Introduction
High-grade cervical intra-epithelial neoplasia (CIN
2/3) is known to be attributable to persistent infection with
high-risk human papillomavirus (HR-HPV). The standard treatment
consists in ablation or excision, HPV infection leads to raised
frequency of conization once CIN 2/3 have been identified. However,
obstetrical complications and recurrence may occur after
conization: The relative risk of premature delivery, premature
rupture of membranes, and intra-uterine growth retardation ranged
from to 1.5 to 2.7 after cold knife and loop excision (1,2). In a
population-based cohort study in Norway which mixed data on
conization and pregnancy outcomes, the rate of premature delivery
increased from 7% in women giving birth before conization to 17% in
women giving birth after conization (3). The relative risks of late abortion
<24 weeks and premature delivery between 24 and 27 weeks were
4.0 and 4.4, respectively (3).
Conization treats the lesion, but does not
systematically treat the infection and patients may relapse. In a
study of 610 women followed up after conization, 37% were still
positive for HR-HPV at 5 months. The average cumulative rate of
recurrent high-grade lesions was 7%. The risk of recurrence was
higher for HPV16 (37%) than for other HR-HPV (11%) and for low-risk
(LR)-HPV (1.5%) (4).
Prophylactic vaccines do not cover the population
globally and have no therapeutic effect. In a randomized trial
including HR-HPV positive women at entry, vaccinated either with a
bivalent vaccine Cervarix® or control, the viral
clearance did not significantly differ at 12 months (49 vs. 50%)
between the two groups (5).
Quadrivalent vaccine Gardasil® was also not effective to
prevent CIN2/3 in women HPV positive by PCR (6).
Non-surgical treatment may be a useful option for
women to preserve future reproductive outcome and avoid HPV
persistence.
Targeted immunotherapy is one of these options.
Research into and development of targeted immunotherapy is active,
challenging and ongoing. A number of reviews have been published on
this topic recently (7–9). Main study reports were missing in one
review, whereas others mixed phase 1 and 2 studies, including both
invasive cervical cancer and CIN2/3. The objectives of this review
are to present the products according to their development process,
to describe phase 2/3 study results exclusively on high-grade CIN
and to question the future of targeted immunotherapy.
HPV biology
HPVs are non-enveloped, double-stranded, circular
deoxyribonucleic acid (DNA) viruses belonging to the Papovaviridae
family. Their genome encodes six ‘early’ proteins (E1, E2, E4, E5,
E6 and E7) and two ‘late’ proteins (L1 and L2). The former proteins
co-operate with cellular gene products to enable viral DNA
replication, whereas the latter proteins make up the structural
components of the viral capsid associated with the packaging of new
virions. HPV DNA normally replicates in episomal form. It may be
incorporated into host DNA, and this frequently leads to the
deletion of a number of viral genes, including several early (E2,
E4 and E5) and late (L1 and L2) genes. E6 and E7 are thus the main
proteins expressed in the infected cells. Since E2 is a
transcriptional repressor of E6 and E7, loss of E2 brings about
upregulation of the E6 and E7 genes. The E6 and E7 proteins
interact with vital cell cycle regulatory proteins, the p53 and
retinoblastoma proteins respectively. The uncontrolled expression
of E6 and E7 proteins brings about the disruption of cell cycle
regulation and genomic instability, and thus participates in the
progression of HPV related oncogenesis (10).
Concept and types of targeted
immunotherapy
Two different stages of the oncogenic infection
process are targeted by vaccine-mediated immune strategies: Initial
and established infection. To prevent initial infection, the
prophylactic vaccines induce neutralizing antibody responses
against L1 late protein inhibiting HPV from binding and entering
the cell. Since these vaccines are currently used in clinical
practice, they will not be examined in this review (5,6).
Targeted immunotherapy aims to eliminate or diminish infected cells
by priming cytotoxic T cells against infected cells and
upregulating major histocompatibility (MHC) Class I expression. The
design of these vaccines is predicated on the presence of episomal
replicating virus or integrated viral sequences. Immunotherapy
mainly targets E6 and E7 proteins since infected basal epithelial
cells and cervical cancer cells do not strongly express L1 and/or
L2 capsid antigens (11).
Furthermore, as HPV-16 along with CIN2/3 is responsible for around
half of all invasive cancer globally most clinical studies have
concentrated on E6 and E7 proteins of this genotype (12,13). The
types of targeted immunotherapy of CIN2/3, according to the various
formulations reported in the literature and/or registered on the
ClinicalTrials.gov website are shown in Table I. Technologies to create therapeutic
HPV vaccines include peptide/protein-based vaccines, nucleic
acid-based vaccines (DNA only, as ribonucleic acid (RNA)-based
vaccines are not available for CIN), live vector-based vaccines
(bacterial or viral), and whole cell vaccines derived from
dendritic cells or even tumor cells (although not available for
CIN) (7–9,14,15).
 | Table I.Description of targeted immunotherapy
formulations. |
Table I.
Description of targeted immunotherapy
formulations.
