Introduction
In most prostate cancer cases, androgen ablation is
effective initially because the prostate cancer depends on
androgens for growth (1). However,
some of the cancers eventually recur, at which point they are
termed ‘castration-resistant prostate cancer (CRPC)’ and can no
longer be treated by conventional treatment (1). The mechanisms underlying this
resistance were thought to be androgen receptor amplification,
gain-of-function mutation, and novel splice variant expression
(2). However, recent studies have
shown that CRPC development still depends on the intratumoral
generation of the potent androgen, dihydrotestosterone (DHT)
(3–5). Moreover, the androgen levels in the
prostate tissue of CRPC patients are comparable with levels in
healthy men (6–8), thus accounting for the continued
expression of androgen-dependent genes after androgen ablation
(9). These findings are consistent
with the reportedly low number of complete clinical responses
observed in a trial of neoadjuvant androgen deprivation therapy
(10). However, the effectiveness of
some next-generation, androgen-modulating drugs (enzalutamide and
abiraterone acetate) has been reported (11–13).
Circulating testosterone is converted into DHT by
5α-reductase (SRD5A). Three 5α-reductase isoforms (SRD5A types I,
II, and III) have been detected in the prostate (14). In normal prostate tissue, DHT was
produced mainly by SRD5A. SRD5A type I is reportedly overexpressed
in the primary and metastatic sites of CRPC patients and bypasses
testosterone, using instead androstenedione as a substrate to
produce 5α-androstanedione, which is then converted to DHT
(15). SRD5A type I is therefore
thought to play a critical role in the progression of prostate
cancer.
SRD5A inhibitors can inhibit the conversion of
testosterone to DHT. Finasteride can selectively inhibit SRD5A type
II to decrease the incidence of prostate cancer but lacks clear
activity against CRPC. Dutasteride is a dual inhibitor of SRD5A
types I and II and is reportedly effective in the treatment of
benign prostatic hyperplasia and preventing prostate cancer
(16,17). However, its efficacy against CRPC is
still unclear (18). In the present
study, we assessed the efficacy of dutasteride against CRPC.
Patients and methods
Patients
Between 2010 and 2013, 41 patients at the Tokyo
Metropolitan Tama Medical Center received the diagnosis of CRPC,
which progressed despite the administration of luteinizing
hormone-releasing hormone agonists, androgen receptor antagonist
(flutamide or bicalutamide), or an orchiectomy.
The institutional review board of the Tokyo
Metropolitan Tama Medical Center approved the study protocol. Each
patient provided written informed consent.
Treatment
Dutasteride 0.5 mg/day was given as additional
treatment until the disease reached a progressive state.
Luteinizing hormone-releasing hormone agonists and dexamethasone
were continued after dutasteride administration. A partial response
was defined as ≥2 PSA values obtained at least four weeks apart
exceeding 50% of the baseline value with no evidence of disease
progression on imaging. A progressive disease was defined as a 25%
increase in the serum PSA level over the last pre-registration
measurement confirmable at least four weeks later. In patients
showing a decrease in serum PSA levels during dutasteride
administration, a progressive disease was defined as a confirmed
increase of 25% to ≥5 ng/ml over the nadir (19).
Statistical analysis
The distribution of the recurrence-free survival
(RFS) rate was constructed using the Kaplan-Meier method.
Univariate and multivariable logistic regression were performed to
assess clinicopathologic characteristics associated with PSA
response. Statistical analyses were performed using the
JMP® software package. P<0.05 was considered
statistically significant.
Results
Patient characteristics
The patient and disease characteristics are shown in
Table I. The median age of the
patients was 77.3 years (range, 63–90). Bone metastases were
recognized in 12 patients. All patients received dexamethasone. Of
24 (59%) patients who had previously undergone chemotherapy, 11
(27%) patients had received docetaxel, and 24 (59%) had received
estramustine. The median follow-up period was 4.8 months with a
range of 1.5–16.2 months.
