Introduction
The trend in the treatments for advanced head and
neck squamous cell carcinoma (HNSCC) has shifted from radical
surgery to organ preservation due, in large part, to the two
milestone studies conducted in the 1990s: The Department of
Veterans Affairs Laryngeal Cancer Study Group (VALCSG) (1) and European Organization for Research
and Treatment of Cancer (EORTC) 24891 (2). These two studies clearly demonstrated
that laryngeal preservation is feasible with combined use of
induction chemotherapy and radiation without compromising patients'
survival in advanced laryngeal and hypopharyngeal carcinoma. As a
result, organ-preservation was adopted as the main goal of the
clinical studies and whereby unprecedented dose-intensification has
been conducted mainly through two forms of modalities: Concurrent
chemoradiotherapy (CRT) (e.g., clinical trials led by the Radiation
Therapy Oncology Group [RTOG]) or sequential therapy (ST) composed
of induction chemotherapy and CRT (e.g., GORTEC and Tax 324
protocols) (3–6). Because of the further improved
laryngeal preservation, these dose-intensified organ-preserving
strategies (DIOPSs) are currently proposed as the standard for
organ preservation (7,8). However, it is becoming apparent that
DIOPSs involve critical issues. Firstly, only a limited number of
advanced HNSCC patients benefit from DIOPSs, because these types of
experimental therapies are feasible only in a select subset of
patients (i.e., patients with good general condition) who can
tolerate these heavy regimens. This limitation may be related to
the recent surprising results of large surveys based on the
Surveillance, Epidemiology, and End Results (SEER) or the National
Cancer Data Base (NCDB), which demonstrated a worsening survival
trend in patients with laryngeal cancer and only a marginal
improvement in those with hypopharyngeal cancer (9,10).
Secondly, it is obvious that current DIOPSs have reached the upper
limit of human tolerance in terms of late toxicitiy, as exemplified
in the recently published long-term results of RTOG 91–11. On this
regimen, which employs concurrent 100 mg/m2 of CDDP
tri-weekly, as much as 43% of the patients with preserved larynx
developed laryngo-esophageal dysfunction, and which eventually
accounted for the high rate of tumor-unrelated deaths (11–13).
Thirdly, it became practically infeasible to compare the treatment
results of radical surgery with organ-preserving treatments in
randomized control studies, due to the strong dogma: Similar
survival is achievable by either DIOPS or radical surgery on the
basis of VALCSG and EORTC 24891 studies (1,2)
conducted more than 20 years ago. Consequently, the survival
benefit of radical surgery appears to have been overly
underestimated despite considerable technical advancements and the
improved multimodality setting in which surgery is conducted.
Contrary to the trend of DIOPSs, we have treated
HNSCC patients using a distinctive platform, in which 30–40 GY of
induction CRT is used as a selection tool for organ preservation
(14,15). In this algorithm-based
‘chemoradioselection’ strategy, only patients who demonstrate good
response to induction CRT (i.e., ‘chemoradioselected’: CRS),
proceed to organ preservation arm and then receive further CRT up
to 60–70 Gy. For the remaining non-responders (i.e.,
‘non-chemoradioselected’: N-CRS), radical surgery is recommended.
Mainly using moderate intensity CRT regime, we obtained quite
satisfactory laryngeal preservation and survival in T2 glottic
carcinoma and overall survival in oropharyngeal carcinoma with
minimal toxicity (14,16–18). In
a recent pilot study on advanced hypopharyngeal carcinoma (19), we reported the utility of this
protocol; chemoradioselection can segregate tumors for
organ-preservation with use of moderate intensity CRT from those
that would be better treated by radical surgery. Interestingly
irrespective of clinical stage, CRS patients demonstrated quite
favorable survival and organ preservation with moderate intensity
CRT that seldom caused laryngo-esophageal-dysfunction, whereas
radical surgery appeared to exhibit survival benefit in the N-CRS.
In this context, the aim of this study is to verify the utility of
this strategy in a larger scale study employing patients with
advanced hypopharyngeal and laryngeal cancer and to explore the
potential of this strategy as a novel platform for the treatment of
advanced HNSCC which may address the issues associated with the
current DIOPSs mentioned above.
Patients and methods
Patients eligibility and protocol
All patients had pathologically confirmed,
previously untreated stage III or IV laryngeal and hypopharyngeal
cancer according to the UICC TNM classification (2002 and 2007).