| Formulations | Main
characteristics | Description |
|---|
| Peptide-based
vaccines | Safe | HPV-16 E7 peptides
(Peninsula Labs Inc, CA, USA) (20) |
|
| Stable | HPV-16 E6/E7
peptides and incomplete Freund's adjuvant (Leiden |
|
| Easy to
produce | University Medical
Center, The Netherlands) (21) |
|
| MHC specific |
|
|
| Poor
immunogenicity |
|
|
| Adjuvants
required |
|
| Protein-based
vaccines | Safe | PD-E7 fusion
protein: HPV-16 E7 and AS02B adjuvant (GSK Biologicals, Belgium)
(26,27) |
|
| Stable |
|
|
| Easy to
produce | HPV-16 E6/E7 fusion
protein and ISCOMATRIX adjuvant (Iscotec AB, Sweden) (28) |
|
| Not MHC
specific |
|
|
| Low
immunogenicity | SGN-000101: HPV-16
E7 and heat shock protein (Nventa, CA, USA) (29–31) |
|
| Adjuvants
required |
|
|
|
| TVGV-1: PEK fusion
protein and GPI-0100 adjuvant (TheVax Genetics Vaccine Co., FL,
USA) |
| DNA-based
vaccines | Safe | ZYC101a: HPV-16 and
HPV-18 E6/E7 (MGI Pharma, MN, USA) (32,33) |
|
| Stable |
|
|
| Easy to
produce | VGX-3100: HPV-16
and HPV-18 E6/E7 (Inovio Pharmaceuticals, PA, USA) (34,35) |
|
| Low
immunogenicity |
|
|
| No neutralizing
antibody | pNGVL4a-CRT/E7
(Detox): HPV-16 E7 (NCI RAID program, USA) (36) |
|
| protection |
|
|
| Repeat
administration possible |
pNGVL4a-Sig/E7(Detox)/Hsp70: HPV-16 E7
(NCI, USA) (37) |
|
|
| GX-188E: HPV-16 and
HPV-18 E6/E7 (Genexine Inc., Korea) |
|
|
| VB10: HPV-16 E6/E7
(Vaccibody AS, Norway) |
| Natural viral
vectors | Strong
immunogenicity | TA-HPV: Recombinant
vaccinia vector encoding HPV-16 and HPV− |
|
| Neutralizing
antibodies | 18 E6/E7 (Xenova
group PLC, United Kingdom) (40,41) |
|
| Repeat
administration potency reduction | MVA-E2: Modified
vaccine of Ankara encoding bovine papillomavirus E2 (Lemery,
Mexico) (42,43) |
|
|
| MVA-E6-E7: Modified
vaccine of Ankara encoding HPV-16 E6/E7 |
| Synthetic viral
vectors | Risk of
toxicity | (Transgene, France)
(44,45) |
|
| Difficulty of
production | HPV-16 L1-E7 CVLP
(Medigene, Germany) (46,47) |
|
|
| Lovaxin C:
Attenuated Listeria monocytogenes vector encoding |
|
|
| HPV-16 E7 (Advaxis,
NJ, USA) (49) |
| Bacterial
vectors |
| GLBL101c:
Attenuated Lactobacillus casei vector encoding HPV-16 |
|
|
| E7 (Genolac BL
Corp, Japan) (50) |
Peptide/protein-based vaccines
Peptide-based vaccines
Peptides derived from HPV antigens may be
administered directly to vaccinate against HPV. Dendritic cells
take up HPV antigenic proteins which are presented to human
leukocyte antigen (HLA) molecules along with the MHC class I and/or
class II pathways to stimulate an immune response against the
pathogen. The polymorphic character of HLA molecules necessitates
the identification of specific immunogenic epitopes of HPV antigens
before developing the vaccine. Adjuvants such as chemokines,
cytokines and costimulatory molecules must be used to improve
vaccine potency (16).
Phase I/II human clinical trials, have shown that
peptide vaccines are safe and well-tolerated in patients with
advanced cervical cancer. Detection of T helper responses and
inconstantly HPV-specific cytotoxic T lymphocyte responses, and
cytokine production have been observed (14,17–19).
However, no response in terms of tumor control was reported.
In a phase I study, 18 women with CIN2/3 related to
HPV16 were vaccinated with HPV-16 E7 peptides developed using amino
acids 12–20 +/-86–93 encoded by the E7 gene by Peninsula Labs, Inc
(Belmont, CA). Subcutaneous administration of the vaccine took
place 4 times at 3-week intervals at escalating doses (100 to 2,000
µg per dose). Conization was performed at 12 weeks. Complete
clinical and histological regression (no CIN) was observed in 3
patients (17%) whereas the response was partial in 6 patients
(33%). HPV-16 DNA cleared on smears in 12 patients (67%). However,
a HPV-16 RNA signal remained positive in all tissue specimens. An
E7-specific cytokine release was observed in 10 patients (62%), but
immune response was not correlated with regression of the lesion
(20).
The peptide-based vaccine developed by the Leiden
University Medical Center in the Netherlands has been studied the
most since good efficacy and tolerance have been reported in
patients with vulvar intra-epithelial neoplasia (21). This vaccine contains 9 HPV-16 E6 and
4 HPV-16 E7 synthetic peptides of 25–35 amino acids along with an
overlap of 10–14 amino acids, in an emulsion with incomplete
Freund's adjuvant (Montanide ISA-51, Seppic). In a randomized trial
including 9 patients with high grade CIN and HPV-16, the vaccine
was administered twice subcutaneously at 3-week intervals (300 µg
per peptide) (22). A conization was
performed at 7 weeks. The main side effects observed were flu-like
symptoms and reactions at injection sites. In all patients, a
marked HPV-specific IFNy-associated T-cell response was observed
using ELISPOT. However, no HPV clearance was detected at the time
of conization and it was too early to assess the histological
impact of the vaccine. Inclusion rates were affected by
motivational problems leading to the postponement of treatment of
CIN2/3 and caused the study to be abandoned prematurely. For this
reason, the same investigators conducted a randomized trial in 51
patients with low-grade premalignant disorders (LSIL or persistent
HPV infection) related to at least one HR-HPV (33% HPV-16) with a
two-year follow-up (23). The design
was complex with a double subsequent randomization (vaccine vs.
placebo) at inclusion and after one year. Adverse effects did not
exceed grade 2 and were mainly flu-like symptoms (26%).
HPV-specific memory T-cell responses were detected at one year
after vaccination and reactivity was maintained for at least 2
years. Among 37 patients vaccinated, the rates of cytological
regression, persistence and progression were 51, 43 and 3%
respectively at one year. The corresponding rates were 78, 22 and
0% respectively in the placebo group (n=9). HPV-16 cleared in 3/8
vaccinated patients and in 1/2 placebo patients. Thus, no
significant difference was observed between both groups.
Protein-based vaccines
Protein-based vaccines pose no safety risk and are
as easy to produce as peptide vaccines. In addition, they carry all
possible antigen HLA epitopes and thus do not require the
identification of patient HLA types. Protein-based vaccines tend to
induce better antibody responses than cytotoxic T cell responses
(16). They also suffer from low
immunogenicity, which means that adjuvant and fusion protein
strategies are required to improve vaccine effectiveness (24,25).
A fusion protein PD-E7 is made up of a mutated
HPV-16 E7 associated with the first 108 amino acids of
Haemophilus influenzae protein D, formulated in the
GlaxoSmithKline Biologicals adjuvant AS02B (26). In a phase I/II clinical trial, the
vaccine was given to 9 patients (7 CIN3 and 2 CIN1, HPV-16
related), 3 intramuscular injections every 2-weeks. Conization was
performed 8 weeks after vaccination in patients with CIN3. Patients
exhibited significant E7-specific cytotoxic T lymphocytes (CTL)
responses. Following vaccination, no viral clearance or lesion
regression was detected in CIN3 patients. This could be due to the
fact that histological assessment took place early and the
induction of only low-level immunization (27).
A phase I study tested a vaccine containing an
HPV-16 E6/E7 fusion protein in association with the ISCOMATRIX
adjuvant. The vaccine was administered subcutaneously 3 times every
3 weeks at escalating doses (20 to 200 µg per dose) in 23 patients
with CIN2/3. HPV16 was positive at inclusion in 65% of patients. A
biopsy was carried out 2 weeks after the last injection.
Immunization was shown to be safe and resulted in a humoral
response with E6E7 specific IgG antibody and enhanced
CD8+ T cell responses to both E6 and E7 antigens. All
patients exhibited a reduction in HPV16 viral load on biopsy. No
correlation between virological response and immunity was found. As
the length of follow-up was very short, only 1 patient (4%) had a
histological response (no CIN) (28).