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
| Variables | Average range |
|---|
| Age, years | 77.3 |
|
| 63–90 |
| PSA at initiation of
dutasteride, ng/ml | 197.3 |
|
| 17–1,297 |
|
| Variables | No. of patients
(%) |
|
| Gleason score at
diagnosis |
|
| 4–6 | 1 (2) |
| 7 | 12 (30) |
| 8–9 | 21 (51) |
|
Unknown | 7 (17) |
| Prior treatment |
|
| Orchiectomy | 12 (29) |
| LHRH agonist | 29 (71) |
| Bicalutamide | 41 (100) |
| Flutamide | 41 (100) |
| Dexamethasone | 41 (100) |
| Estramustine | 24 (59) |
| Docetaxel | 11 (27) |
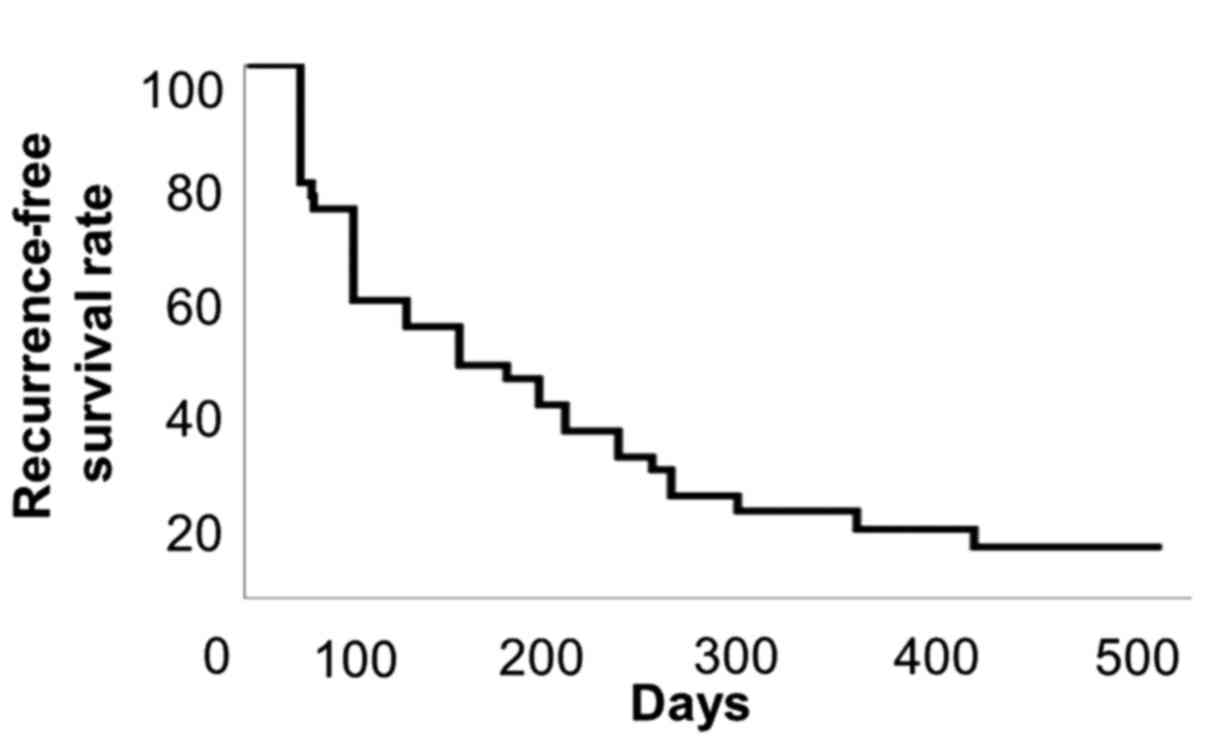
The median progression-free survival was 3.7 months
(Fig. 1). No significant adverse
events were encountered.
