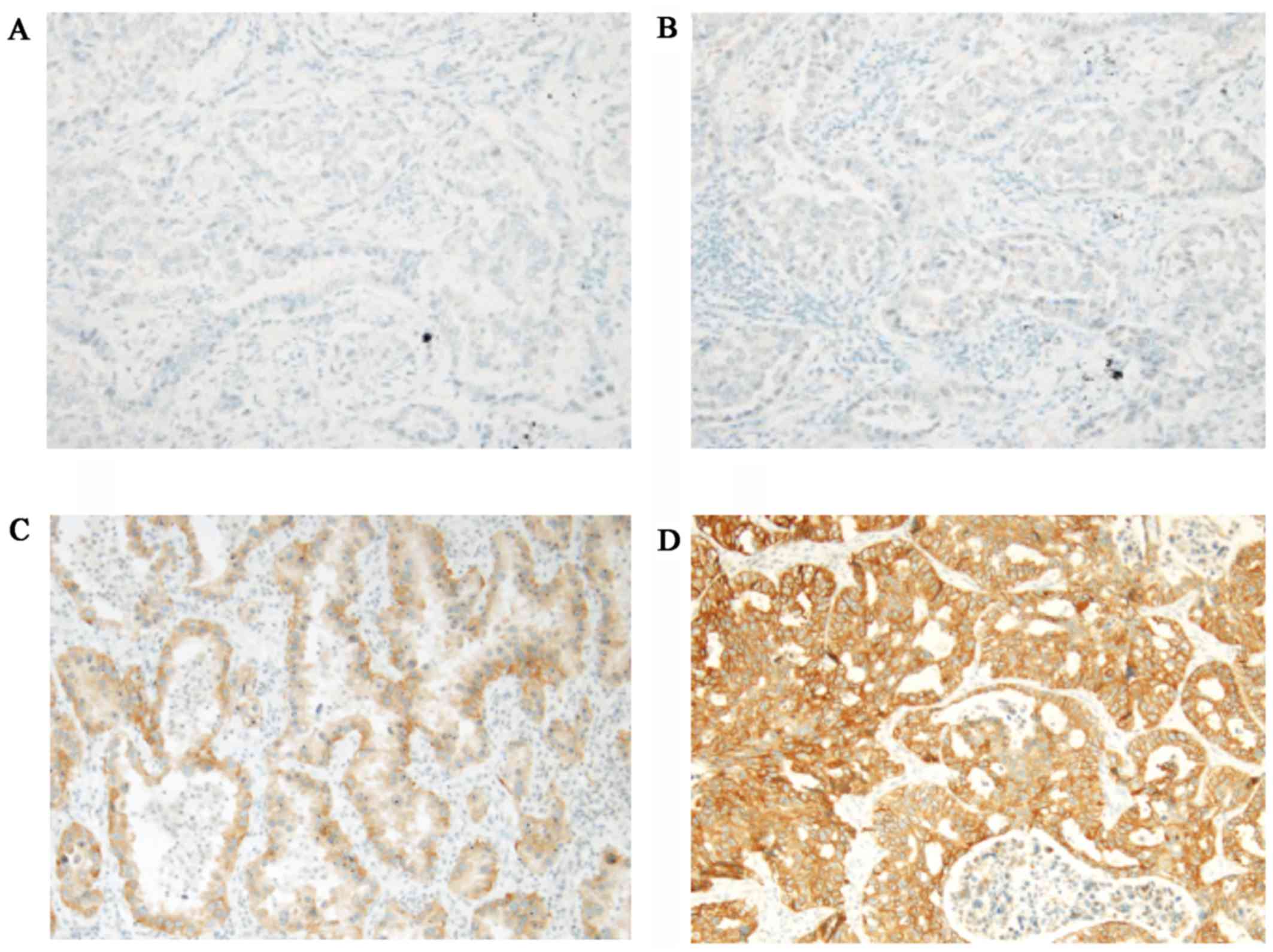
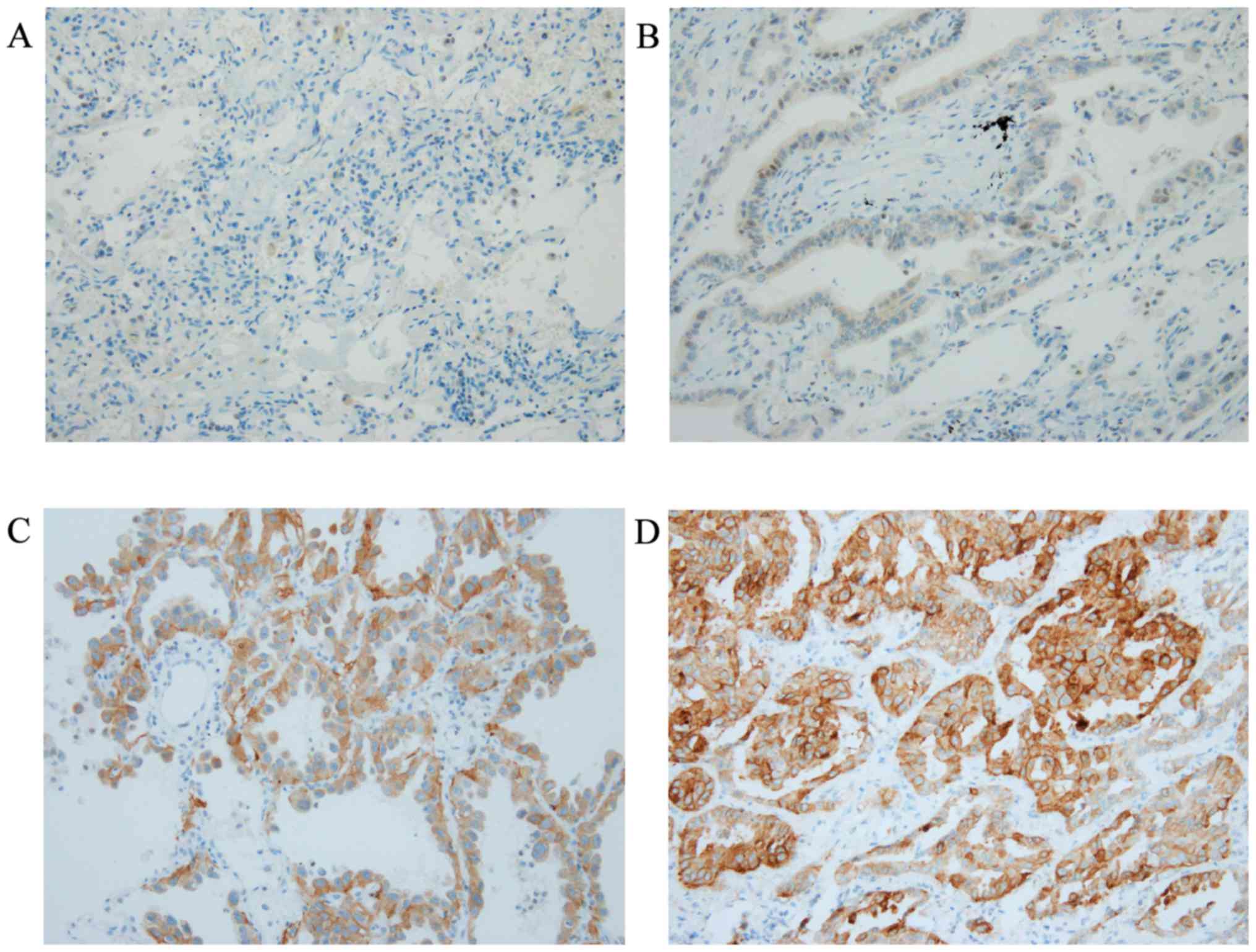
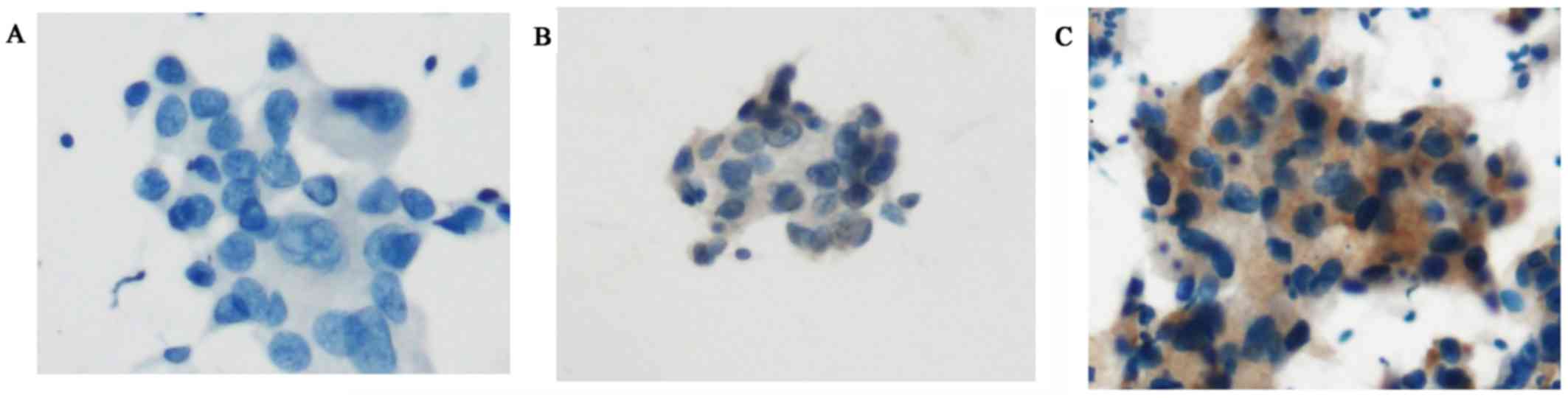
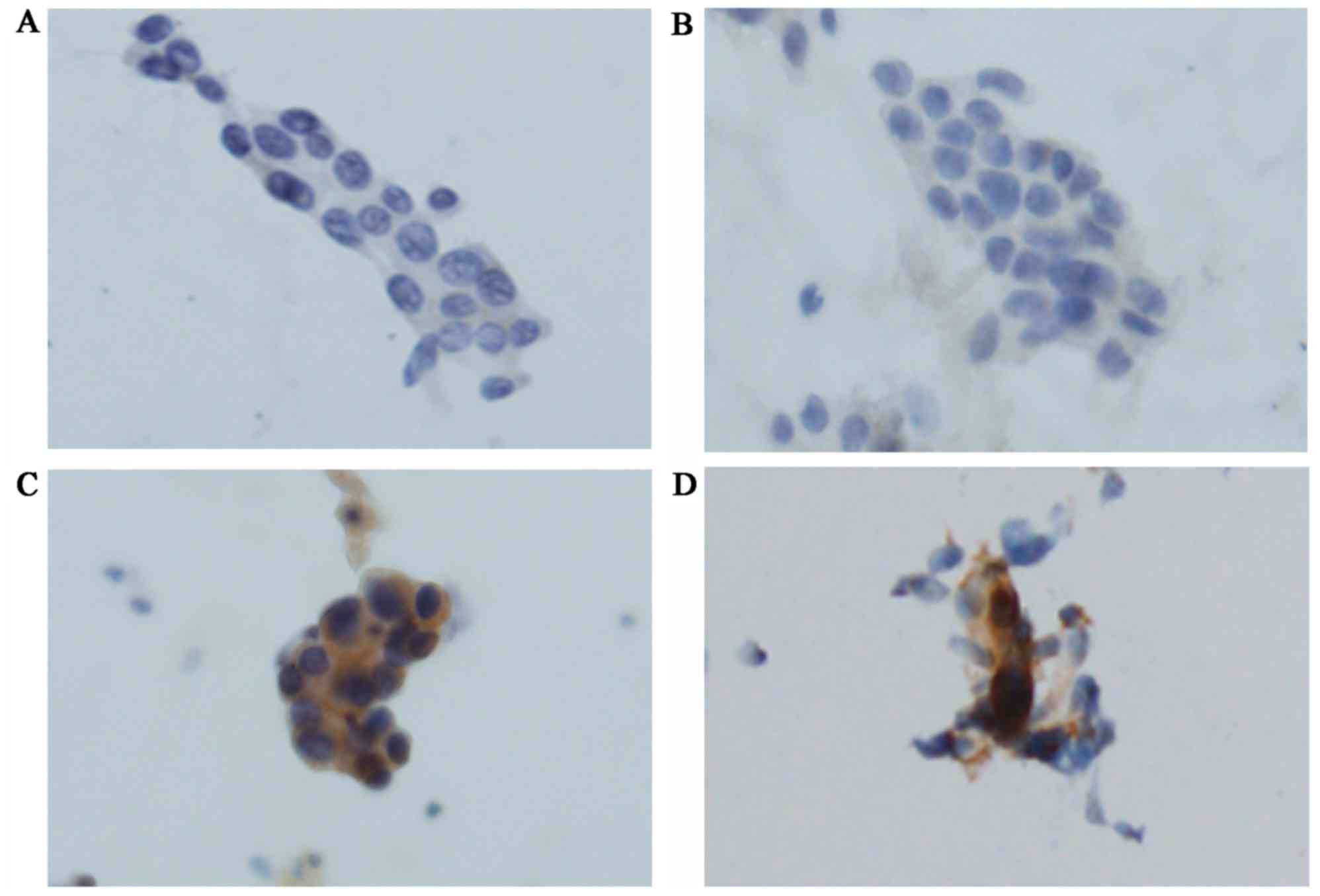
|
1
|
Fact Sheet WHO: 297, . https://www.who.inta. WHO Fact Sheet 297. November
8–2016
|
|
2
|
Travis WD, Rekhtman N, Riley GJ, Geisinger
KR, Asamura H, Brambilla E, Garg K, Hirsch FR, Noguchi M, Powell
CA, et al: Pathologic diagnosis of advanced lung cancer based on
small biopsies and cytology: A paradigm shift. J Thorac Oncol.
5:411–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al:
EGF receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:pp. 13306–13311. 2004,
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitsudomi T and Yatabe Y: Mutations of the