SGN-00101 vaccine (Stressgen, Nventa) is a fusion
protein consisting of heat shock protein (Hsp) from
Mycobacterium bovis and HPV 16 E7, administered
subcutaneously (500 µg per dose) following two different
protocols.
In the first study, 21 patients with CIN2/3 (18
CIN3), 24% being positive for HPV16, received 4 injections 3 weeks
apart and conization was performed at 15 weeks (29). Among the 20 patients assessed, the
clinical and histological response was complete (no CIN or CIN1) in
8 patients (40%). In eleven patients (55%) the disease was stable
and in one, the cancer progressed due to the extension of the
lesion. No difference in response was found according to HPV type.
A specific cell immune response assessed by ELISPOT was found in 9
of 17 patients (53%). Among the 8 patients with complete response,
5 exhibited specific cell immunity suggesting a good correlation
between efficacy and immunity. However, HPV clearance was
associated with neither clinical nor immune response as only one of
19 patients (5%) cleared HPV after follow-up.
In the second study, 58 patients with CIN3, 57%
being positive for HPV16, received 3 injections 4 weeks apart and
conization was performed at 6 months (30). The clinical and histological response
was complete (no CIN or CIN1) in 13 patients (23%) and partial
(reduction in lesion size by colposcopy >50%) in 32 patients
(55%). Eleven patients (19%) had stable disease and 2 had
microinvasive carcinoma. Serum IgG levels against HPV-16 E7 were
found to have modestly increased after vaccination and tended to be
correlated with a positive therapeutic effect (31). More complete responses, as well as
significant higher HPV-16 E7 IgG levels, were observed in patients
with recurring disease compared to patients who had never undergone
conization. No difference in response was found according to HPV
type.
In a third study (NCT00054041), patients with CIN3
received 3 subcutaneous injections of SGN-00101 4 weeks apart and
had large loop excision of the transformation zone under colposcopy
at week 15. The study aimed to determine the efficacy of SGN-00101,
with regard to complete histologic regression. The study was
completed in January 2013, but no results were available in
2017.
TVGV-1 is composed of lyophilized PEK fusion protein
(PE-E7-KDEL3 fusion protein made with HPV-16 E7 peptide) and
GPI-0100. GPI-0100 is a semi-synthetic triterpene glycoside,
originating in natural saponins, acting as an adjuvant for vaccines
to improve the immunogenicity of proteins. A phase 2a double-blind,
randomized, parallel group, dose-ranging study (NCT02576561) was
conducted in 2015. The objective was to evaluate the safety and
efficacy of three doses of TVGV-1 vaccine in comparison with its
adjuvant, GPI-0100, in patients with histologically confirmed
HPV-induced cervical CIN2/3. The outcome was the absence of CIN2/3
assessed by conization at 9 months. This study is currently
recruiting participants.
DNA-based vaccines
DNA vaccines are safe, stable, and reasonably easy
to produce. They do not elicit neutralizing antibody production,
and any given patient can receive it over and over again. Genomic
instability could theoretically be caused by DNA integrating the
host genome, but no evidence of DNA integration in human tissues
has been shown. DNA vaccines are made of E6 and/or E7 DNA and are
not highly immunogenic since DNA is not naturally able to amplify
or disperse from transfected cells to surrounding cells in
vivo (16).
Among several DNA vaccines investigated in clinical
trials, ZYC-101 appears to be the most advanced studied. ZYC-101
(ZYCOS, Inc., currently owned by MGI Pharma) is a microencapsulated
DNA vaccine encoding multiple HLA-A2-restricted E7-derived
epitopes. In a preliminary study including 15 women with CIN2/3, 5
had complete histological responses and 11 showed HPV specific
T-cell responses (32). ZYC-101a,
also called amolimogen, is the evolution of ZYC-101, encoding
HPV-16 and HPV-18 E6- and E7-derived epitopes. The vaccine was
administered by intra-muscular route 3 times every 3 weeks at two
doses (100 or 200 µg) in a randomized trial to compare the efficacy
of the vaccine (n=86) vs. placebo (n=41) (33).
All patients had CIN2/3 and 56% were positive for
HPV-16 or HPV-18. A conization was performed at 6 months. A
histological response (no CIN or CIN1) was observed in 37
vaccinated patients (43%) and 11 controls (27%). In the subgroup of
patients aged under 25 (n=43), the histological response was
significantly higher (70%) after immunotherapy than in the control
group (23%). Neither the dose of vaccine nor the HPV genotype
influenced results. The vaccine was well tolerated. No virological
or immune data were available for this study.
This vaccine reached phase 3 and a multicenter,
double-blind, randomized, placebo-controlled study was carried out
in 2005 (NCT00264732). Inclusion criteria were women between the
ages of 13 and 25 with a CIN 2/3 identified by a colposcopically
directed punch biopsy and a colposcopically visible lesion that
does not involve more than 75% of the cervix. The efficacy of ZYC
101a was assessed by colposcopy and biopsy, 6 months after the
vaccination. However, no results were available in 2017, suggesting
abandonment of the publication process.
VGX-3100, a DNA vaccine containing plasmids
targeting HPV 16 and 18 E6 and E7 proteins was used in clinical
trials employing electroporation technology which consists in
administering the vaccine through an intramuscular injection then
electroporation with several devices delivering a small electrical
charge. In a phase 1 clinical trial, T cell and antibody responses
were observed in 78% of patients vaccinated using VGX-3100
(34).
In a phase 2 multicenter clinical trial, 167
patients with CIN2/3 were randomized (3:1) to receive 6 mg VGX-3100
or placebo at 0, 4, and 12 weeks. Regression to CIN1 or normal
pathology 36 weeks after the first dose constituted the primary
efficacy endpoint. Fifty-three (49.5%) of 107 VGX-3100 recipients
and 11 (30.6%) of 36 placebo recipients were found to have
histopathological regression (P=0.034) in the per-protocol
analysis. Similar significant difference was observed in the
modified intention-to-treat analysis. Reactions at the injection
site were observed in the majority of women, however in the
VGX-3100 group erythema was the sole significantly more frequent
side-effect (78.4%) compared to the placebo group (57.1%),
(P=0.007) (35).
In a pilot study, patients with HPV-16 CIN2/3
received a DNA vaccine pnGVL4a-CRT/E7 (Detox) via different
administration routes: Intradermally, intramuscularly, or
intralesionally (36). Thirty-two
patients were enrolled and toxicity and immunogenicity were
evaluated at 2 years. In 8 out of 27 (30%) women who were given all
vaccinations and underwent conization, histologic regression to CIN
1 or less was observed. Twenty-two out of 32 patients (69%)
experienced adverse events (grade 1 or less in severity)
specifically related to the vaccine. In subject-matched
comparisons, there was an increase in intraepithelial
CD8+ T cell infiltrates following vaccination in
patients belonging to the intralesional administration cohort.