PSA kinetics and maximal change
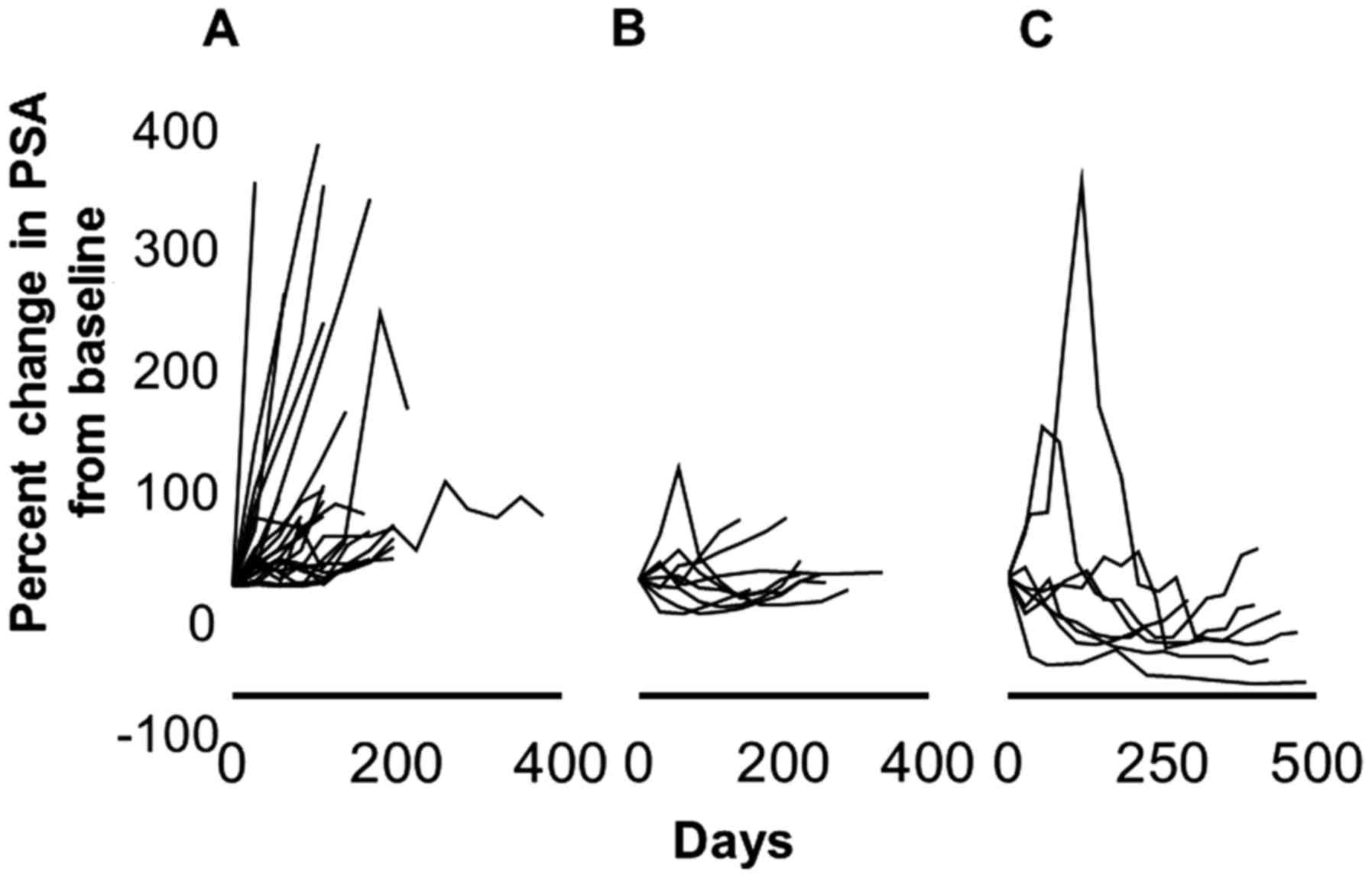
Fig. 2 shows the PSA
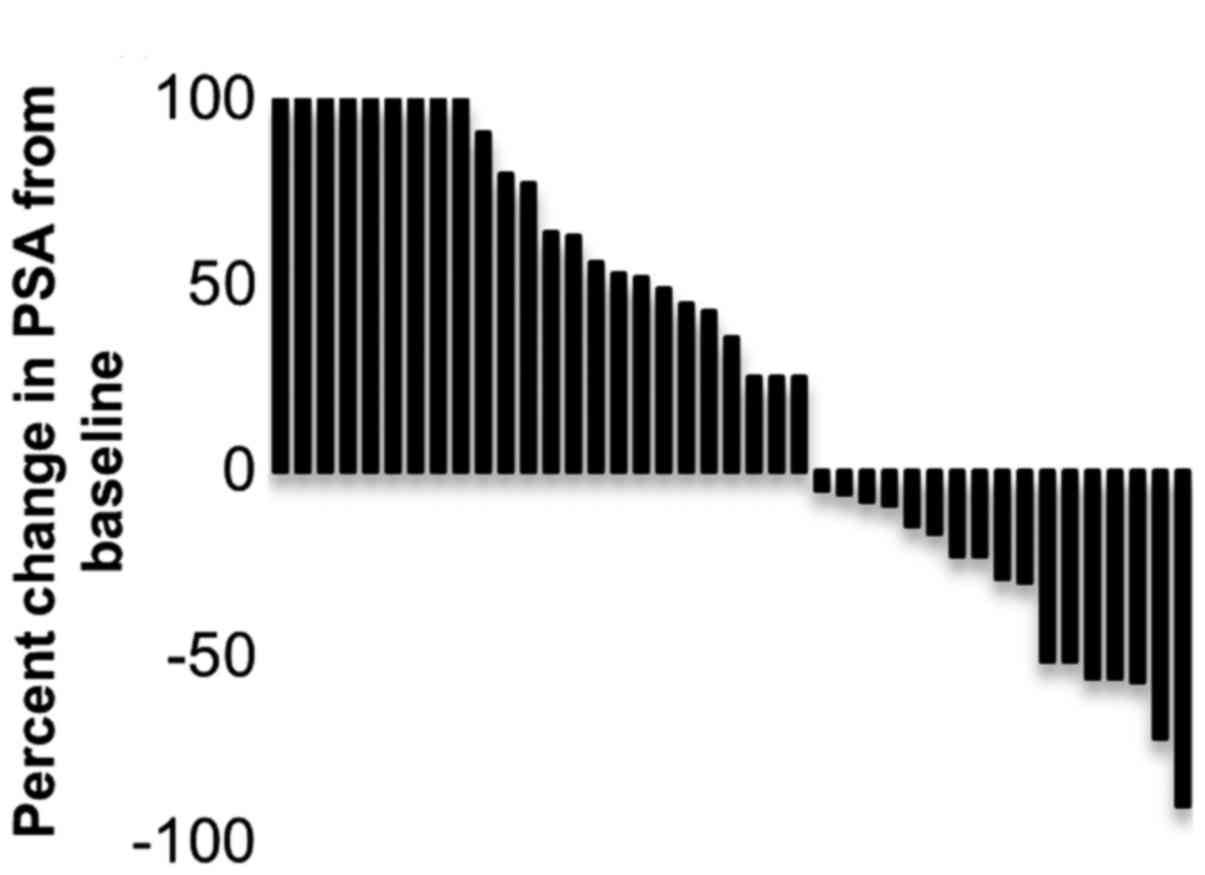
kinetics during the administration of dutasteride. Fig. 3 shows the maximum decrease in PSA
from the baseline for each patient. Seventeen (41%) patients showed
a decline in their PSA level from the baseline due to the
administration of dutasteride. The median PSA decrease was 23%
(range, 4.3–89.8%), and the median duration of response was four
months (range, 1–10). The PSA response rate (defined as >50%
decline in PSA from baseline) was recognized in 7 (17%) patients.
The median duration of response was three months (range, 2–10). In
three cases, PSA initially increased during therapy but decreased
later to values below the baseline. A decrease in PSA was
recognized three months and six months after dutasteride
administration in one and six patients, respectively. No
significant adverse effect was recognized.
Univariate logistic regression analysis was used to
detect factors predicting PSA response (Table II). PSA at initiation of
dutastereide, N stage and M stage were statistically significant
predictors of PCa at biopsy (P≤0.05). In multivariate models, only