Clinical staging and the diagnosis of double cancer were done by
endoscopy, contrast enhanced computed tomography, magnetic
resonance imaging, ultrasonography, and fluorodeoxyglucose positron
emission tomography. Patients who had distant metastasis and/or
synchronous cancer were excluded. T4 cases were also excluded,
because use of organ preserving treatment in this stage is
controversial, especially for hypopharyngeal tumors (20,21). All
patients were required to have an Eastern Cooperative Oncology
Group Organization performance status ≦2, and adequate medical and
laboratory parameters required for CRT. The choice of platinum
agent was determined by the creatinine clearance (Ccr): Cis-platin
(CDDP) was used for those with Ccr ≧60 ml/min and palapalatin
(CBDCA) for those with Ccr <60 ml/min. Enrolled patients were
treated according to the algorithm-based chemoradioselection
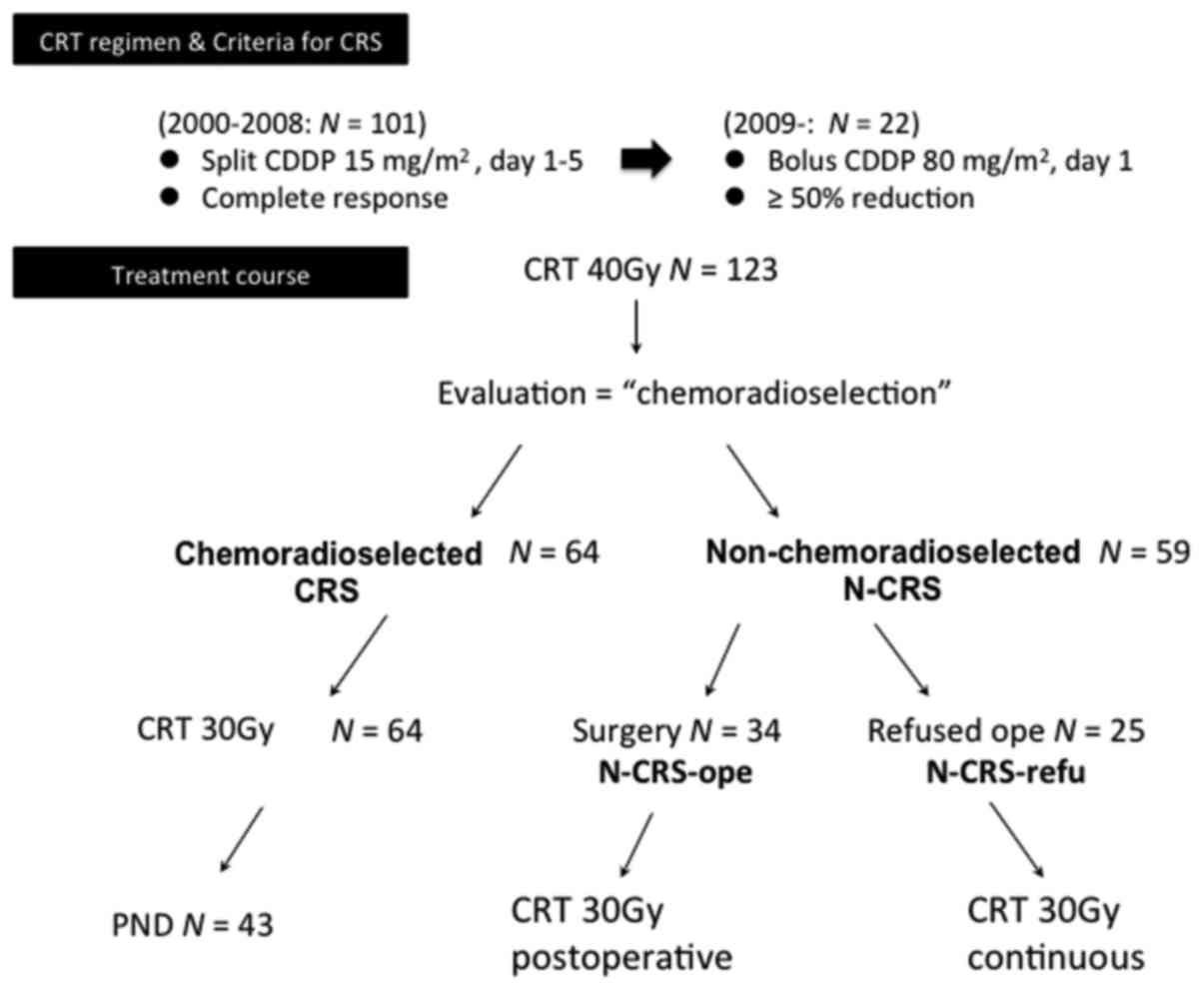
protocol. From 2000 to 2008, split CDDP (15 mg/m2/day)
or CBDCA (AUC=1/day) was administered from day 1 to 5,
concomitantly with external beam of irradiation (2.0 Gy/day); since
2009 CDDP (80 mg/m2/) or CBDCA (AUC=5) on day 1 was
administered. After 40 Gy of CRT, the tumor response was evaluated
either clinically or pathologically. With the split CDDP regimen,
complete response at the primary site was considered as
chemoradioselected (CRS). For patients treated with bolus CDDP,
≥50% reduction of primary tumor was used as the criteria for CRS
(Fig. 1). The CRS patients continued
to receive additional CRT up to 70 GY and received planned neck
dissection (PND) for residual N. For the N-CRS patients, radical
surgery was recommended and conducted (N-CRS-ope), when consent for
total laryngectomy was obtained. However, to those who refused
surgery (N-CRS-refu), continuous CRT was administered up to 70 GY.
This algorithm-based protocol was approved by the Institutional
Review Board and all patients gave documented informed consent.
Patients characteristics
From 2000, March to 2012, September 123 patients
with stage III and IV HPC were registered for this algorithm-based
chemoradioselection protocol. All 123 patients were followed up for
more than 36 months; median follow up period was 63 months (range
4–168). Their average age was 64. The patient characteristics are
summarized in Table 1.
 | Table I.Patient characteristics
(N=123). |
Table I.