Sig/E7 (Detox)/Hsp70 is a DNA vaccine encoding an
endoplasmic reticulum signal sequence (Sig), associated with an
attenuated form of HPV 16 E7 fused to Hsp70. The vaccine was
administered by intramuscular route 3 times, 4 weeks apart at
escalating doses (0.5 to 3 mg per dose) in 15 patients with CIN2/3
all HPV16 positive (37). A
conization was performed at 15 weeks. A histological response (no
CIN) was observed in 0/6 patients at low dose and in 3/9 patients
(33%) at high dose. E6E7 specific IgG antibodies were found in 13
to 20% of patients at entry, but were not boosted after
vaccination. Cell mediated immunity was variable after vaccination.
No correlation with histological response was observed. Adjuvating
treatments like TLR7 agonist, imiquimod, were tested in association
with the vaccine to increase its immunogenicity. The efficacy and
safety of Sig/E7 (Detox)/Hsp70 administered with topical imiquimod
were assessed in a phase 1 clinical trial (NCT00788164). Results
were not available in 2017.
A Korean open-label, dose-escalation, single-center,
phase 1 study examined how safe GX-188E, a DNA-based therapeutic
vaccine, administered via electroporation in subjects with HPV-16
or HPV-18-associated CIN3 (NCT01634503) was. Each patient was given
an intramuscular 1, 2 or 4 mg injection of GX-188E by
electroporation 3 times every 4 weeks. The results were assessed 24
weeks after the third injection. The study was completed in 2014,
but no results were available in 2017. A multi-center randomized,
double-blind, placebo-controlled, phase 2 clinical trial evaluated
the safety and efficacy of GX-188E at a dose of 1 mg by
electroporation 3 times every 4 weeks (NCT02596243). The outcome
measures were the rate of histopathological regression of cervical
lesions to CIN1 or less and the clearance of HPV 16 or 18 at 36
weeks. This study is scheduled to end in 2018.
A phase 1–2 prospective multi-center study in
Germany evaluated the safety, the immunogenicity, and the efficacy
of VB10 in 16 patients with HPV16 related CIN2/3 lesions
(NCT02529930). The vaccine was administered intramuscularly every 3
weeks 3 times apart. This study is still recruiting participants in
2017.
Live-vector-based vaccines
Live-vector-based vaccines classically include viral
vectors (adenovirus, vaccinia virus) and bacterial vectors
(listeria, lactobacillus). Like protein-based vaccines, these
vaccines deliver the antigen to dendritic cells, thus expressing
HPV E6 and/or E7 in order to treat HPV-associated malignancies.
Live-vector-based vaccines exhibit strong immunogenicity due to
their ability to replicate inside host cells and facilitate the
spread of antigen inside cells. However, the production of
neutralizing antibodies in the host during vaccination could
diminish the efficacy of repeat immunizations. Using live vectors
may also be associated with a risk of toxicity (16).
Natural viral vectors
Among viral vectors, the vaccinia virus is the most
highly developed because of its high efficiency of antigen-specific
immunotherapy. The other vectors tested, such as the Semliki Forest
virus encoding E7, have been shown to induce strong immune
responses in mice, but failed to demonstrate any clinical
responses, possibly because of the immune tolerance of HPV-infected
cells (38,39).
TA-HPV, a recombinant vaccinia vector encoding an
HPV-16/18 E6/E7 fusion protein was assessed in Phase 1/2 clinical
trials. It was well tolerated and induced T-cell-mediated immune
responses in women with cervical cancer although no trials have
been set up for women with CIN (40,41).
The modified vaccine of Ankara (MVA) has been
developed as a recombinant vaccinia vector carrying nucleotidic
sequences encoding either bovine papillomavirus E2 protein
(MVA-E2), or HPV-16 E6 and E7 proteins (MVA-E6-E7).
After cervical injection of MVA E2 (Lemery, Mexico),
patients produced antibodies against the MVA-E2 vaccine and
developed a specific cytotoxic T lymphocyte (CTL) response against
HPV-transformed cells (42).
The originality of the MVA E2 recombinant vaccinia
virus is that it activates the immune system against E2 antigen,
which has an important part to play by increasing the expression of
E6 and E7. However, these studies did not demonstrate that MVA E2
induced an E2-specific immune response. Moreover, the agent does
not focus exclusively on HPV16, as containing bovine
papillomavirus. The vaccine was administered by the intra-cervical
route 6 times, 1 week a part. Thirty-four patients with CIN3, all
related to HPV 16 or 18, were treated and assessed by conization at
12 weeks (43). The clinical
response was complete (no more lesion) in 19 patients (56%) and
partial (reduction in lesion size by colposcopy >50%) in 11
patients (32%). A histological response (no CIN or CIN1) was
obtained in 20 patients (59%). All patients had a humoral and cell
mediated immunity as shown by MVA E2 IgG antibodies and specific
cytotoxic T lymphocytes. HPV cleared in 12 patients (35%) and the
viral load was reduced by 95% in 5 patients (15%). Histology was
well correlated with virology, as 15 of 20 CIN1-had a viral load
reduced by 95 to 100%.
MVA-E6-E7 (Transgene, France) is administered
subcutaneously 3 times, 1 week apart. In a phase 2 study including
21 patients with CIN2/3, all positive for HPV-16, the efficacy of
vaccination was assessed by colposcopy, cytology, and virology at 6
months (44). Conization was
performed if any anomalies or discordances were found. Follow-up
extended to 12 months. A complete clinical and/or histological
response (no CIN or CIN1) was observed in 10 patients (48%). HPV16
cleared on smears in 9 patients (43%). Histology was well
correlated with virology, as 7 of 10 patients with no CIN or CIN1
cleared HPV16 mRNA. No recurrence of CIN2/3 was seen at 1 year.
Following treatment with TG4001, no patient developed or enhanced
an IgG antibody response to E6 or E7. Cell immune data was not
available in this study.
A multi-center randomized, double-blind,
placebo-controlled, phase 2b clinical trial was conducted to assess
the efficacy and the safety of MVA-E6-E7 in 206 patients with
HPV-related CIN 2/3 (45). The
vaccine's safety profile is just as good as in former trials.