PSA at initiation of dutastereide was an independent predictor for
PSA resposnse (Table II).
 | Table II.Univariable and multivariable logistic
regression models to predict PSA response |
Table II.
Univariable and multivariable logistic
regression models to predict PSA response
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age | 1.12 | 0.58 |
|
|
|
| 0.92–3.87 |
|
|
|
| PSA at diagnosis | 0.88 | 0.072 |
|
|
|
| 0.69–2.81 |
|
|
|
| PSA at initiation of
dutasteride | 0.75 | 0.039 | 0.67 | 0.041 |
|
| 0.29–0.85 |
| 0.13–0.79 |
|
| Time to CRPC | 2.12 | 0.71 |
|
|
|
| 0.81–3.01 |
|
|
|
| T stage | 0.84 | 0.21 |
|
|
| T1-2 vs
T3-4 | 0.62–2.12 |
|
|
|
| N stage | 0.72 | 0.042 | 1.92 | 0.31 |
| N0 vs
N1 | 0.52–0.91 |
| 0.67–2.31 |
|
| M stage | 0.62 |
|
|
|
| M0 vs
M1 | 0.23–0.91 | 0.028 | 0.94 | 0.11 |
|
|
|
| 0.31–1.91 |
|
Table III shows the
PSA response rates in prior treatments. PSA decreased in three
patients after treatment with docetaxel (27%). However, with ≤50%
decrease in PSA, the response rate was lower than in patients who
did not receive docetaxel.
 | Table III.Response rate according to previous
treatment. |
Table III.