Patient characteristics
(N=123).
| Characteristic | No. of patients
(%) |
|---|
| Sex | 113 (92) |
| Male |
|
|
Female | 10 (8) |
| Primary site |
|
|
Hypopharynx | 59 (48) |
|
Larynx | 64 (52) |
| T classification |
|
| T1 | 13 (11) |
| T2 | 58 (47) |
| T3 | 52 (42) |
| N classification |
|
| N0 | 19 (15) |
| N1 | 25 (20) |
| N2a | 2 (2) |
| N2b | 49 (40) |
| N2c | 19 (15) |
| N3 | 9 (7) |
| TNM Stage | 44 (36) |
| III |
|
| IVa | 70 (57) |
| IVb | 9 (7) |
Adverse effects
Adverse effects were evaluated according to the
Common Terminology Criteria for Adverse Events version 3.0.
End-points and statistical
analyses
The primary endpoints were overall survival (OS) and
laryngo-esophageal dysfunction free survival (LEDFS). Use of LEDFS
as an endpoint was proposed by the Larynx Preservation Consensus
Panel in 2009, in which, death, local recurrence, total or partial
laryngectomy, tracheostomy, and feeding tube/gastrostomy insertion
after ≧2 years were considered as a composite event (22). Thus, LEDFS reflects the ‘functional’
laryngeal preservation in surviving patients more accurately than
laryngectomy free survival (LFS) that has been used conventionally.
In order to clarify the efficacy of chemoradioselection, OS and
LEDFS in specific subgroups of patients (e.g., treatment courses
and clinicopathological factors) were also calculated. For these
analyses, Kaplan-Meier curves were generated and the between-group
differences were assessed by log-rank test for statistical
significance. The cause of death and the results of laryngeal
preservation were assessed by Chi-square test or Fisher's exact
test. Multivariate Cox proportional hazard model were used to
calculate the effects of T, N, primary sites and treatment courses
on OS. Values of P<0.05 were considered statistically
significant.
Results
Treatment courses
The treatment courses of patients are demonstrated
in Fig. 1. After 40 Gy of CRT, 64
(52%) of patients were classified as CRS, while 59 (48%) of
patients as N-CRS. CRS patients were further treated by additional
30 Gy of CRT and 43 patients received PND. Among the N-CRS
population, 34 patients underwent radical surgery (N-CRS-ope),
while 25 patients refused operation (N-CRS-refu). Additional 30 Gy
