Introduction
Malignant pleural mesothelioma (MPM) is relatively
rare (1–5). The World Health Organization has
classified MPM into three types, namely epithelioid, sarcomatoid
and biphasic types. Only 10% of MPMs are classified as sarcomatoid
malignant pleural mesothelioma (SMPM), which is associated with a
worse prognosis (6).
Distant metastases usually appear during the late
stages of the disease. However, reported cases of intracranial
metastases are extremely rare, with only few published articles to
date on intracranial metastases from MPM (7–11), and
only 1 reported case of multiple intracranial metastases from SMPM
(8).
We herein report a case of multiple intracranial
metastases from a giant SMPM in a 41-year-old male patient with no
history of asbestos exposure, and review previously published cases
of SMPM (Table I).
 | Table I.Previously published cases of
sarcomatoid malignant pleural mesothelioma. |
Table I.
Previously published cases of
sarcomatoid malignant pleural mesothelioma.
| Authors | Year of
publication | MPM | SMPM | Sex | Age, years | Intracranial
metastasis | Survival time,
months | (Refs.) |
|---|
| Makimoto et
al | 2014 | – | 1 | Male | 74 | No | 8 | (2) |
| Mah et al | 2004 | – | 1 | Male | 67 | Yes | ≥3 | (10) |
| Winfree et
al | 2004 | – | 1 | Female | 71 | Yes | 8 | (11) |
| Balduyck et
al | 2010 | 329 | 28 | M/F (26/2) | 65.6 | No | 5 | (14) |
| Brenner et
al | 1982 | 123 | 31 | – | 56 | No | 12 | (18) |
| Law et al | 1982 | 115 | 25 | – | 59 | No | – | (20) |
| Hillerdal et
al | 2007 | – | 1 | Male | 57 | No | – | (26) |
| Kim et
al | 2016 | – | 1 | Female | 65 | No | ≥7 | (27) |
Case report
A 41-year-old male patient presented to the
Department of Cardiothoracic Surgery of the Affiliated Hospital of
North Sichuan Medical College (Nanchong, China) in May, 2016 with a
chief complaint of left-sided chest pain for 1 month. The patient's
height was ~178 cm and he weighed 70 kg, he was a non-smoker and
had no past history of exposure to carcinogenic chemicals, such as
asbestos. The past medical history was unremarkable and there was
no family history of cancer. There was no history of cough, fever,
or hemoptysis, and the vitals on admission were normal. Physical