Histological resolution (no CIN) at 6 months was significantly
higher in vaccinated patients 11/55 (20%) than in controls 1/27
(4%) (P=0.049) in HPV-16 mono-infected patients, as well as in
patients infected by all HPV genotypes, 32/129 (25%) vs. 6/63 (10%)
respectively (P=0.013). The viral clearance was significantly
higher in vaccinated patients 20/52 (38%) than controls 2/23 (9%)
(P=0.009) in HPV-16 mono-infected patients, as well as in patients
infected by all HPV genotypes, 45/121 (37%) vs. 8/58 patients
(14%), (P=0.001). However, the trial did not meet its primary
endpoint of 60% resolution at six-months of CIN 2/3 in the HPV-16
mono-infected population. Therefore, Transgene did not further
develop TG4001 for this indication.
Synthetic viral vectors
Chimeric virus-like particles (CVLP) can be produced
using the same technology as for HPV prophylactic vaccines since
they are easy to manufacture, able to compact DNA, and target
specific cell receptors. HPV-16 L1-E7 CVLP consists of a
carboxy-terminally truncated HPV16 L1 protein fused to the
amino-terminal part of the HPV-16 E7 protein and self-assembled by
recombinant expression of the fusion protein (MediGene). HPV16
L1-E7 CVLP has been shown to induce E7-specific cellular immunity
in mice (46). A randomized,
double-blind, placebo-controlled clinical trial has been conducted
in HPV-16 related CIN 2/3 patients. CVLP was administered
subcutaneously 4 times over 12 weeks at two doses (75 or 250 µg) in
23 patients with CIN2/3, while 12 patients received placebo
(47). All were assessed by
colposcopy and biopsy at 7 months. A histological response (no CIN
or CIN1) was observed in 9 vaccinated patients (39%) and 3 controls
(25%). HPV16 cleared on biopsy in 6/16 vaccinated patients (37%)
and 1/7 controls (14%). Histology was well correlated with
virology, as 85% of patients responding to immunotherapy (no CIN or
CIN1) cleared HPV16 DNA. HPV16 E7 antibodies negative at baseline
were positive in 10 vaccinated patients (42%). An E7 specific T
cell response was observed in 22% of vaccinated patients, but was
not correlated with histological response. Antibodies with high
titers against HPV 16 L1 and low titers against HPV 16 E7 were
induced.
Bacterial vectors
Listeria-based vaccines have been shown to be able
to produce CD8+ and CD4+ immune responses as
well as inducing tumor-regression in animal models (48). Lm-LLO-E7, also called ADXS11-001 or
Lovaxin C (Advaxis, Princeton, NJ, USA) consists in a live
attenuated Listeria monocytogenes vector secreting HPV-16 E7 fused
to listerioly-sin 0. This vaccine was safe in phase 1 trials and
induced immunological responses in patients with advanced cervical
cancer (49). An American
randomized, single-blind, placebo controlled phase 2 study was
conducted to evaluate the safety and efficacy of Lovaxin C for
CIN2/3 therapy. The vaccine was administered intravenously at
escalating doses twice, 4 weeks a part (NCT01116245). No results
are available as the study was stopped prematurely due to lack of
enrollment.
An attenuated Lactobacillus casei expressing
modified full-length HPV16 E7 protein was evaluated in 10 patients
with HPV16-associated CIN3. The vaccine was administered orally.
After 9 weeks of therapy, the lesion downgraded to CIN2 in 70% of
patients. E7 cell-mediated immune responses in cervical lymphocytes
were directly correlated to the pathological downgrade. E7-specific
mucosal immunity in cervical lesions in the uterus were elicited by
the vaccine (50).
Conclusion
The present review has identified eighteen vaccines
for treating CIN at various stages of development, and primarily
described phase 2/3 studies. Most of them were well tolerated.
Adverse reactions were principally injection site reactions and
flu-like symptoms less than grade 2.
Most of the vaccines investigated were demonstrated
to bring about E6 or E7 specific T cell response, but did not show
a correlation linking histological or virological response and
immune T cell responses.
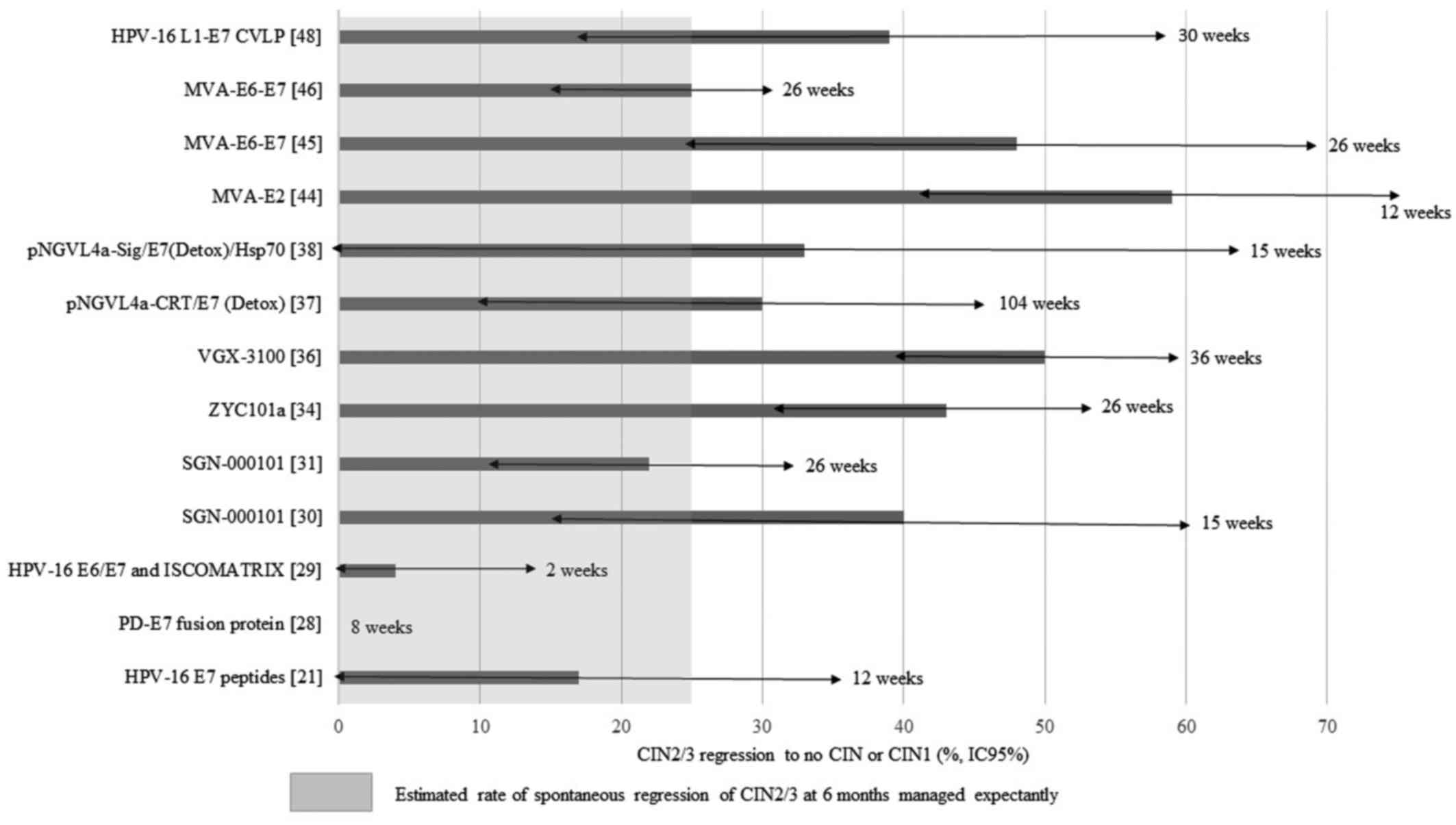
The efficacy of vaccines defined by regression of
CIN2/3 to no CIN or CIN1 ranged from 17 to 59% after a minimum of a
twelve-week follow-up (Fig. 1). An
additional value is occurred by some of the vaccines, as the
expected spontaneous regression rate of CIN2/3 provided by
randomized studies (placebo arm) is 10–30% (33,35,45).