of CRT was given either as postoperatively to N-CRS-ope or in
continuation to N-CRS-refu. There were 101 patients who received
split CDDP, while 22 patients received bolus CDDP.
Adverse effects
None of the patients developed grade 5 toxicity.
Only one patient developed grade 4 toxicity (granulocytopenia and
neutropenia). Cumulative grade 3 adverse effects included
leucopenia (N=13); neutropenia (N=6);
thrombocytopenia (N=3); dermatitis (N=4); mucositis
(N=13); anolexia (N=1); pneumonia (N=2);
dysphagia (N=5); renal dysfunction (N=1); and liver
dysfunction (N=1). Comparable rates of adverse effects
(≥grade 3) were observed in the split CDDP (38%) and bolus CDDP
(43%) groups.
Patients survival
There were a total of 50 deaths (21 in the CRS
patients, 15 in the N-CRS-ope, and 14 in the N-CRS-refu groups).
Out of the 21 deaths in the CRS arm, 7 were tumor-related deaths
[loco-regional failure (N=3); distant metastases
(N=4)], while 14 were tumor-unrelated deaths (metachronous
cancer (N=4); pneumonia (N=1); hepatitis
(N=1); and others (N=8). Out of the 15 deaths in
N-CRS-ope arm, 7 were tumor-related deaths [loco-regional failure
[N=3]; distant metastasis (N=4)] and 8 were
tumor-unrelated deaths [metachronous cancer (N=4); heart
failure (N=1); and pneumonia (N=3)]. Out of the 14
deaths in the N-CRS-refu arm, 11 were tumor-related deaths
[loco-regional failure (N=8); distant metastasis
(N=3)] and 3 were tumor-unrelated deaths [metachronous
cancer (N=2); others (N=1)]. Thus, patients in the
N-CRS-refu arm experienced significantly (P=0.0038,
Chi-square-test) higher tumor-related death rate (11/25, 44%) as
compared to that in the CRS (10/64, 10%) and the N-CRS-ope (7/34,
20%) arms. The rate of distant metastasis in the N-CRS-ope (4/34,
11%) and the N-CRS-refu (3/25, 12%) arms was approximately 2 times
higher than that in the CRS (4/64, 6%) arm, although the difference
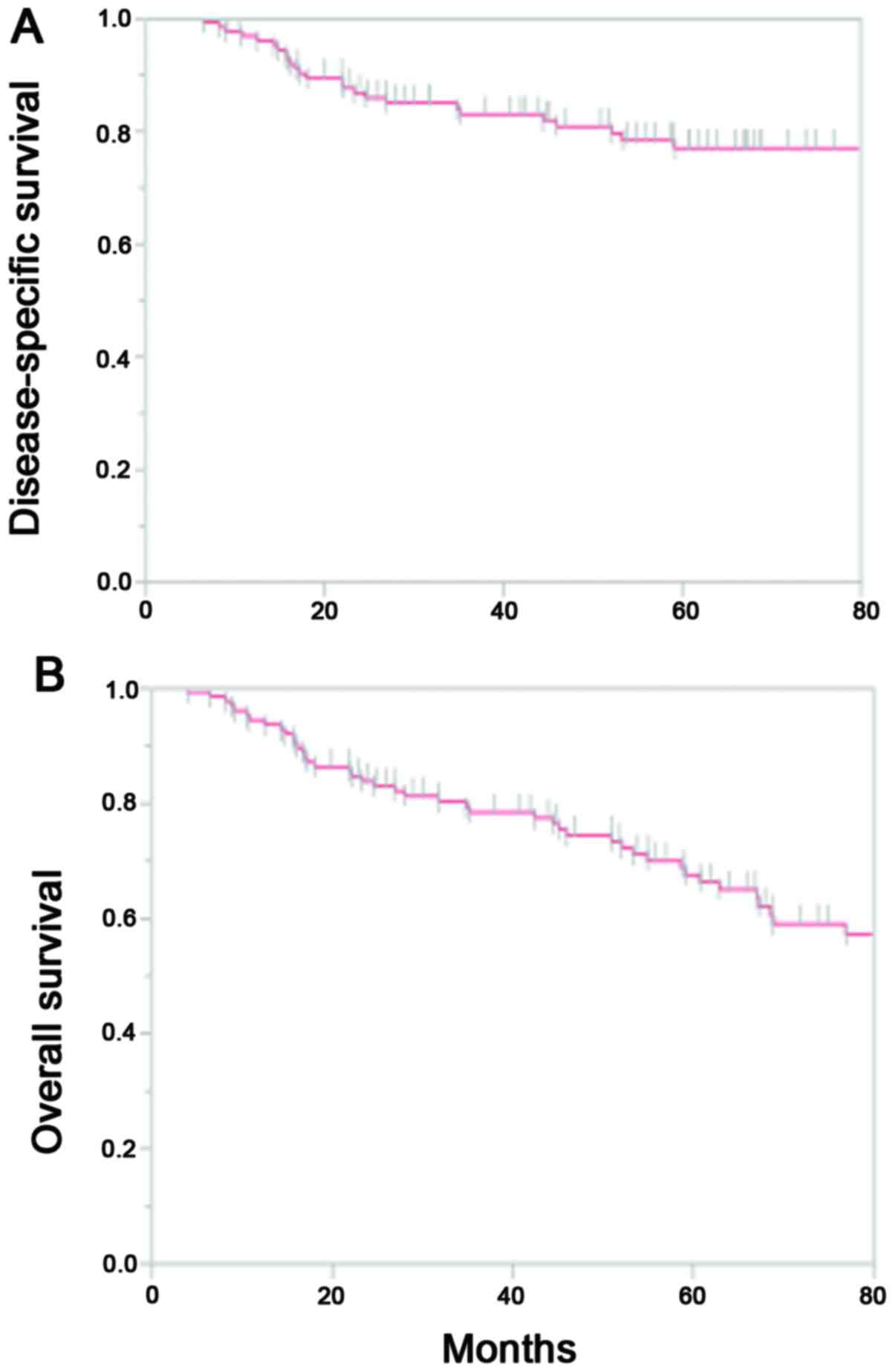
was not statistically significant. Collectively, the 5-year
cumulative disease-specific survival rate (DSS) and OS rates were
77% and 65%, respectively (Fig. 2).
We then analyzed the effects of 4 candidate prognostic factors [T
(T1, 2 vs. T3); N (N0, 1 vs. N2, 3); primary site (larynx vs.
hypopharynx); and treatment [planned: i.e., CRS + N-CRS-ope vs.