examination revealed no lymphadenopathy. The findings on lung
function tests were within the normal range [forced expiratory
volume in 1 sec (FEV1) 4.41 l, forced vital capacity (FVC) 5.28 l,
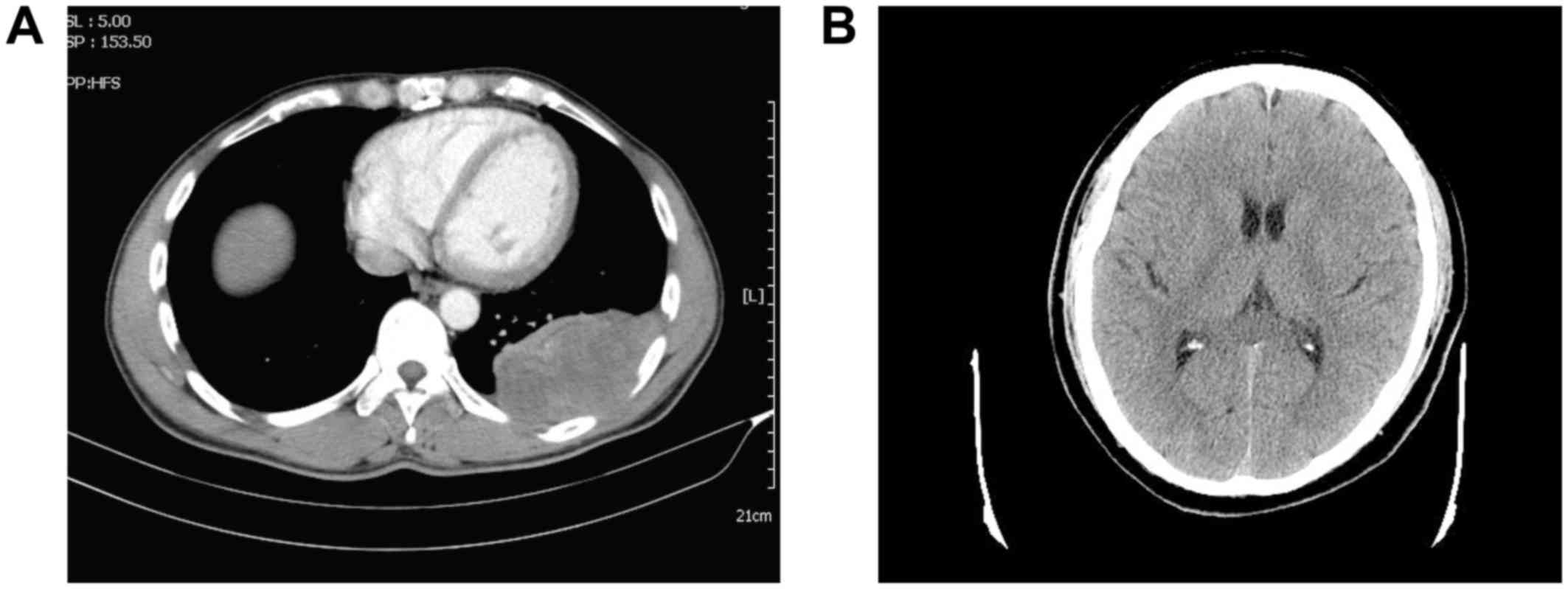
and a FEV1/FVC ratio of 0.83]. A chest computed tomography (CT)
scan revealed locally thickened left visceral and parietal pleura,
associated with intermingled pulmonary infiltrative shadowing. The
tumor had smooth margins with a wide tumor base. A cranial CT scan
revealed no abnormal masses in the brain (Fig. 1B). A bone scan using positron
emission CT detected no invasion or distant metastasis. On
three-dimensional CT imaging, the ribs were not invaded. Doppler
ultrasound of the abdomen and cervical area revealed no lymph node
or distant metastatic lesions in other organs. The findings on
CT-guided percutaneous biopsy of the mass were not significant.
The patient underwent surgery on April 10, 2016. A
20-cm incision was made at the left 6th intercostal space and a
mass originating from the pleura was identified. Extrapleural
pneumonectomy (EPP) was performed as the tumor invaded the left
inferior lobe. The solid tumor was sized ~12×10×8 cm and it was
yellowish-white on cross-section. To confirm the ribs were not
invaded, part of the left 5th and 6th ribs was removed for
intraoperative frozen section biopsy, and the results was negative
for invasion. The duration of entire procedure was ~2 h. The
intraoperative blood loss was ~500 ml and the patient received a
transfusion of 2 units of whole blood.
Pathological examination of the pleural mass was
performed. The examination of hematoxylin and eosin-stained
sections revealed papillary formations or sheets of spindle cells.
Cellular atypia and nuclear fission were observed on high
magnification. Bubble-like cells were also identified focally. On
immunohistochemical examination, the tumor was pan-cytokeratin
(CK)+, CK7+, CK18+, epithelial
membrane antigen−, calretinin−,
CK5/6−, P63−, there was no obvious loss of
INI-1 expression, CD34−, Wilms tumor-1−,
CD31−, ERG−, leukocyte common
antigen−, Ki-67+ (30%) and S-100+
(partially). These characteristics were consistent with the
diagnosis of SMPM (Fig. 2).