epidermal growth factor receptor gene and related genes as
determinants of epidermal growth factor receptor tyrosine kinase
inhibitors sensitivity in lung cancer. Cancer Sci. 98:1817–1824.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pao W and Ladanyi M: Epidermal growth
factor receptor mutation testing in lung cancer: Searching for the
ideal method. Clin Cancer Res. 13:4954–4955. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ellison G, Zhu G, Moulis A, Dearden S,
Speake G and McCormack R: EGFR mutation testing in lung cancer: A
review of available methods and their use for analysis of tumour
tissue and cytology samples. J Clin Pathol. 66:79–89. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu J, Kane S, Wu J, Benedettini E, Li D,
Reeves C, Innocenti G, Wetzel R, Crosby K, Becker A, et al:
Mutation-specific antibodies for the detection of EGFR mutations in
non-small-cell lung cancer. Clin Cancer Res. 15:3023–3028. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brevet M, Arcila M and Ladanyi M:
Assessment of EGFR mutation status in lung adenocarcinoma by
immunohistochemistry using antibodies specific to the two major
forms of mutant EGFR. J Mol Diagn. 12:169–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kato Y, Peled N, Wynes MW, Yoshida K,
Pardo M, Mascaux C, Ohira T, Tsuboi M, Matsubayashi J, Nagao T, et
al: Novel epidermal growth factor receptor mutation-specific
antibodies for non-small cell lung cancer: Immunohistochemistry as
a possible screening method for epidermal growth factor receptor
mutations. J Thorac Oncol. 5:1551–1558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawahara A, Yamamoto C, Nakashima K, Azuma
K, Hattori S, Kashihara M, Aizawa H, Basaki Y, Kuwano M, Kage M, et
al: Molecular diagnosis of activating EGFR mutations in non-small
cell lung cancer using mutation-specific antibodies for
immunohistochemical analysis. Clin Cancer Res. 16:3163–3170. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kitamura A, Hosoda W, Sasaki E, Mitsudomi
T and Yatabe Y: Immunohistochemical detection of EGFR mutation
using mutation-specific antibodies in lung cancer. Clin Cancer Res.
