Introduction
Leiomyosarcomas are typically malignant soft tissue
tumors composed of spindle cells that exhibit distinct smooth
muscle differentiation. Leiomyosarcomas usually occur in the
uterus, retroperitoneum, skin, or soft tissues; however, in
exceedingly rare cases, they may present as primary bone tumors.
Bone leiomyosarcomatous tumors tend to develop at a median age of
47 years (range, 9–87 years) (1),
with a minor predominance in men. Patients typically complain of
pain and occasionally present with pathological fractures (1). Characteristic plain radiographs show an
osteolytic lesion involving the cortical and medullary bone
(2).
We herein present the case of a patient with bone
leiomyosarcoma displaying osteoclast-like giant cells and report
the significant therapeutic effect of denosumab, a
receptor-activator of nuclear κB ligand (RANKL) inhibitor that
blocks osteoclastogenesis.
Case report
A 64-year-old woman complaining of left shoulder
pain with resulting impairment in daily activities underwent
radiography and computed tomography. The imaging examinations
revealed an expanding lytic lesion of the left clavicle along with
multiple osteolytic lesions in other regions, including the skull,
right scapula, humeri, ribs, cervical and thoracic vertebra, right
ilium and femora. On tumor staging studies, there was no evidence
of pulmonary metastasis or other primary tumors. The patient became
increasingly bed-confined due to multifocal bone pain. An iliac
bone tumor biopsy revealed atypical spindle cell proliferation with
active mitosis (>20/10 high-power fields) and partial necrosis.
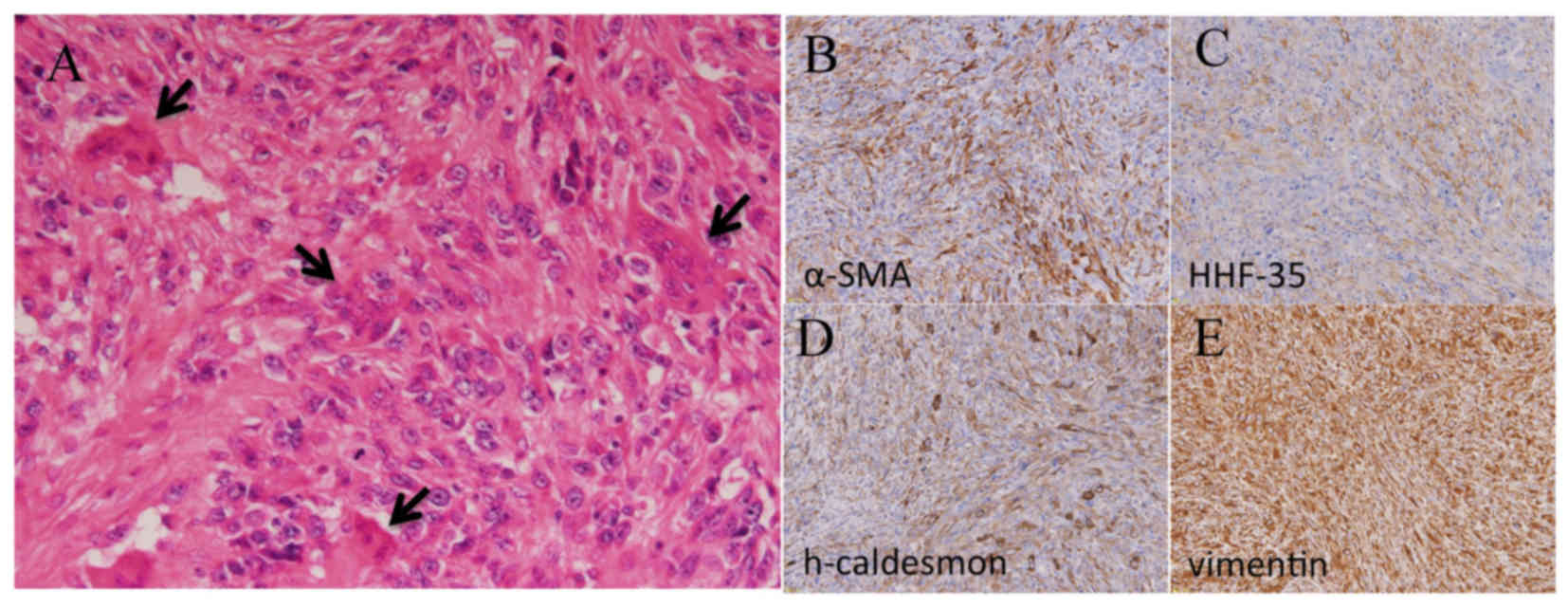
Osteoclast-like giant cells were occasionally observed (Fig. 1). Immunohistochemical analyses
revealed expression of α-smooth muscle actin (smooth
muscle-specific actin), h-caldesmon and vimentin (Fig. 1). These observations confirmed the
diagnosis of bone leiomyosarcoma.