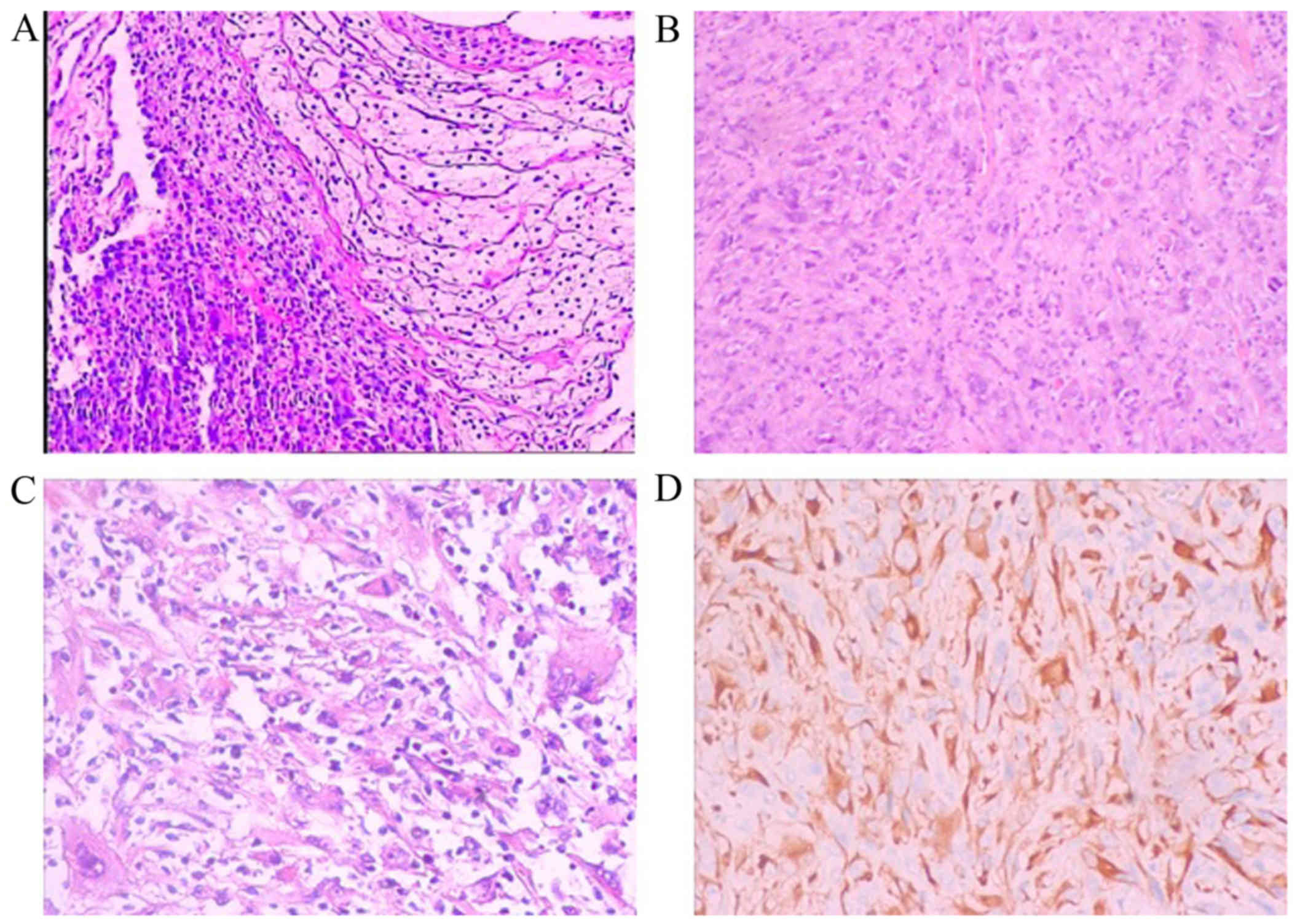 | Figure 2.Pathology results of the pleural mass.
Papillary formations or sheets of spindle cells were observed, with
cellular atypia, nuclear fission and focal bubble-like cells
observed on high magnification. Hematoxylin and eosin staining,
magnification (A) ×50, (B) ×100 and (C) ×200. (D)
Immunohistochemical examination revealed that the tumor cells were
positive for PCK, CK7, CK18; magnification, ×100. The tumor cells
were negative for epithelial membrane antigen, calretinin, CK5/6,
P63, CD34, Wilms tumor-1, CD31, ERG and leukocyte common
antigen. |
Postoperative adjuvant chemotherapy was not
performed due to financial difficulties. Five months after the
surgery, the patient visited our hospital with new complaints of
paralysis of the left leg and chest pain. A follow-up chest
contrast-enhanced CT revealed recurrence at the site of the
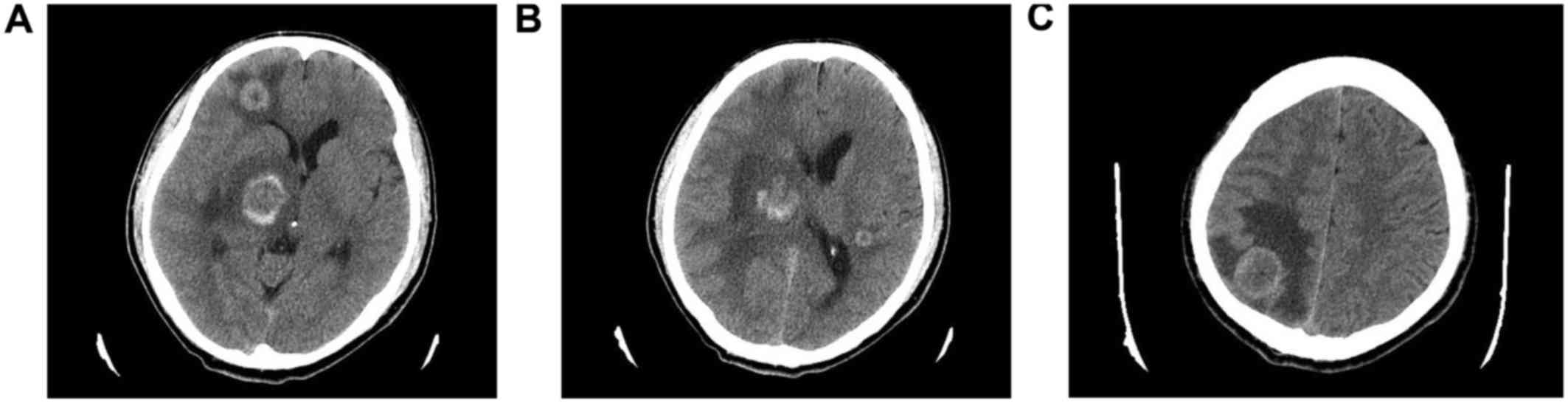
excision. A cranial CT scan was also performed and revealed 4
intracranial metastatic lesions: A 0.5-cm mass located in the
posterior horn of the lateral ventricle of the left temporal lobe,
and three more lesions in the frontal lobe, parietal lobe and basal
ganglia region of the right cerebrum, sized ~1, 2 and 2 cm,
respectively. There was edema surrounding the mass lesions
(Fig. 3A-C). The patient was
discharged without any treatment and succumbed to the disease 1
week later.
The present case report was approved by the Ethics
Committee of the Affiliated Hospital of North Sichuan Medical
College, and written informed consent was obtained from the patient
for publication of this case report and the accompanying
images.
Discussion
Approximately 80% of patients with MPM have a
history of asbestos exposure (12).
The period between asbestos exposure and the onset of MPM is
reported to be ~30-40 years (13).
MPM originates from the mesothelium of the parietal pleura and is
associated with a poor prognosis (2,12), with
a median survival period of 11.5–15.3 months. However, SMPM carries
a significantly worse prognosis, with a median survival of 4.2–5.0