However, the definitions of inclusion criteria (both
CIN2 and CIN3) and of pathological response (no CIN or CIN1)
commonly used in clinical reports remain debatable. The diagnosis
of CIN2 is subjective in that it depends on the colposcopy which
guides the biopsy and on the variability of the pathological
examination. Although CIN2 and CIN3 are included in the same high
grade lesion group, CIN2 is known to be a heterogeneous entity
including both real CIN3 and also condylomatous lesion or CIN1
(51). It is for this reason that
the reproducibility of the diagnosis is low for CIN2. To avoid any
possible confusion, some published studies and on-going clinical
trials include only true CIN3 (29,30,50);
others considering that no CIN was the only response criterion
(20,28,37,45).
Colposcopy also has inherent limitations and may
lead to the misdiagnose some CIN2/3. The sensitivity of colposcopy
is classically over 65%, and may exceed 80% in countries where
cervical cancer programs are available (52). However, specificity is lower as
colposcopy also detects more clinically insignificant low grade
lesions (53).
Some clinical trials have assessed viral regression
and clinical response after immunotherapy, but the results are
controversial. Only 3 studies showed a correlation between
histological and virological response (43,44,47). Two
studies demonstrated a high proportion of HPV clearance, while the
pathological response rate was low (20,28). One
study failed to demonstrate viral clearance when lesions were cured
(29).
None of the clinical trials reported in this paper
suggest the production and marketing of targeted immunotherapy
against CIN2/3 in the near future. However, as long as
immunotherapies can demonstrate efficacy over observation with no
major side effects, research and development of this concept should
be encouraged. Indeed, HPV related diseases are dramatically
increasing, especially in young women where current US guidelines
no longer recommend surgical conization for women aged 21–24 with
CIN2 (54). In addition,
prophylactic vaccines are not covering the population worldwide,
either because the license is not available or the coverage remains
low, as in France where vaccination is individual and not organized
(55). Alternatives to conization
are necessary in order to preserve reproductive outcome, avoid HPV
persistence and facilitate colposcopic surveillance. Unfortunately
some vaccines are no longer being investigated for this indication,
whereas other have regressed to phase 1/2 due to the addition of
new adjuvants to increase immunogenicity and hopefully
efficacy.
References
|
1
|
Kyrgiou M, Athanasiou A, Paraskevaidi M,
Mitra A, Kalliala I, Martin-Hirsch P, Arbyn M, Bennett P and
Paraskevaidis E: Adverse obstetric outcomes after local treatment
for cervical preinvasive and early invasive disease according to
cone depth: Systematic review and meta-analysis. BMJ.
354:i36332016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arbyn M, Kyrgiou M, Simoens C, Raifu AO,
Koliopoulos G, Martin-Hirsch P, Prendiville W and Paraskevaidis E:
Perinatal mortality and other severe adverse pregnancy outcomes
associated with treatment of cervical intraepithelial neoplasia:
Meta-analysis. BMJ. 337:a12842008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Albrechtsen S, Rasmussen S, Thoresen S,
Irgens LM and Iversen OE: Pregnancy outcome in women before and
after cervical conisation: Population based cohort study. BMJ.
337:a13432008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kreimer AR, Guido RS, Solomon D, Schiffman
M, Wacholder S, Jeronimo J, Wheeler CM and Castle PE: Human
papillomavirus testing following loop electrosurgical excision
procedure identifies women at risk for posttreatment cervical
intraepithelial neoplasia grade 2 or 3 disease. Cancer Epidemiol
Biomarkers Prev. 15:908–914. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hildesheim A, Herrero R, Wacholder S,
Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin
G, Porras C, et al: Costa rican HPV vaccine trial group: effect of
human papillomavirus 16/18 l1 viruslike particle vaccine among
young women with preexisting infection: A randomized trial. JAMA.
298:743–753. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
FUTURE IIStudy Group, . Quadrivalent
vaccine against human papillomavirus to prevent high-grade cervical
lesions. N Engl J Med. 356:1915–1927. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Young JL, Jazaeri AA, Darus CJ and
Modesitt SC: Cyclooxygenase-2 in cervical neoplasia: A review.
Gynecol Oncol. 109:140–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim HS, Kim T, Kim MK, Suh DH, Chung HH
and Song YS: Cyclooxygenase-1 and −2: Molecular targets for
cervical neoplasia. J Cancer Prev. 18:123–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grabosch SM, Shariff OM, Wulff JL and Helm
CW: Non-steroidal anti-inflammatory agents to induce regression and
prevent the progression of cervical intraepithelial neoplasia.
Cochrane Database Syst Rev. 9:CD0041212014.