Response rate according to previous
treatment.
| Variables | No. of patients
(%) |
|---|
| PSA response
rate |
|
| Without
chemotherapy (17) | 10 (59) |
| Any PSA decline from
baseline |
|
|
Estramustine (13) | 4
(31) |
|
Estramustine + Docetaxel
(11) | 3
(27) |
| >50% PSA decline
from baseline |
|
| Without
chemotherapy (17) | 5
(29) |
|
Estramustine (13) | 2
(15) |
|
Estramustine + Docetaxel
(11) | 0 (0) |
Discussion
Dutasteride is known to be effective in preventing
prostate cancer and treating low-risk prostate cancer. However, the
role of SRD5A inhibitors in the progression of prostate cancer to
CRPC has not been well studied (18). The development of CRPC is still
dependent on DHT, and some next-generation drugs targeting the
androgen signaling pathway were reportedly effective (11–13). We
therefore assessed the effects of dutasteride in patients with CRPC
by assessing changes in PSA levels.
The present study showed that 41% of patients with
progressive CRPC showed a decrease in their PSA level, with 17%
showing a decrease greater than 50%. The median duration of the PSA
response was four months.
The mainstay of treatment for CRPC patients is
chemotherapy. However, in some cases chemotherapy cannot be
performed due to performance status, age, adverse effects, or
complications. For these patients the only recourse has thus far
only been palliative care. Our results show that there may be
another treatment option available.
Notably, 8 (47%) of 17 patients exhibiting any
response showed an initial increase followed by a decrease in their
serum PSA level. The reason for the initial increase in PSA level
is unclear. One possibility is that dutasteride required several
months to produce its effects, a possibility made more likely by
the fact that the drug requires about six months to shrink benign
prostate hyperplasia. Another possibility is that dutasteride
treatment caused a surge in PSA like that seen in chemotherapy
(20). These results suggest that
dutasteride should be continued for at least three months, even
when the serum PSA level increases.
The present study has some limitations inherent in a
retrospective analysis. The prospective evaluation of the objective
response on imaging, the symptomatic response, and toxicity, was
not performed. It is possible that the impact of dutasteride on PSA
endpoints may not translate into a worthwhile therapeutic effect on
clinically meaningful endpoints. Prospective clinical studies are
required to test this possibility.
Of note, the PSA response was recognized in three
CRPC patients after chemotherapy using docetaxel. In two patients,
a PSA decrease from baseline continued for more than six months. A
study by Sartor did not include chemotherapy-resistant CRPC
patients (21). While such patients
do have some treatment options, controlling docetaxel-resistant
CRPC remains difficult. These findings suggested that dutasteride
may provide a promising option in the treatment of CRCP.
In summary, dutasteride was demonstrably effective
in some CRPC cases and may be a promising therapeutic option for
patients with CRPC. Prospective randomized trials are necessary to
confirm the efficacy of dutasteride.
References
|
1
|
Marech I, Vacca A, Ranieri G, Gnoni A and
Dammacco F: Novel strategies in the treatment of
castration-resistant prostate cancer (Review). Int J Oncol.
40:1313–1320. 2012.PubMed/NCBI
|
|
2
|
Nacusi LP and Tindall DJ: Targeting
5α-reductase for prostate cancer prevention and treatment. Nat Rev
Urol. 8:378–384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Locke JA, Guns ES, Lubik AA, Adomat HH,
Hendy SC, Wood CA, Ettinger SL, Gleave ME and Nelson CC: Androgen
levels increase by intratumoral de novo steroidogenesis during
progression of castration-resistant prostate cancer. Cancer Res.
68:6407–6415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Montgomery RB, Mostaghel EA, Vessella R,
Hess DL, Kalhorn TF, Higano CS, True LD and Nelson PS: Maintenance
of intratumoral androgens in metastatic prostate cancer: A
mechanism for castration-resistant tumor growth. Cancer Res.