unplanned: i.e., N-CRS-refu] on OS using both univariate and
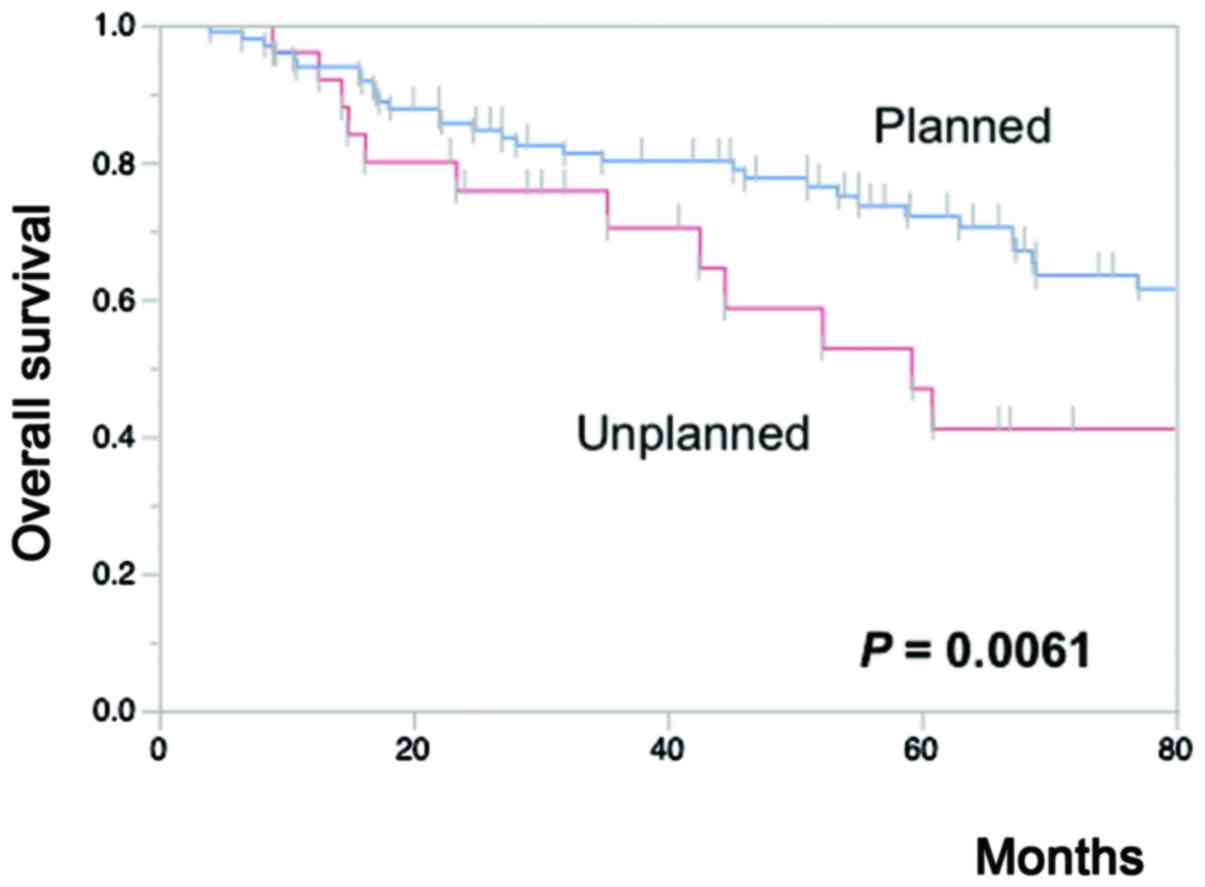
multivariate models. The 5-year OS in the CRS (73%) and the
N-CRS-ope arms (70%) was significantly better (P=0.0193) than that
(47%) in the N-CRS-refu arm. In other words, unplanned-treatment
(i.e., NCR-refu) was associated with significantly (P=0.0061) worse
5-year OS (47%) as compared to that associated with
planned-treatment (i.e., CRS+N-CRS-ope arms, 72%) (Fig. 3). In contrast, the Kaplan-Meier
curves produced by T (T1, 2 vs. T3), N (N0, 1 vs. N2, 3), and
primary site (larynx vs. hypopharynx) did not display significant
differences. This tendency was also confirmed in the Cox
proportional hazard model, in which only unplanned treatment (i.e.,
N-CRS-refu arm) showed a significant correlation with poor OS (HR:
2.583, 95% CI: 1.313–4.354, P=0.007) (Table II). We then additionally analyzed
the hazard ratios of the 4 factors for OS according to the two
different stratification criteria: Patients who received 70 Gy of
CRT without initial laryngectomy (CRS + N-CRS-refu) and those who
were N-CRS (N-CRS-ope + N-CRS-refu). In the former cohort,
unplanned treatment (HR: 3.137, 95% CI: 1.458–6.661, P=0.0039) and
hypopharyngeal tumor (HR: 2.458, 95% CI 1.137–5.392, P=0.0223) were
associated with significantly increased risk of death. In the
latter cohort, only unplanned treatment was a significant risk
factor (HR: 7.638, 95% CI: 2.294–27.542, P=0.0008).
 | Table II.Hazard ratios (HR) using multivariate
Cox proportional hazard model. |
Table II.
Hazard ratios (HR) using multivariate
Cox proportional hazard model.
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|---|
| Class | Factor | HR | 95% CI | P-value |
|---|
| Treatment | Planned | Ref. |
|
|
|
| Unplanned | 2.583 | 1.313–4.854 | 0.007 |
| T stage | 1–2 | Ref. |
|
|
|
| 3 | 0.691 | 0.363–1.297 |
|
|
|
| 0.2518 |
|
|
| N stage | 0–1 | Ref. |
|
|
|
| 2–3 | 1.017 | 0.577-.1.816 |
|
|
|
| 0.9511 |
|
|
| Primary site | Larynx | Ref. |
|
|
|
| Hypopharynx | 0.584 | 0.306–1.097 |
|
|
|
| 0.0949 |
|
|
Laryngeal preservation
Sixteen out of 64 (25%) in the CRS arm developed
tumor recurrences. Fourteen of these underwent surgery including
total laryngectomy, of which 9 were salvaged (i.e., survived),
while 5 patients were died of disease unrelated to laryngeal or
hypopharyngeal cancer. Thus, the surgical salvage rate in patients
with recurrence was 64% (9/16). The N-CRS-refu arm experienced
significantly (P=0.0114, Fisher's exact test) higher recurrence
rate in the larynx and hypopharynx (56%, 14/25) as compared to that
in the CRS-group. Seven patients proceeded to surgery and 6 of
these were salvaged, while one patient died of other cancer; the
surgical salvage rate in this cohort was 43% (6/14). No
laryngo-esophageal dysfunction was observed in the survivors with
preserved larynx. Based on these data, the cumulative LEDF survival
rate of all patients were 41% at 5-years. The CRS arm demonstrated
significantly (P<0.0001) better 5-year (69%) LEDS than the
N-CRS-refu arm (3.6%). In the Kaplan-Meier curves produced by the
group (T, N, and primary site), the T classification alone had a
significant effect on LEDFS; T3 tumors were associated with
significantly (P=0.0012) lower LEDFS (25%) than T1 and T2 tumors
(53%).