|
|
10
|
Zur Hausen H: Papillomaviruses and cancer:
From basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Badaracco G and Venuti A: Human
papillomavirus therapeutic vaccines in head and neck tumors. Expert
Rev Anticancer Ther. 7:753–766. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li N, Franceschi S, Howell-Jones R,
Snijders PJ and Clifford GM: Human papillomavirus type distribution
in 30,848 invasive cervical cancers worldwide: Variation by
geographical region, histological type and year of publication. Int
J Cancer. 128:927–935. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tjalma WA, Fiander A, Reich O, Powell N,
Nowakowski AM, Kirschner B, Koiss R, O'Leary J, Joura EA, Rosenlund
M, et al: Differences in human papillomavirus type distribution in
high-grade cervical intraepithelial neoplasia and invasive cervical
cancer in Europe. Int J Cancer. 132:854–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Poelgeest MI, Welters MJ, van Esch EM,
Stynenbosch LF, Kerpershoek G, van Persijn van Meerten EL, van den
Hende M, Löwik MJ, Berends-van der Meer DM, Fathers LM, et al:
HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of
patients with advanced or recurrent HPV16-induced gynecological
carcinoma, a phase II trial. J Transl Med. 11:882013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su JH, Wu A, Scotney E, Ma B, Monie A,
Hung CF and Wu TC: Immunotherapy for cervical cancer: Research
status and clinical potential. BioDrugs. 24:109–129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hung CF, Ma B, Monie A, Tsen SW and Wu TC:
Therapeutic human papillomavirus vaccines: Current clinical trials
and future directions. Expert Opin Biol Ther. 8:421–439. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ressing ME, van Driel WJ, Brandt RM,
Kenter GG, de Jong JH, Bauknecht T, Fleuren GJ, Hoogerhout P,
Offringa R, Sette A, et al: Detection of T helper responses, but
not of human papillomavirus-specific cytotoxic T lymphocyte
responses, after peptide vaccination of patients with cervical
carcinoma. J Immunother. 23:255–266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Driel WJ, Ressing ME, Kenter GG,
Brandt RM, Krul EJ, van Rossum AB, Schuuring E, Offringa R,
Bauknecht T, Tamm-Hermelink A, et al: Vaccination with HPV16
peptides of patients with advanced cervical carcinoma: Clinical
evaluation of a phase I–II trial. Eur J Cancer. 35:946–952. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Welters MJ, Kenter GG, Piersma SJ, Vloon
AP, Löwik MJ, Berends-van der Meer DM, Drijfhout JW, Valentijn AR,
Wafelman AR, Oostendorp J, et al: Induction of tumor-specific CD4+
and CD8+ T-cell immunity in cervical cancer patients by a human
papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer
Res. 14:178–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muderspach L, Wilczynski S, Roman L, Bade
L, Felix J, Small LA, Kast WM, Fascio G, Marty V and Weber J: A
phase I trial of a human papillomavirus (HPV) peptide vaccine for
women with high-grade cervical and vulvar intraepithelial neoplasia
who are HPV 16 positive. Clin Cancer Res. 6:3406–3416.
2000.PubMed/NCBI
|
|
21
|
Kenter GG, Welters MJ, Valentijn AR, Lowik
MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM,
Offringa R, Drijfhout JW, et al: Vaccination against HPV-16
oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med.
361:1838–1847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Vos van Steenwijk PJ, Ramwadhdoebe TH,
Löwik MJ, van der Minne CE, Berends-van der Meer DM, Fathers LM,
Valentijn AR, Oostendorp J, Fleuren GJ, Hellebrekers BW, et al: A
placebo-controlled randomized HPV16 synthetic long-peptide
vaccination study in women with high-grade cervical squamous
intraepithelial lesions. Cancer Immunol Immunother. 61:1485–1492.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Vos van Steenwijk PJ, van Poelgeest MI,
Ramwadhdoebe TH, Löwik MJ, Berends-van der Meer DM, van der Minne
CE, Loof NM, Stynenbosch LF, Fathers LM, Valentijn AR, et al: The
long-term immune response after HPV16 peptide vaccination in women
with low-grade pre-malignant disorders of the uterine cervix: A
placebo-controlled phase II study. Cancer Immunol Immunother.
63:147–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui Z and Huang L: Liposome-polycation-DNA
(LPD) particle as a carrier and adjuvant for protein-based
vaccines: Therapeutic effect against cervical cancer. Cancer
Immunol Immunother. 54:1180–1190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stewart TJ, Drane D, Malliaros J, Elmer H,
Malcolm KM, Cox JC, Edwards SJ, Frazer IH and Fernando GJ:
ISCOMATRIX adjuvant: An adjuvant suitable for use in anticancer
vaccines. Vaccine. 22:3738–3743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hallez S, Simon P, Maudoux F, Doyen J,
Noël JC, Beliard A, Capelle X, Buxant F, Fayt I, Lagrost AC, et al:
Phase I/II trial of immunogenicity of a human papillomavirus (HPV)
type 16 E7 protein-based vaccine in women with oncogenic
HPV-positive cervical intraepithelial neoplasia. Cancer Immunol
Immunother. 53:642–650. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Simon P, Buxant F, Hallez S, Burny A, Fayt
I, Anaf V and Noël JC: Cervical response to vaccination against
HPV16 E7 in case of severe dysplasia. Eur J Obstet Gynecol Reprod
Biol. 109:219–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frazer IH, Quinn M, Nicklin JL, Tan J,
Perrin LC, Ng P, O'Connor VM, White O, Wendt N and Martin J: Phase
1 study of HPV16-specific immunotherapy with E6E7 fusion protein
and ISCOMATRIX adjuvant in women with cervical intraepithelial
neoplasia. Vaccine. 23:172–181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roman LD, Wilczynski S, Muderspach LI,
Burnett AF, O'Meara A, Brinkman JA, Kast WM, Facio G, Felix JC,
Aldana M and Weber JS: A phase II study of Hsp-7 (SGN-00101) in
women with high-grade cervical intraepithelial neoplasia. Gynecol
Oncol. 106:558–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Einstein MH, Kadish AS, Burk RD, Kim MY,
Wadler S, Streicher H, Goldberg GL and Runowicz CD: Heat shock
fusion protein-based immunotherapy for treatment of cervical
intraepithelial neoplasia III. Gynecol Oncol. 106:453–460. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Doorslaer K, Reimers LL, Studentsov
YY, Einstein MH and Burk RD: Serological response to an HPV16 E7
based therapeutic vaccine in women with high-grade cervical
dysplasia. Gynecol Oncol. 116:208–212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sheets EE, Urban RG, Crum CP, Hedley ML,
Politch JA, Gold MA, Muderspach LI, Cole GA and Crowley-Nowick PA:
Immunotherapy of human cervical high-grade cervical intraepithelial
neoplasia with microparticle-delivered human papillomavirus 16 E7
plasmid DNA. Am J Obstet Gynecol. 188:916–926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garcia F, Petry KU, Muderspach L, Gold MA,
Braly P, Crum CP, Magill M, Silverman M, Urban RG, Hedley ML and
Beach KJ: ZYC101a for treatment of high-grade cervical
intraepithelial neoplasia: A randomized controlled trial. Obstet
Gynecol. 103:317–326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bagarazzi ML, Yan J, Morrow MP, Shen X,
Parker RL, Lee JC, Giffear M, Pankhong P, Khan AS, Broderick KE, et
al: Immunotherapy against HPV16/18 generates potent TH1 and
cytotoxic cellular immune responses. Sci Transl Med.
4:155ra1382012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Trimble CL, Morrow MP, Kraynyak KA, Shen
X, Dallas M, Yan J, Edwards L, Parker RL, Denny L, Giffear M, et
al: Safety, efficacy and immunogenicity of VGX-3100, a therapeutic
synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6
and E7 proteins for cervical intraepithelial neoplasia 2/3: A
randomised, double-blind, placebo-controlled phase 2b trial.