68:4447–4454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stanbrough M, Bubley GJ, Ross K, Golub TR,
Rubin MA, Penning TM, Febbo PG and Balk SP: Increased expression of
genes converting adrenal androgens to testosterone in
androgen-independent prostate cancer. Cancer Res. 66:2815–2825.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geller J, Albert JD, Nachtsheim DA and
Loza D: Comparison of prostatic cancer tissue dihydrotestosterone
levels at the time of relapse following orchiectomy or estrogen
therapy. J Urol. 132:693–696. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohler JL, Gregory CW, Ford OH III, Kim D,
Weaver CM, Petrusz P, Wilson EM and French FS: The androgen axis in
recurrent prostate cancer. Clin Cancer Res. 10:440–448. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Titus MA, Schell MJ, Lih FB, Tomer KB and
Mohler JL: Testosterone and dihydrotestosterone tissue levels in
recurrent prostate cancer. Clin Cancer Res. 11:4653–4657. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mostaghel EA, Page ST, Lin DW, Fazli L,
Coleman IM, True LD, Knudsen B, Hess DL, Nelson CC, Matsumoto AM,
et al: Intraprostatic androgens and androgen-regulated gene
expression persist after testosterone suppression: Therapeutic
implications for castration-resistant prostate cancer. Cancer Res.
67:5033–5041. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gleave ME, La Bianca S and Goldenberg SL:
Neoadjuvant hormonal therapy prior to radical prostatectomy:
Promises and pitfalls. Prostate Cancer Prostatic Dis. 3:136–144.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ryan CJ, Smith MR, de Bono JS, Molina A,
Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng
S, et al: Abiraterone in metastatic prostate cancer without
previous chemotherapy. N Engl J Med. 368:138–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scher HI, Fizazi K, Saad F, Taplin ME,
Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et
al: Increased survival with enzalutamide in prostate cancer after
chemotherapy. N Engl J Med. 367:1187–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin L and Hu Q: CYP17
inhibitors-abiraterone, C17,20-lyase inhibitors and multi-targeting
agents. Nat Rev Urol. 11:32–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uemura M, Tamura K, Chung S, Honma S,
Okuyama A, Nakamura Y and Nakagawa H: Novel 5 alpha-steroid
reductase (SRD5A3, type-3) is overexpressed in hormone-refractory
prostate cancer. Cancer Sci. 99:81–86. 2008.PubMed/NCBI
|
|
15
|
Chang KH, Li R, Papari-Zareei M, Watumull
L, Zhao YD, Auchus RJ and Sharifi N: Dihydrotestosterone synthesis
bypasses testosterone to drive castration-resistant prostate
cancer. Proc Natl Acad Sci USA. 108:pp. 13728–13733. 2011;
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andriole GL, Bostwick DG, Brawley OW,
Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL,
Teloken C, Tindall DJ, et al: Effect of dutasteride on the risk of
prostate cancer. N Engl J Med. 362:1192–1202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barkin J, Guimaraes M, Jacobi G, Pushkar
D, Taylor S and van Vierssen Trip OB: Alpha-blocker therapy can be
withdrawn in the majority of men following initial combination
therapy with the dual 5alpha-reductase inhibitor dutasteride. Eur
Urol. 44:461–466. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tindall DJ and Rittmaster RS: The
rationale for inhibiting 5alpha-reductase isoenzymes in the
prevention and treatment of prostate cancer. J Urol. 179:1235–1242.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petrylak DP, Macarthur RB, O'Connor J,
Shelton G, Judge T, Balog J, Pfaff C, Bagiella E, Heitjan D, Fine
R, et al: Phase I trial of docetaxel with estramustine in
androgen-independent prostate cancer. J Clin Oncol. 17:958–967.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sella A, Sternberg CN, Skoneczna I and
Kovel S: Prostate-specific antigen flare phenomenon with
docetaxel-based chemotherapy in patients with androgen-independent
prostate cancer. BJU Int. 102:1607–1609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sartor O, Nakabayashi M, Taplin ME, Ross
RW, Kantoff PW, Balk SP and Oh WK: Activity of dutasteride plus
ketoconazole in castration-refractory prostate cancer after
progression on ketoconazole alone. Clin Genitourin Cancer.
7:E90–E92. 2009. View Article : Google Scholar : PubMed/NCBI
|