Split CDDP vs. bolus CDDP
Although preliminary, we examined whether bolus CDDP
could potentate the efficacy of chemoradioselection over split
CDDP. The bolus CDDP group demonstrated higher rates of CRS (68%)
as compared to that in the split CDDP group (51%). The bolus group
also showed a tendency for higher 3-year OS and LEDFS (90 and 64%)
than split CDDP (76% and 45%). In T1 and T2 cases, bolus CDDP
improved the 5-year LEDFS from 61 to 80%. However, surprisingly,
the LEDFS of T3 in the bolus CDDP dropped sharply to 29% at 2-year
post-treatment, which is rather worse than that of overall T3 data
(38%). These findings indicate that bolus CDDP may contribute to
the improvement in overall survival and laryngeal preservation in
T1and T2, but not T3.
Discussion
Over the last decade, there has been a hot debate
about the superiority of CRT and ST as DIOPS for treatment of
advanced HNSCC (8,23,24).
However, a recent meta-analysis clearly demonstrated that induction
chemotherapy has no survival benefit over CRT in advanced HNSCC
(25) and therefore CRT is expected
to be the future mainstay of treatment. On the other hand, it is
obvious that current standard CRT with high-dose CDDP, in which 100
mg/m2 of CDDP is administered every three weeks, needs
to be optimized to reduce the severe toxicity, which is frequently
observed in both the acute and the long-term phase (11–13). In
this context, the quite favorable 5-year OS (73%) and 5-year LEDFS
(69%) observed in this CRS cohort in the present study appears to
offer one solution. Thus, for those who show good response to
induction CRT, total 150 (split regimen) or 160 (bolus regimen)
mg/m2 of CDDP may be sufficient to achieve favorable
survival without impairment of laryngeal function. Interestingly
these CDDP doses were considerably lower than the 200
mg/m2 dose recommended for maintenance of the
oncological results in the standard tri-week CDDP or in the
recently proposed weekly (40 mg/m2) CDDP protocol
(26–28) and therefore more applicable to the
elderly patients and/or patients with poor general condition. This
result suggests that a majority of the CRS tumors are composed of
less aggressive tumors which are amenable to cure with the moderate
intensity CRT alone; this inference is also supported by the
significantly lower rates of tumor-related death in the CRS arm as
compared to that in the N-CRS arm. Thus, chemoradioselection
provides an accurate measure of the biological aggressiveness of
each tumor, and may be a useful strategy for optimization of the
intensity of CRT. This method is comparable with chemoselection,
which entails one or a few cycles of induction chemotherapy as a
tool to select patients for organ preservation (20,21,29).
However, given the conclusions of the above-mentioned meta-analysis
(25) and the time- and
cost-effectiveness, chemoradioselection is thought to be a better
method than chemoselection.
In this study, the premise of VALCSG and EORTC
(1,2), i.e., DIOPS is a better option than
radical surgery in patients with advanced laryngeal and
hypopharyngeal cancer, because of better quality of life and
comparable survival rates, was questioned. The N-CRS-ope showed
markedly better 5-year OS (70%) at the expense of the larynx, which
otherwise remained at 47% as shown by the N-CRS-ref, which
indicates that under selected conditions, radical surgery confers
survival benefit over CRT. Because similar rates of distant
metastases were observed in these two populations, the rates of
loco-regional failure appear to be the main cause of this
difference; the N-CRS-refu demonstrated as high as the 32% of
loco-regional failure, which probably caused by the high local
recurrence (56%) and poor salvage (43%), whereas in the N-CRS-ope
arm the incidence of loco-regional failure was only 9%.
Interestingly, only refusal of surgery (and not T, N, and primary
site) was an independent predictor of adverse prognosis in the
N-CRS population, as was clearly demonstrated in our multivariate
analysis. This result implies that independent of the tumor stage
and site, chemoradioselection can accurately segregate patients who
would be better treated with curative-intent surgery at a
relatively early phase of treatment. Moreover, in a similar
analysis of the overall population, the refusal of surgery alone
correlated with increased risks of death, which validates our
algorithm-based treatment decision (i.e., organ preservation vs.
surgery). In the cohort of patients who were initially treated with
CRT alone (i.e., the CRS plus CRS-ref), hypopharyngeal cancer and
refusal of surgery was associated with poor prognosis. The former
result confirms a general fact that when treated with similar
modalities hypopharyngeal cancer shows poorer prognosis than
laryngeal cancer. Whereas the latter finding indicates that
irrespective of the tumor stage and site, continuation of CRT for
poor responders eventually culminates in poor survival. Given that
this similar phenomenon has long been recognized in the field of ST
and has been used as the logical basis for chemoselection (20,21,29), it
is quite puzzling that CRT strategy has stuck to the policy of
definitive CRT and salvage surgery without adequate attention to
the biological selection so far. Collectively, our results suggest
that poor responders should be treated by radical surgery with
curative-intent at a relatively early phase of CRT rather than by
definitive CRT, which increases the risk of the local failure and
eventually compromises survival.
