk HPV in Chinese
women with abnormal cervical cytology findings at baseline. Int J
Gynaecol Obstet. 110:137–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Castle PE, Walker JL, Schiffman M and
Wheeler CM: Hormonal contraceptive use, pregnancy and parity and
the risk of cervical intraepithelial neoplasia 3 among oncogenic
HPV DNA-positive women with equivocal or mildly abnormal cytology.
Int J Cancer. 117:1007–1012. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng X: Chinese medicine Paiteling
treatment of condyloma acuminatum. The curative effect of 392
cases. China science. 15:282006.
|
|
18
|
Chen Rui ZJ and Liaoqin Ping: The clinical
effects of paiteling on Cervical intraepithelial neoplasia grade 1
and 2. Chin J Pract Gynecol Obestet. 3:2011.
|
|
19
|
Tang Huagui XH, Wanyu, Xie Yane and Liu
Xuefeng: Ou 2007 The Significance of Prognosis in different age
groups eliminated CIN after Leep treatment by detection of HPV. J
Hunan Norm Univ (Medical Sciences). 2007.
|
|
20
|
Li Rui-zhen LW, Changhuai, Zhang Zhihong
Liu Yanqiu, Zhou Leiming and Weng Ruifang: Wu HPV clearance related
factors after cervical intraepithelial neoplasia surgery. Chin J
Matern Child Health Res. 2009:42009.
|
|
21
|
Alonso I, Torne A, Puig-Tintore LM, Esteve
R, Quinto L, Campo E, Pahisa J and Ordi J: Pre- and post-conization
high-risk HPV testing predicts residual/recurrent disease in
patients treated for CIN 2–3. Gynecol Oncol. 103:631–636. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim YT, Lee JM, Hur SY, Cho CH, Kim YT,
Kim SC and Kang SB: Clearance of human papillomavirus infection
after successful conization in patients with cervical
intraepithelial neoplasia. Int J Cancer. 126:1903–1909.
2010.PubMed/NCBI
|
|
23
|
Yuquan ZMMXZ: Recent residual or
recrudescent development of CIN after surgical cone. Chin J Clin
Oncol. 34:32007.
|
|
24
|
Nagai Y, Maehama T, Asato T and Kanazawa
K: Persistence of human papillomavirus infection after therapeutic
conization for CIN 3: Is it an alarm for disease recurrence?
Gynecol Oncol. 79:294–299. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo-Qin H: Clinical observation of
paiteling on treatment of cervicitis combined with high-risk HPV
infection. Chin J Woman Child Health Res. 23:32012.
|
|
26
|
Rositch AF, Koshiol J, Hudgens MG,
Razzaghi H, Backes DM, Pimenta JM, Franco EL, Poole C and Smith JS:
Patterns of persistent genital human papillomavirus infection among
women worldwide: A literature review and meta-analysis. Int J
Cancer. 133:1271–1285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong H, Liu Y, Tang Y and Liang F:
Research on the circumstance of human papillomavirus gene subtypes
changing into negativity. J Mol Diagn Therapy. 4:32012.(In
Chinese).
|