Denosumab (120 mg/day) was administered
subcutaneously for 4 weeks, in addition to daily supplementation
with calcium and vitamin D. Furthermore, the right scapula,
cervical and thoracic vertebrae, left clavicle, femora and right
humerus were subjected to standard radiation therapy as palliative
care. The patient did not undergo adjuvant chemotherapy or
surgery.
After 10 weeks of denosumab administration, the pain
symptoms resolved and the patient recovered from the bedridden
status. There were no reported denosumab-related side effects. The
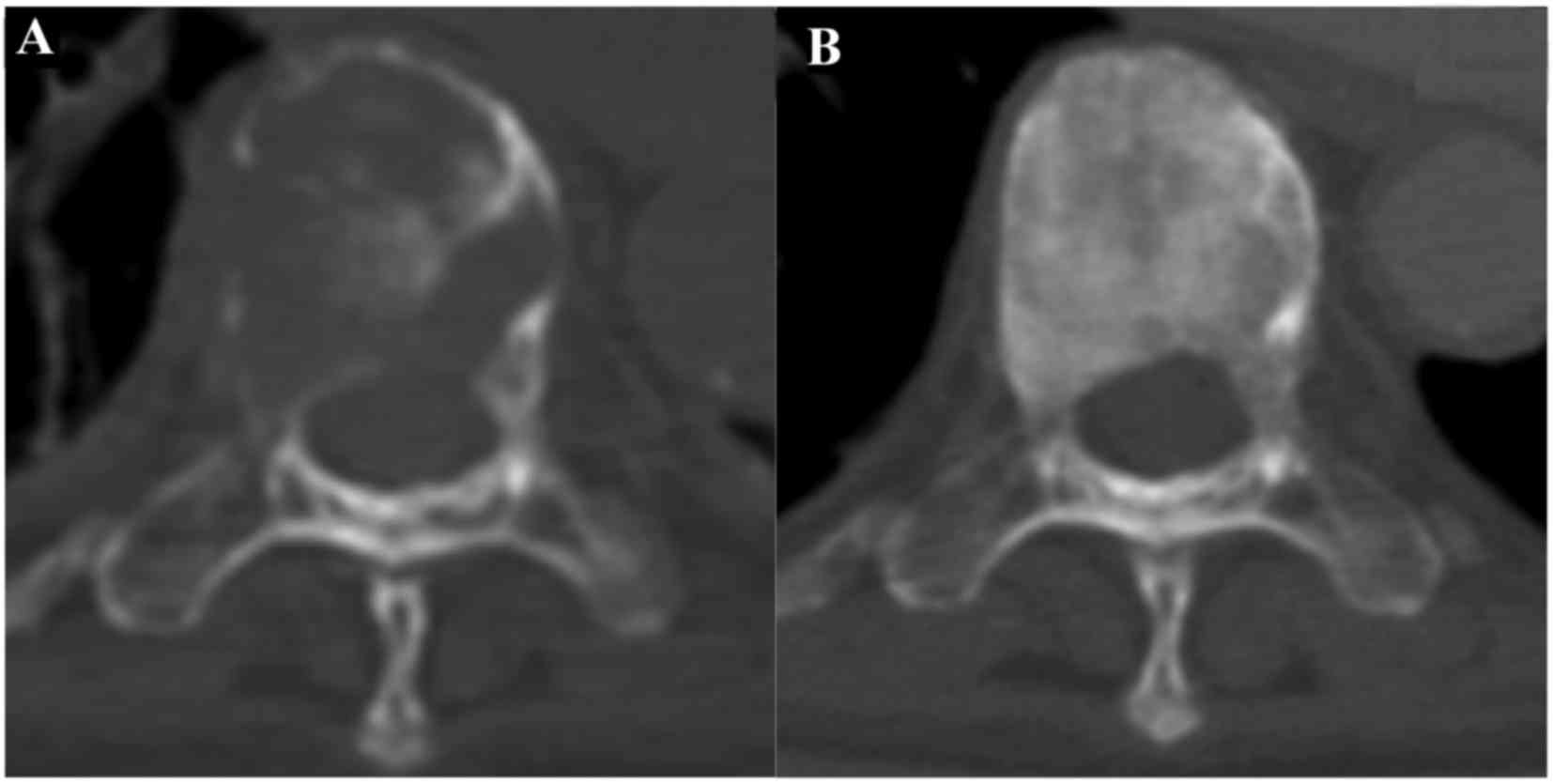
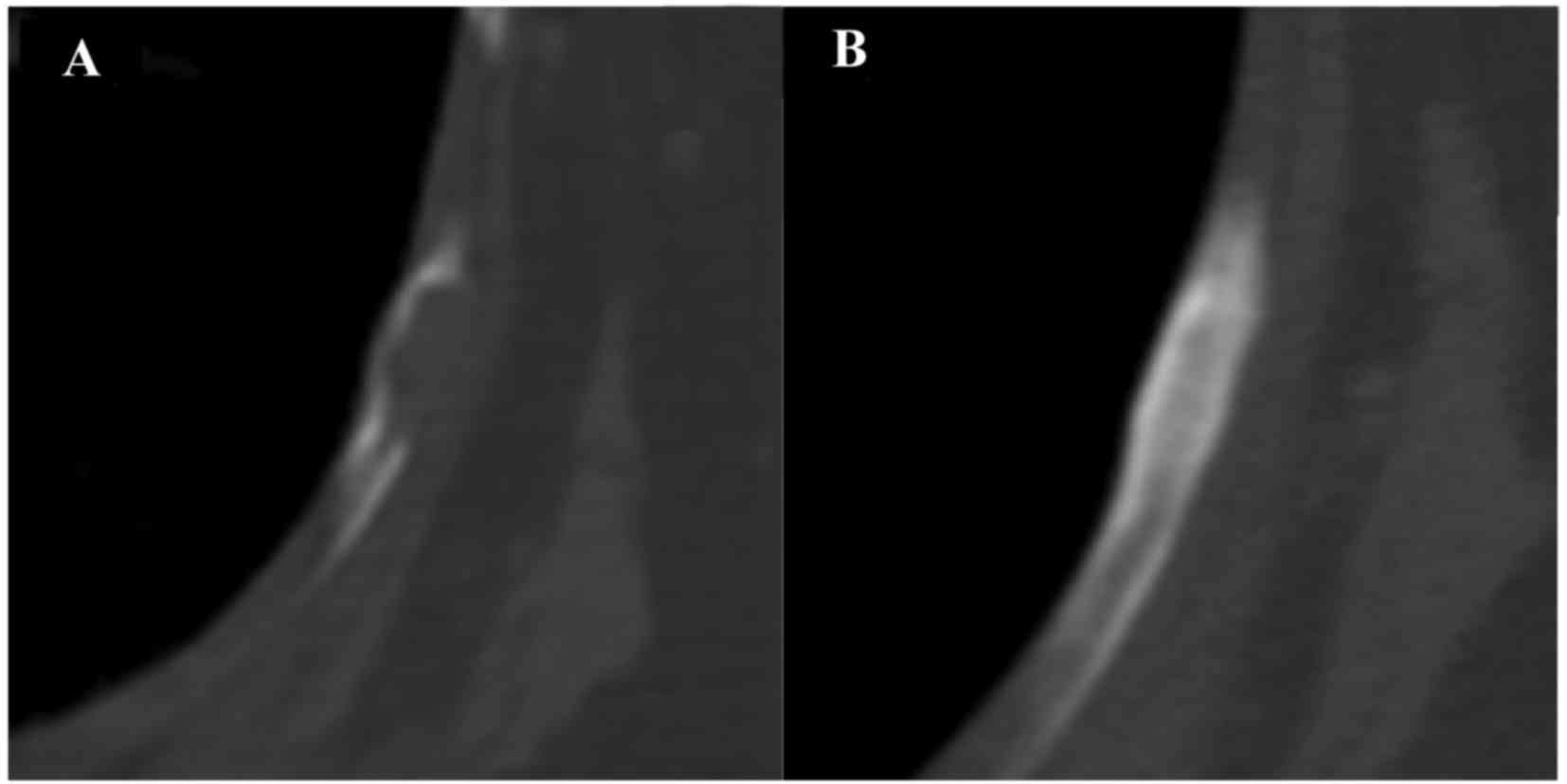
first follow-up computed tomography examination, performed 2 months
post-denosumab treatment, revealed that all tumor growth had
stabilized. Osteosclerotic changes were observed in multiple
osteolytic lesions following denosumab and radiation combination
therapy and denosumab therapy alone (Figs. 2 and 3). However, after 14 months of denosumab
administration, a new lumbar vertebral metastasis was detected. At
56 months since denosumab administration, the patient had developed
multiple metastases that had led to multiple organ failure, and
finally succumbed to gastrointentinal bleeding.