In this algorithm-based strategy, improvement of the
overall LEDFS (41%), which is mainly worsened by poor results of T3
tumors (25%), is an essential issue to be addressed. We altered the
regimen from split to bolus CDDP, with the expectation that a
substantial increase in the rate of chemoradioselection may lead to
the improved results including an increase in LEDFS in T3 cases.
Our preliminary data suggests that this alteration succeeded in
increasing the rate of chemoradioselection and improved overall OS
and LEDFS. However, in T3 cases the improvement in LEDFS was not
observed. These results indicate the superiority of bolus CDDP as
well as its limitation for laryngeal preservation, particularly in
advanced T. It is obvious that further dose-intensification
inevitably leads to the enhanced toxicity and tends to diminish the
utility of chemoradioselection. Thus, addition of a
molecular-targeted approach to our strategy seems to be a rational
method to address this problem. Recently we found that induction of
a putative cancer stem cell marker, CD44 variant 9 (CD44v9), was a
major hurdle to chemoradioselection. Thus, addition of CD44v9
targeting (e.g., sulfasalazine) to chemoradioselection may be a
promising approach to enhance the efficacy of chemoradioselection
and thereby improve survival and laryngeal preservation (30).
In conclusion, our algorithm-based
‘chemoradioselection’ might provide a novel platform for the
treatment of advanced HNSCC and help optimize the currently
employed excessive treatment intensity. We have started a
multi-institutional prospective study to verify the utility of this
protocol on a larger scale.
Acknowledgement
The present study was supported in part by fund from
Grants-in-Aid for Scientific Research (C): 24592600 to Muneyuki
Masuda.
References
|
1
|
Department of Veterans Affairs Laryngeal
Cancer Study Group, . Wolf GT, Fisher SG, Hong WK, Hillman R,
Spaulding M, Laramore GE, Endicott JW, McClatchey K and Henderson
WG: Induction chemotherapy plus radiation compared with surgery
plus radiation in patients with advanced laryngeal cancer. N Engl J
Med. 324:1685–1690. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lefebvre JL, Chevalier D, Luboinski B,
Kirkpatrick A, Collette L and Sahmoud T: Larynx preservation in
pyriform sinus cancer: Preliminary results of a European
organization for research and treatment of cancer phase III trial.
EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst.
88:890–899. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Posner MR, Hershock DM, Blajman CR,
Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM,
Cullen K, Ervin TJ, et al: Cisplatin and fluorouracil alone or with
docetaxel in head and neck cancer. N Engl J Med. 357:1705–1715.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: Cisplatin, fluorouracil, and docetaxel in unresectable
head and neck cancer. N Engl J Med. 357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adelstein DJ, Saxton JP, Rybicki LA,
Esclamado RM, Wood BG, Strome M, Lavertu P, Lorenz RR and Carroll
MA: Multiagent concurrent chemoradiotherapy for locoregionally
advanced squamous cell head and neck cancer: Mature results from a
single institution. J Clin Oncol. 24:1064–1071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanna GJ, Haddad RI and Lorch JH:
Induction chemotherapy for locoregionally advanced head and neck
cancer: Past, present, future? Oncologist. 18:288–293. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haddad R, O'Neill A, Rabinowits G, Tishler
R, Khuri F, Adkins D, Clark J, Sarlis N, Lorch J, Beitler JJ, et
al: Induction chemotherapy followed by concurrent chemoradiotherapy
(sequential chemoradiotherapy) versus concurrent chemoradiotherapy
alone in locally advanced head and neck cancer (PARADIGM): A
randomised phase 3 trial. Lancet Oncol. 14:257–264. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoffman HT, Porter K, Karnell LH, Cooper
JS, Weber RS, Langer CJ, Ang KK, Gay G, Stewart A and Robinson RA:
Laryngeal cancer in the United States: Changes in demographics,
patterns of care, and survival. Laryngoscope. 116 9 Pt 2 Suppl
111:S1–S13. 2006. View Article : Google Scholar
|
|
10
|
Newman JR, Connolly TM, Illing EA, Kilgore
ML, Locher JL and Carroll WR: Survival trends in hypopharyngeal
cancer: A population-based review. Laryngoscope. 125:624–629. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Machtay M, Moughan J, Trotti A, Garden AS,
Weber RS, Cooper JS, Forastiere A and Ang KK: Factors associated
with severe late toxicity after concurrent chemoradiation for
locally advanced head and neck cancer: An RTOG analysis. J Clin
Oncol. 26:3582–3589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Argiris A, Brockstein BE, Haraf DJ,
Stenson KM, Mittal BB, Kies MS, Rosen FR, Jovanovic B and Vokes EE:
Competing causes of death and second primary tumors in patients
with locoregionally advanced head and neck cancer treated with
chemoradiotherapy. Clin Cancer Res. 10:1956–1962. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Corry J, Peters LJ and Rischin D:
Optimising the therapeutic ratio in head and neck cancer. Lancet
Oncol. 11:287–291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumamoto Y, Masuda M, Kuratomi Y, Toh S,
Shinokuma A, Chujo K, Yamamoto T and Komiyama S: ‘FAR’
chemoradiotherapy improves laryngeal preservation rates in patients
with T2N0 glottic carcinoma. Head Neck. 24:637–642. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Masuda M, Kamizono K, Uryu H, Fujimura A
and Uchi R: Roles of therapeutic selective neck dissection in
multidisciplinary treatment. Journal, 2012Neck Dissection -
Clinical Application and Recent Advances. In Tech; Croatia: pp.