months (14,15). Only early-stage EPP may prolong the
survival of patients with MPM (16);
however, as MPM is either asymptomatic or associated with a
non-specific presentation, early diagnosis is usually difficult
(2,12).
Distant metastases of MPM by hematogenous spread are
estimated to occur in >10% of the cases at later stages of the
disease (17). The most frequently
involved organs are the liver, adrenal gland, kidney and
contralateral lung (18–22). There have only been few reports on
intracranial metastasis of MPM (7–11).
Hurmuz et al reported a case of multiple intracranial
metastases from MPM, but not SMPM (9). Falconieri et al summarized 93
cases of MPM with distant metastases, among which only 3
intracranial metastases were observed. All 3 cases were SMPMs, and
only 1 case without CNS symptoms involved multiple intracranial
metastases (8).
The incidence of the three subtypes of MPM is as
follows: Epithelioid subtype, ~60%; sarcomatoid subtype, ~10%; the
biphasic subtype, exhibiting a mixed histological pattern, accounts
for the remainder of the cases (23). The epithelioid subtype has the best
prognosis, whereas the sarcomatoid has the worst (23). Wagner et al reported that,
among 200 cases of MPM, there were only 25 SPM cases and they all
exhibited short survival (24).
Pathological confirmation of SMPM is difficult
preoperatively (25). With
immunohistochemistry, calretinin is the most commonly used marker
for MPM, which is often positive in epithelioid MPM, but negative
in SMPM (26–28). In the present case, calretinin was
negative, consistently with previous reports.
There was recurrence in the thorax and multiple
intracranial metastatic tumors ~5 months after EPP. There is
currently no widely accepted curative approach to intracranial
metastases of MPM. Surgery or stereotactic radiosurgery would be
considered as treatments for solitary intracranial metastasis from
MPM (11); however, there has been
no documented treatment for multiple intracranial metastases from
MPM. Whole-brain irradiation or/and chemotherapy would be
considered as reasonable treatment options.