RANKL expression
RANKL gene expression was evaluated in a
biopsy of the iliac bone metastasis in the present case, and in
surgically resected samples from giant cell tumors of bone (GCTB,
n=6), aneurysmal bone cysts (ABC, n=2), fibrous dysplasia (FD, n=6)
and bone metastases from breast cancer (BC; n=3).
Total RNA was prepared from iliac bone biopsies
using ISOGEN reagent (Nippon Gene; Tokyo, Japan) according to the
manufacturer's recommendations. cDNA synthesis was performed using
the PrimeScript™ RT reagent kit (TaKaRa Bio; Tokyo,
Japan). Quantitative polymerase chain reaction analysis was
performed using SYBR Premix Ex Taq II in a Thermal Cycler Dice
Real-Time System TP800 (TaKaRa Bio; Otsu, Japan). RANKL was
selected as the target gene (forward primer:
5′-GCCTTTCAAGGAGCTGTGCAA−3′, reverse primer:
5′-ATCTAACCATGAGCCATCCACCAT-3′) and GAPDH was selected as
the reference gene (forward primer: 5′-GCACCGTCAAGGCTGAGAAC-3′,
reverse primer: 5′-TGGTGAAGACGCCAGTGGA-3′). A standard curve was
generated using the SaOS2 osteosarcoma cell line, and was used to
calculate gene copy numbers. RPMI8226, a myeloma cell line, was
used as the calibrator. The target gene expression level was
calculated as the ratio of the copy number of the target gene to
that of the reference gene. Finally, the relative level of
expression was calculated as [copy number of the target gene
(RANKL)/copy number of the reference gene
(GAPDH)]/copy number of the target gene (RANKL) in
the RPMI8226 cell line.
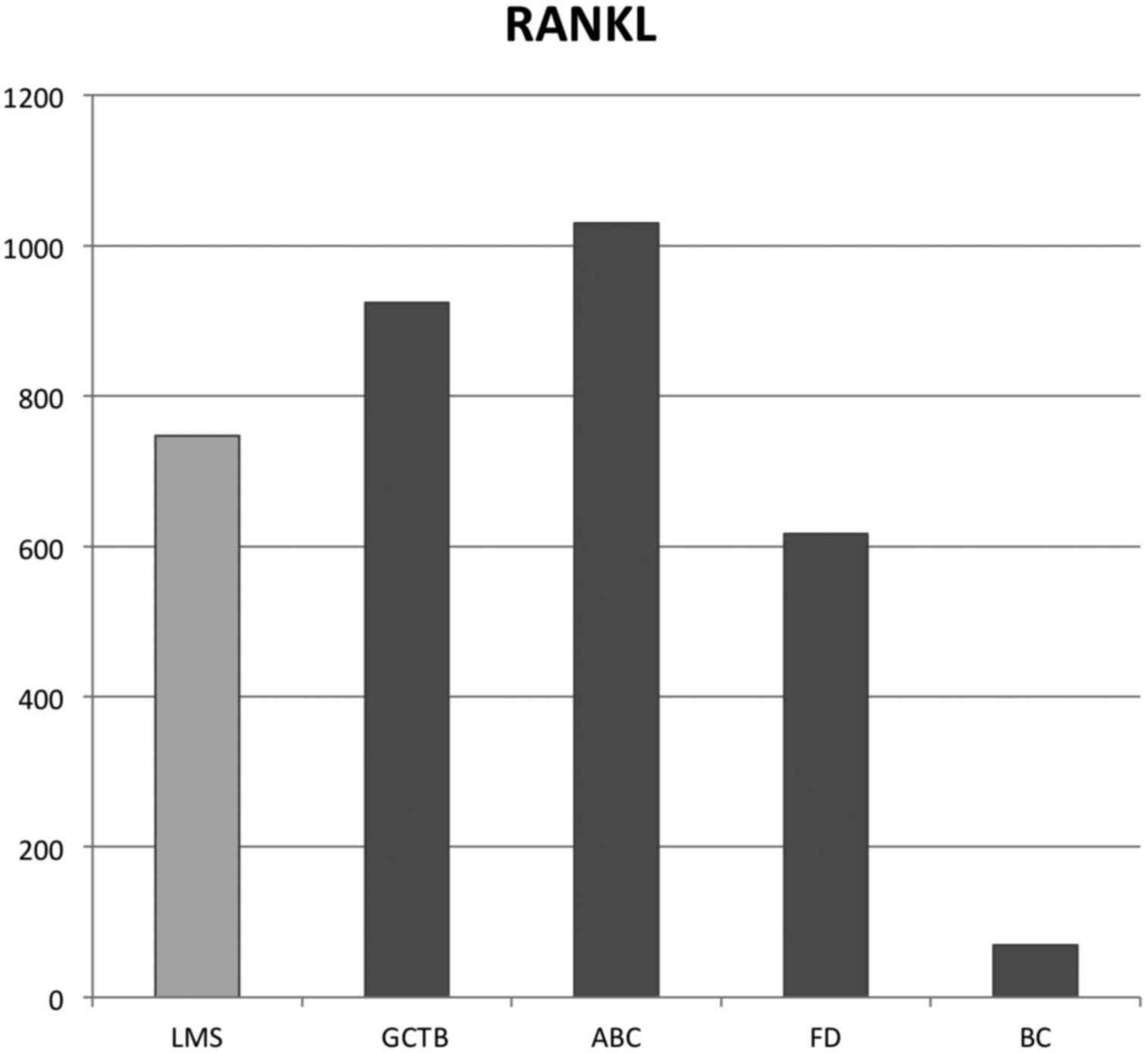
In the present case, RANKL gene expression
was 747-fold higher compared with that of the calibrator. The
results for the other tumor types are shown in Table I. The median fold changes for
RANKL relative expression levels were 994-, 1,030-, 616- and