Lancet. 386:2078–2088. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alvarez RD, Huh WK, Bae S, Lamb LS Jr,
Conner MG, Boyer J, Wang C, Hung CF, Sauter E, Paradis M, et al: A
pilot study of pNGVL4a-CRT/E7 (detox) for the treatment of patients
with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3).
Gynecol Oncol. 140:245–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Trimble CL, Peng S, Kos F, Gravitt P,
Viscidi R, Sugar E, Pardoll D and Wu TC: A phase I trial of a human
papillomavirus DNA vaccine for HPV16+ cervical intraepithelial
neoplasia 2/3. Clin Cancer Res. 15:361–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Riezebos-Brilman A, Regts J, Freyschmidt
EJ, Dontje B, Wilschut J and Daemen T: Induction of human papilloma
virus E6/E7-specific cytotoxic T-lymphocyte activity in
immune-tolerant, E6/E7-transgenic mice. Gene Ther. 12:1410–1414.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hsieh CJ, Kim TW, Hung CF, Juang J, Moniz
M, Boyd DA, He L, Chen PJ, Chen CH and Wu TC: Enhancement of
vaccinia vaccine potency by linkage of tumor antigen gene to gene
encoding calreticulin. Vaccine. 22:3993–4001. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Borysiewicz LK, Fiander A, Nimako M, Man
S, Wilkinson GW, Westmoreland D, Evans AS, Adams M, Stacey SN,
Boursnell ME, et al: A recombinant vaccinia virus encoding human
papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy
for cervical cancer. Lancet. 347:1523–1527. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kaufmann AM, Stern PL, Rankin EM, Sommer
H, Nuessler V, Schneider A, Adams M, Onon TS, Bauknecht T, Wagner
U, et al: Safety and immunogenicity of TA-HPV, a recombinant
vaccinia virus expressing modified human papillomavirus (HPV)-16
and HPV-18 E6 and E7 genes, in women with progressive cervical
cancer. Clin Cancer Res. 8:3676–3685. 2002.PubMed/NCBI
|
|
42
|
Gutierrez CM Corona, Tinoco A, Navarro T,
Contreras ML, Cortes RR, Calzado P, Reyes L, Posternak R, Morosoli
G, Verde ML and Rosales R: Therapeutic vaccination with MVA E2 can
eliminate precancerous lesions (CIN 1, CIN 2 and CIN 3) associated
with infection by oncogenic human papillomavirus. Hum Gene Ther.
15:421–431. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
García-Hernández E, González-Sánchez JL,
Andrade-Manzano A, Contreras ML, Padilla S, Guzmán CC, Jiménez R,
Reyes L, Morosoli G, Verde ML and Rosales R: Regression of
papilloma high-grade lesions (CIN 2 and CIN 3) is stimulated by
therapeutic vaccination with MVA E2 recombinant vaccine. Cancer
Gene Ther. 13:592–597. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brun JL, Dalstein V, Leveque J, Mathevet
P, Raulic P, Baldauf JJ, Scholl S, Huynh B, Douvier S, Riethmuller
D, et al: Regression of high-grade cervical intraepithelial
neoplasia with TG4001 targeted immunotherapy. Am J Obstet Gynecol.
204:169.e1–e8. 2011. View Article : Google Scholar
|
|
45
|
Transgene: Transgene reports randomized
phase 2b data with its therapeutic HPV vaccine TG4001 in women with
CIN2/3 intraepithelial cervical neoplasia. https://www.transgene.fr/wp-content/uploads/PR/208_en.pdfNovember
11–2017
|
|
46
|
Sharma C, Dey B, Wahiduzzaman M and Singh
N: Human papillomavirus 16 L1-E7 chimeric virus like particles show
prophylactic and therapeutic efficacy in murine model of cervical
cancer. Vaccine. 30:5417–5424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kaufmann AM, Nieland JD, Jochmus I, Baur
S, Friese K, Gabelsberger J, Gieseking F, Gissmann L, Glasschröder
B, Grubert T, et al: Vaccination trial with HPV16 L1E7 chimeric
virus-like particles in women suffering from high grade cervical
intraepithelial neoplasia (CIN 2/3). Int J Cancer. 121:2794–2800.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Souders NC, Sewell DA, Pan ZK, Hussain SF,
Rodriguez A, Wallecha A and Paterson Y: Listeria-based vaccines can
overcome tolerance by expanding low avidity CD8+ T cells capable of
eradicating a solid tumor in a transgenic mouse model of cancer.
Cancer Immun. 7:22007.PubMed/NCBI
|
|
49
|
Maciag PC, Radulovic S and Rothman J: The
first clinical use of a live-attenuated Listeria monocytogenes
vaccine: A Phase I safety study of Lm-LLO-E7 in patients with
advanced carcinoma of the cervix. Vaccine. 27:3975–3983. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kawana K, Adachi K, Kojima S, Taguchi A,
Tomio K, Yamashita A, Nishida H, Nagasaka K, Arimoto T, Yokoyama T,
et al: Oral vaccination against HPV E7 for treatment of cervical
intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific
mucosal immunity in the cervix of CIN3 patients. Vaccine.
32:6233–6239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Stoler M, Bergeron C, Colgan T, Ferenczy
A, Herrington C, Kim K, Loening T, Schneider A, Sherman M, Wilbur
D, et al: Squamous cell tumours and precursors. WHO Classification
of tumours of the uterine cervix. IARC Press; pp. 172–181. 2014
|
|
52
|
Decker KM, McLachlin CM and Lotocki R;
Pan-canadian cervical cancer screening network monitoring program
performance working group, : Performance measures related to
colposcopy for canadian cervical cancer screening programs:
Identifying areas for improvement. J Obstet Gynaecol Can.
37:245–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kyrgiou M, Kalliala IE, Mitra A,
Fotopoulou C, Ghaem-Maghami S, Martin-Hirsch PP, Cruickshank M,
Arbyn M and Paraskevaidis E: Immediate referral to colposcopy
versus cytological surveillance for minor cervical cytological
abnormalities in the absence of HPV test. Cochrane Database Syst
Rev. 1:CD0098362017.PubMed/NCBI
|
|
54
|
Partridge EE, Abu-Rustum N, Giuliano A,
Massad S, McClure J, Dwyer M and Hughes M; National comprehensive
cancer network, : Cervical cancer screening. J Natl Compr Canc
Netw. 12:333–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Denis F, Cohen R, Stahl JP, Martinot A,
Dury V, Le Danvic M and Gaudelus J: Papillomavirus vaccination in
France according to 2008 to 2012 Vaccinoscopie(®) data.
Med Mal Infect. 44:18–24. 2014. View Article : Google Scholar : PubMed/NCBI
|