The most common symptoms of SMPM include coughing,
hemoptysis, weight loss, chest pain, dyspnea, fatigue, and fever
due to recurring pneumonia (3). In
the present case, the patient presented with only chest pain.
Smoking may also be a risk factor, but our patient was not a
smoker. The CT findings suggested MPM, which usually affects the
lungs. The recommended treatment included surgery, radiation and
chemotherapy; however, only surgery was performed due to financial
constraints. Supportive treatment may relieve some of the symptoms.
Prognosis in MPM may be difficult to assess consistently, due to
the great variability in the time before diagnosis and the rate of
disease progression. Our patient only survived for ~5 months after
surgery, as the disease exhibited an aggressive clinical
course.
EPP appears to be the only radical treatment option
for locally advanced MPM, and may be able to eradicate macroscopic
disease in selected patients. However, the long-term survival
remains unsatisfactory due to the high incidence of recurrence,
particularly locoregional treatment failure, and more effective
treatments are urgently required (16).
Cytological assessment of pleural effusion may not
be sufficiently sensitive and specific (29). In addition, fine-needle biopsy is not
primarily recommended, as it is associated with low sensitivity
(~30%). In the present case, CT-guided percutaneous needle biopsy
was performed, but the findings were not significant. Deng et
al suggested that SMPM may only be confirmed by full-thickness
biopsy (30).
In conclusion, SMPM has not been extensively
investigated due to the scarcity of reported cases. We herein
present a case of multiple intracranial metastases from a giant
SMPM, emphasizing the rare metastatic pattern, aggressive clinical
course and poor response to treatment, along with a review of the
previously published relevant literature.
Acknowledgements
The authors would like to thank Dr Xiaoguang Guo of
the Department of Pathology, Nanchong Central Hospital, The Second
Clinical Institute of North Sichuan Medical College (Nanchong,
China) for the support and assistance.
Glossary
Abbreviations
Abbreviations:
|
MPM
|
malignant pleural mesothelioma
|
|
SMPM
|
sarcomatoid malignant pleural
mesothelioma
|
|
CT
|
computed tomography
|
|
ECT
|
emission computed tomography
|
|
EPP
|
extrapleural pneumonectomy
|
|
CNS
|
central nervous system
|
References
|
1
|
Crotty TB, Myers JL, Katzenstein AL,
Tazelaar HD, Swensen SJ and Churg A: Localized malignant
mesothelioma. A clinicopathologic and flow cytometric study. Am J
Surg Pathol. 18:357–363. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Makimoto G, Fujiwara K, Fujimoto N,
Yamadori I, Sato T and Kishimoto T: Phrenic nerve paralysis as the
initial presentation in pleural sarcomatoid mesothelioma. Case Rep
Oncol. 7:389–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakano T, Hamanaka R, Oiwa K, Nakazato K,
Masuda R and Iwazaki M: Localized malignant pleural mesothelioma.
Gen Thorac Cardiovasc Surg. 60:468–474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stahel RA, Weder W and Felip E; ESMO
Guidelines Working Group, : Malignant pleural mesothelioma: ESMO
clinical recommendations for diagnosis, treatment and follow-up.
Ann Oncol. 19 Suppl 2:ii43–ii44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanzi S, Tiseo M, Internullo E, Cacciani
G, Capra R, Carbognani P, Rusca M, Rindi G and Ardizzoni A:
Localized malignant pleural mesothelioma: Report of two cases. J
Thorac Oncol. 4:1038–1040. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang H, Testa JR and Carbone M:
Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr
Treat Options Oncol. 9:147–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davies MJ, Ahmedzai S, Arsiwala SS and
Leverment JN: Intracranial metastases from malignant pleural
mesothelioma. Scand J Thorac Cardiovasc Surg. 29:97–99. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Falconieri G, Grandi G, DiBonito L,
Bonifacio-Gori D and Giarelli L: Intracranial metastases from
malignant pleural mesothelioma. Report of three autopsy cases and
review of the literature. Arch Pathol Lab Med. 115:591–595.
1991.PubMed/NCBI
|
|
9
|
Hurmuz P, Zorlu F, Cansiz C and Emri S:
Malignant pleural mesothelioma with brain metastasis. J BUON.