69-fold for GCTB, ABC, FD and bone metastasis from BC, respectively
(Fig. 4).
 | Table I.Clinical characteristics and RANKL
expression of each tumor sample. |
Table I.
Clinical characteristics and RANKL
expression of each tumor sample.
| Diagnosis | Age, years | Anatomical
location | RANKL relative
expression levels |
|---|
| LMS | 64 | Ilium | 747 |
| GCTB1 | 46 | Radius | 1,531 |
| GCTB2 | 35 | Femur | 243 |
| GCTB3 | 70 | Tibia | 318 |
| GCTB4 | 53 | Tibia | 2,047 |
| GCTB5 | 16 | Humerus | 4,831 |
| GCTB6 | 22 | Femur | 298 |
| ABC1 | 20 | Humerus | 441 |
| ABC2 | 23 | Femur | 1,618 |
| FD1 | 37 | Tibia | 281 |
| FD2 | 58 | Rib | 952 |
| FD3 | 8 | Femur | 186 |
| FD4 | 58 | Ilium | 3,737 |
| FD5 | 23 | Fibula | 1,122 |
| FD6 | 29 | Femur | 215 |
| BC1 | 60 | Humerus |
69 |
| BC2 | 63 | Vertebra | 117 |
| BC3 | 64 | Femur |
6 |
All the procedures were conducted following
international and national regulations, in accordance with the
Declaration of Helsinki.
Informed consent was obtained from all subjects for
participation in the study, the use of their tissue and publication
of the data.
Discussion
Bone leiomyosarcoma is a rare primary malignant bone
tumor. In 1965, Evans and Sanerkin were the first to describe
primary bone leiomyosarcoma in the proximal tibia of a 73-year-old
man (3). Bone leiomyosarcoma is a
smooth muscle tumor composed of spindle cells characterized
immunohistochemically by smooth muscle cell differentiation and the
expression of desmin, h-caldesmon and α-smooth muscle actin.
Osteoclast-like giant cells may be occasionally observed (3,4).
Fletcher reported that other sarcoma types may also display
osteoclast-like giant cells, but that such cells are more commonly
seen in leiomyosarcoma (5).