49–60. 2012
|
|
16
|
Hirata K, Horikoshi N, Aiba K, Okazaki M,
Denno R, Sasaki K, Nakano Y, Ishizuka H, Yamada Y, Uno S, et al:
Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor
drug. Clin Cancer Res. 5:2000–2005. 1999.PubMed/NCBI
|
|
17
|
Nonoshita T, Shioyama Y, Nakamura K,
Nakashima T, Ohga S, Yoshitake T, Ohnishi K, Terashima K and Asai
K: Concurrent chemoradiotherapy with S-1 for T2N0 glottic squamous
cell carcinoma. J Radiat Res. 51:481–484. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohnishi K, Shioyama Y, Nakamura K,
Nakashima T, Ohga S, Nonoshita T, Yoshitake T, Terashima K, Komune
S and Honda H: Concurrent chemoradiotherapy with S-1 as first-line
treatment for patients with oropharyngeal cancer. J Radiat Res.
52:47–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Masuda M, Matsuo M, Aso T, Kiyohara H,
Rikimaru F, Kunitake N and Higaki Y: Utility of algorithm-based
chemoradioselection in the treatment for advanced hypopharyngeal
carcinoma. Head Neck. 37:1290–1296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Strojan P, Haigentz M Jr, Bradford CR,
Wolf GT, Hartl DM, Langendijk JA, Rinaldo A, Eisbruch A, Mendenhall
WM, Forastiere AA, et al: Chemoradiotherapy vs. total laryngectomy
for primary treatment of advanced laryngeal squamous cell
carcinoma. Oral Oncol. 49:283–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Forastiere AA, Weber RS and Trotti A:
Organ preservation for advanced larynx cancer: Issues and outcomes.
J Clin Oncol. 33:3262–3268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lefebvre JL and Ang KK: Larynx
Preservation Consensus Panel: Larynx preservation clinical trial
design: Key issues and recommendations-a consensus panel summary.
Int J Radiat Oncol Biol Phys. 73:1293–1303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cohen EE, Karrison TG, Kocherginsky M,
Huang CH, Agulnik M, Mittal BB, Yunus F, Samant S, Brockstein B,
Raez LE, et al: DeCIDE: A phase III randomized trial of docetaxel
(D), cisplatin (P), 5-fluorouracil (F) (TPF) induction chemotherapy
(IC) in patients with N2/N3 locally advanced squamous cell
carcinoma of the head and neck (SCCHN). J Clin Oncol. 30
suppl:S55002012.
|
|
24
|
Hitt R, Grau JJ, Lopez-Pousa A,
López-Pousa A, Berrocal A, García-Girón C, Irigoyen A, Sastre J,
Martínez-Trufero J, Castelo Brandariz JA, et al: A randomized phase
III trial comparing induction chemotherapy followed by
chemoradiotherapy versus chemoradiotherapy alone as treatment of
unresectable head and neck cancer. Ann Oncol. 25:216–225. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blanchard P, Landais C, Petit C, et al:
Meta-analysis of chemotherapy in head and neck cencer (MACH-NC): an
update on 100 randamized traials and 19,248 patients, on behalf of
MANH-HN group. ESMO Meeting abstract. 950O:2016
|
|
26
|
Ang KK: Concurrent radiation chemotherapy
for locally advanced head and neck carcinoma: Are we addressing
burning subjects? J Clin Oncol. 22:4657–4659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Melotek JM, Cooper BT, Koshy M, Silverman
JS and Spiotto MT: Weekly versus every-three-weeks platinum-based
chemoradiation regimens for head and neck cancer. J Otolaryngol
Head Neck Surg. 45:622016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ho KF, Swindell R and Brammer CV: Dose
intensity comparison between weekly and 3-weekly Cisplatin
delivered concurrently with radical radiotherapy for head and neck
cancer: A retrospective comparison from New Cross Hospital,
Wolverhampton, UK. Acta Oncol. 47:1513–1518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Urba S, Wolf G, Eisbruch A, Worden F, Lee
J, Bradford C, Teknos T, Chepeha D, Prince M, Hogikyan N and Taylor
J: Single-cycle induction chemotherapy selects patients with
advanced laryngeal cancer for combined chemoradiation: A new
treatment paradigm. J Clin Oncol. 24:593–598. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aso T, Matsuo M, Kiyohara H, Taguchi K,
Rikimaru F, Shimokawa M, Segawa Y, Higaki Y, Umeno H, Nakashima T
and Masuda M: Induction of CD44 variant 9-expressing cancer stem
cells might attenuate the efficacy of chemoradioselection and
Worsens the prognosis of patients with advanced head and neck
cancer. PLoS One. 10:e01165962015. View Article : Google Scholar : PubMed/NCBI
|