14:123–125. 2009.PubMed/NCBI
|
|
10
|
Mah E, Bittar RG and Davis GA: Cerebral
metastases in malignant mesothelioma: Case report and literature
review. J Clin Neurosci. 11:917–918. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Winfree CJ, Mack WJ and Sisti MB: Solitary
cerebellar metastasis of malignant pleural mesothelioma: Case
report. Surg Neurol. 61:174–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galetta D, Catino A, Misino A, Logroscino
A and Fico M: Sarcomatoid mesothelioma: Future advances in
diagnosis, biomolecular assessment, and therapeutic options in a
poor-outcome disease. Tumori. 102:127–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carbone M, Kratzke RA and Testa JR: The
pathogenesis of mesothelioma. Semin Oncol. 29:2–17. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balduyck B, Trousse D, Nakas A,
Martin-Ucar AE, Edwards J and Waller DA: Therapeutic surgery for
nonepithelioid malignant pleural mesothelioma: Is it really
worthwhile? Ann Thorac Surg. 89:907–911. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marshall AD, Bayes HK, Bardgett J,
Wedderburn S, Kerr KM and Currie GP: Survival from malignant
mesothelioma: Where are we now? J R Coll Physicians Edinb.
45:123–126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakas A, von Meyenfeldt E, Lau K, Muller S
and Waller D: Long-term survival after lung-sparing total
pleurectomy for locally advanced (International Mesothelioma
Interest Group Stage T3-T4) non-sarcomatoid malignant pleural
mesothelioma. Eur J Cardiothorac Surg. 41:1031–1036. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sussman J and Rosai J: Lymph node
metastasis as the initial manifestation of malignant mesothelioma.
Report of six cases. Am J Surg Pathol. 14:819–828. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brenner J, Sordillo PP, Magill GB and
Golbey RB: Malignant mesothelioma of the pleura: Review of 123
patients. Cancer. 49:2431–2435. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng WF and Berkman AW: Malignant
mesothelioma with bone metastases. Med Pediatr Oncol. 18:165–168.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Law MR, Hodson ME and Heard BE: Malignant
mesothelioma of the pleura: Relation between histological type and
clinical behaviour. Thorax. 37:810–815. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lester T and Xu H: Malignant pleural
mesothelioma with osseous metastases and pathologic fracture of
femoral neck. Appl Immunohistochem Mol Morphol. 16:507–509. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Machin T, Mashiyama ET, Henderson JA and
McCaughey WT: Bony metastases in desmoplastic pleural mesothelioma.
Thorax. 43:155–156. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pass HI, Vogelzang N, Hahn S and Carbone
M: Malignant pleural mesothelioma. Curr Probl Cancer. 28:93–174.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wagner JC, Sleggs CA and Marchand P:
Diffuse pleural mesothelioma and asbestos exposure in the North
Western Cape Province. Br J Ind Med. 17:260–271. 1960.PubMed/NCBI
|
|
25
|
Suter M, Gebhard S, Boumghar M,
Peloponisios N and Genton CY: Localized fibrous tumours of the
pleura: 15 new cases and review of the literature. Eur J
Cardiothorac Surg. 14:453–459. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hillerdal G and Elmberger G: Malignant
mediastinal tumor with bone formation-mesothelioma or sarcoma? J
Thorac Oncol. 2:983–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim KC and Vo HP: Localized malignant
pleural sarcomatoid mesothelioma misdiagnosed as benign localized
fibrous tumor. J Thorac Dis. 8:E379–E384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kushitani K, Takeshima Y, Amatya VJ,
Furonaka O, Sakatani A and Inai K: Differential diagnosis of
sarcomatoid mesothelioma from true sarcoma and sarcomatoid
carcinoma using immunohistochemistry. Pathol Int. 58:75–83. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scherpereel A, Astoul P, Baas P, Berghmans
T, Clayson H, de Vuyst P, Dienemann H, Galateau-Salle F, Hennequin
C, Hillerdal G, et al: Guidelines of the European respiratory
society and the European society of thoracic surgeons for the
management of malignant pleural mesothelioma. Eur Respir J.
35:479–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng CS, Sasada S, Izumo T, Nakamura Y,
Tsuta K and Tsuchida T: Sarcomatoid malignant pleural mesothelioma
confirmed by full-thickness biopsy. Chin Med J (Engl).
126:3391–3392. 2013.PubMed/NCBI
|