Therapeutically, surgical excision with wide margins remains the
gold standard for curative management. Although leiomyosarcomas
appear to be radioresistant (1), the
use of chemotherapy for bone leiomyosarcoma is controversial. The
reported 5-year overall survival rate for bone leiomyosarcoma is
62% (6).
Denosumab is a monoclonal antibody that binds
RANKL and directly inhibits osteoclastogenesis. A phase 2
study demonstrated the safety and efficacy of denosumab
administration in adults and skeletally mature adolescent patients
with GCTB (7). Following exposure to
denosumab in vitro, RANKL expression in the tumor
cells of GCTB was almost entirely eliminated (8). Moreover, Lange et al reported
that the therapeutic administration of denosumab in 2 patients with
spinal ABC resulted in good clinical responses (9).
In the present case of bone leiomyosarcoma, good
clinical and radiological responses to denosumab were observed for
~2 years in the absence of chemotherapy. Pelle et al
demonstrated that RANKL expression in ABC resembled that in
GCTB (10). In the present case,
RANKL expression was higher compared with that observed in
BC, but similar compared with that in GCTB, ABC and FD (Fig. 4). Therefore, in the absence of
chemotherapy, the therapeutic effect of denosumab in the present
case may be explained by the mitigation of the progression of rich
osteoclast-like cell tumors by RANKL-targeted therapy.
In conclusion, denosumab may be a suitable treatment
option for leiomyosarcoma with osteoclast-like giant cells and
other untreatable tumors or cancers rich in osteoclast-like giant
cells, such as GCTB, ABC and FD.
Glossary
Abbreviations
Abbreviations:
|
ABC
|
aneurysmal bone cyst
|
|
BC
|
bone metastasis from breast cancer
|
|
FD
|
fibrous dysplasia
|
|
GCTB
|
giant cell tumor of bone
|
|
RANKL
|
receptor-activator of nuclear κB
ligand
|
References
|
1
|
Adelani MA, Schultenover SJ, Holt GE and
Cates JM: Primary leiomyosarcoma of extragnathic bone:
Clinicopathologic features and reevaluation of prognosis. Arch
Pathol Lab Med. 133:1448–1456. 2009.PubMed/NCBI
|
|
2
|
Berlin O, Angervall L, Kindblom LG, Berlin
IC and Stener B: Primary leiomyosarcoma of bone. A clinical,
radiographic, pathologic-anatomic, and prognostic study of 16
cases. Skeletal Radiol. 16:364–376. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Evans DM and Sanerkin NG: Primary
leiomyosarcoma of bone. J Pathol Bacteriol. 90:348–350. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilkinson N, Fitzmaurice RJ, Turner PG and
Freemont AJ: Leiomyosarcoma with osteoclast-like giant cells.
Histopathology. 20:446–449. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fletcher CD: Leiomyosarcoma with
osteoclast-like giant cells. Histopathology. 22:94–95. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brewer P, Sumathi V, Grimer RJ, Carter SR,
Tillman RM, Abudu A and Jeys L: Primary leiomyosarcoma of bone:
Analysis of prognosis. Sarcoma. 2012:6368492012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chawla S, Henshaw R, Seeger L, Choy E,
Blay JY, Ferrari S, Kroep J, Grimer R, Reichardt P, Rutkowski P, et
al: Safety and efficacy of denosumab for adults and skeletally
mature adolescents with giant cell tumour of bone: Interim analysis
of an open-label, parallel-group, phase 2 study. Lancet Oncol.
14:901–908. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mak IW, Evaniew N, Popovic S, Tozer R and
Ghert M: A translational study of the neoplastic cells of giant
cell tumor of bone following neoadjuvant denosumab. J Bone Joint
Surg Am. 96:e1272014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lange T, Stehling C, Fröhlich B,
Klingenhöfer M, Kunkel P, Schneppenheim R, Escherich G, Gosheger G,
Hardes J, Jürgens H and Schulte TL: Denosumab: A potential new and
innovative treatment option for aneurysmal bone cysts. Eur Spine J.
22:1417–1422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pelle DW, Ringler JW, Peacock JD,
Kampfschulte K, Scholten DJ II, Davis MM, Mitchell DS and Steensma
MR: Targeting receptor-activator of nuclear kappaB ligand in
aneurysmal bone cysts: Verification of target and therapeutic
response. Transl Res. 164:139–148. 2014. View Article : Google Scholar : PubMed/NCBI
|