16:3349–3355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamura H, Mochizuki A, Shinmyo T, Ando
K, Kurimoto N, Yokote K and Takagi M: Immunohistochemical detection
of mutated epidermal growth factor receptors in pulmonary
adenocarcinoma. Anticancer Res. 30:5233–5237. 2010.PubMed/NCBI
|
|
17
|
Kozu Y, Tsuta K, Kohno T, Sekine I,
Yoshida A, Watanabe S, Tamura T, Yokota J, Suzuki K, Asamura H, et
al: The usefulness of mutation-specific antibodies in detecting
epidermal growth factor receptor mutations and in predicting
response to tyrosine kinase inhibitor therapy in lung
adenocarcinoma. Lung Cancer. 73:45–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang G, Fan C, Zhang X, Dong Q, Wang L,
Liu Y, Dai S, Yang L, Zhang Y, Yu J and Wang E: Ascertaining an
appropriate diagnostic algorithm using EGFR mutation-specific
antibodies to detect EGFR status in non-small-cell lung cancer.
PLoS One. 8:e591832013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Simonetti S, Molina MA, Queralt C, de
Aguirre I, Mayo C, Bertran-Alamillo J, Sanchez JJ, Gonzalez-Larriba
JL, Jimenez U, Isla D, et al: Detection of EGFR mutations with
mutation-specific antibodies in stage IV non-small-cell lung
cancer. J Transl Med. 8:1352010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiong Y, Bai Y, Leong N, Laughlin TS,
Rothberg PG, Xu H, Nong L, Zhao J, Dong Y and Li T:
Immunohistochemical detection of mutations in the epidermal growth
factor receptor gene in lung adenocarcinomas using
mutation-specific antibodies. Diagn Pathol. 8:272013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Z, Liu HB, Yu CH, Wang Y, Wang L and
Song Y: Diagnostic value of mutation-specific antibodies for
immunohistochemical detection of epidermal growth factor receptor
mutations in non-small cell lung cancer: A meta-analysis. PLoS One.
9:e1059402014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang T, Nelson RA, Bogardus A and Grannis
FW Jr: Five-year lung cancer survival: Which advanced stage
nonsmall cell lung cancer patients attain long-term survival?
Cancer. 116:1518–1525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Molina JR, Adjei AA and Jett JR: Advances
in chemotherapy of non-small cell lung cancer. Chest.
130:1211–1219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawahara A, Azuma K, Sumi A, Taira T,
Nakashima K, Aikawa E, Abe H, Yamaguchi T, Takamori S, Akiba J and
Kage M: Identification of non-small-cell lung cancer with
activating EGFR mutations in malignant effusion and cerebrospinal
fluid: Rapid and sensitive detection of exon 19 deletion E746-A750
and exon 21 L858R mutation by immunocytochemistry. Lung Cancer.
74:35–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsai TH, Wu SG, Chang YL, Wu CT, Tsai MF,
Wei PF, Yang CH, Yu CJ, Yang PC and Shih JY: Effusion
immunocytochemistry as an alternative approach for the selection of
first-line targeted therapy in advanced lung adenocarcinoma. J
Thorac Oncol. 7:993–1000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawahara A, Taira T, Azuma K, Tominaga M,
Hattori S, Kawahara M, Abe H, Yamaguchi T, Akiba J, Takamori S, et
al: A diagnostic algorithm using EGFR mutation-specific antibodies
for rapid response EGFR-TKI treatment in patients with non-small
cell lung cancer. Lung Cancer. 78:39–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Allo G, Bandarchi B, Yanagawa N, Wang A,
Shih W, Xu J, Dalby M, Nitta H, To C, Liu N, et al: Epidermal
growth factor receptor mutation-specific immunohistochemical
antibodies in lung adenocarcinoma. Histopathology. 64:826–839.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seo AN, Park TI, Jin Y, Sun PL, Kim H,
Chang H and Chung JH: Novel EGFR mutation-specific antibodies for
lung adenocarcinoma: Highly specific but not sensitive detection of
an E746_A750 deletion in exon 19 and an L858R mutation in exon 21
by immunohistochemistry. Lung Cancer. 83:316–323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim CH, Kim SH, Park SY, Yoo J, Kim SK and
Kim HK: Identification of EGFR mutations by immunohistochemistry
with EGFR mutation-specific antibodies in biopsy and resection
specimens from pulmonary adenocarcinoma. Cancer Res Treat.
47:653–660. 2015. View Article : Google Scholar : PubMed/NCBI
|