Introduction
The ampulla of Vater was first described in a
detailed manner by Abraham Vater in 1720 and it consists of three
different anatomical structures: the duodenum, the terminal tract
of the pancreatic duct and the final portion of the common bile
duct (1). These elements have
distinct functions and histological features, comprising
intestinal, pancreatobiliary and gastric mucosal types (2).
Primary ampullary cancer comprises two main
histological subtypes, the pancreatobiliary type and the intestinal
type, but also rarer variants such as signet ring cell,
adenosquamous, mucinous, clear cell, papillary, and neuroendocrine
carcinoma (3,4). Adsay et al (2) classified ampullary cancers into four
subtypes based upon their location which reflects different
prognosis: intra-ampullary, peri-ampullary/duodenal,
ampullary-ductal and ampullary lesions not otherwise specified.
Occasionally, tumours of the pancreatic head or distal bile duct
may extend into the ampullary region, thereby mimicking a primary
ampullary neoplasm.
Several malignancies have been reported to involve
the upper gastrointestinal tract in a secondary manner and among
these lung cancer, breast cancer and malignant melanoma seem to be
the most common primary sites (5).
Within the upper gastrointestinal tract, the stomach is more often
affected by secondary tumours than the duodenum. However,
metastasis to the ampulla of Vater is exceedingly rare, with only
31 cases reported so far (6–33).
Herein we report the case of a 57-year-old patient
who developed ampullary metastasis from renal cell carcinoma 3.5
years following initial diagnosis. Additionally, we conducted a
systematic and comprehensive literature review summarizing all
cases of secondary ampullary tumours documented in MEDLINE/PubMed
until December 2016.
Case report
A 57-year-old male patient presented with subacute
upper gastrointestinal bleeding 3.5 years at the Department of
Gastroenterology, Medical University of Graz (Graz, Austria)
following nephrectomy for renal clear cell carcinoma (pT3b, G2, N0,
M0, R0). The requirement for written informed consent was waived
for the present case report. The patient did not undergo any
further adjuvant treatment but had regular follow-up until the
occurrence of upper gastrointestinal bleeding. Upon gastroscopy,
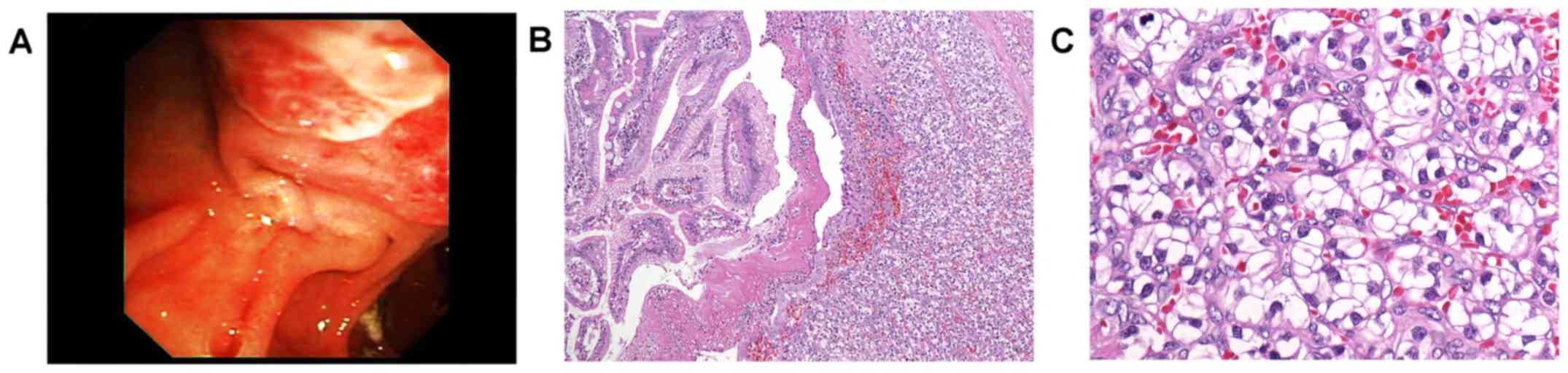
the ampulla of Vater was irregularly enlarged, vulnerable (Fig. 1A), and multiple biopsies were taken.
All other parts of the upper gastrointestinal tract were entirely
normal.
Histology showed well differentiated cancer cells
with clear cytoplasm and small to moderately enlarged,
hyperchromatic nuclei. The tumour cells were arranged in alveolar
pattern (Fig. 1B-C) and were
detected mainly in the submucosa with secondary ulceration of the
mucosal surface. Findings ultimately prompted diagnosis of
secondary ampullary carcinoma, that is, metastatic disease from
renal cell cancer.
Staging including CT of chest, abdomen and pelvis as
well as whole body bone scan did not reveal further metastatic
lesions. The patient underwent pylorus-preserving
pancreaticoduodenectomy (Whipple procedure). Macroscopic workup of
the surgical specimen showed a yellowish tumour mass within the
ampulla, measuring 3 cm in largest diameter. All surgical resection
margins and all dissected lymph nodes were free of cancer. The
postoperative course was uneventful, and the patient discharged on
postoperative day 15. Since then, the patient undergoes regular
controls, including CT scans of the abdomen in regular intervals
and there is now, four years after resection, no evidence of local
recurrence or distant metastasis.
Discussion
We presented a rare case of secondary tumour of the
ampulla of Vater. The corresponding primary tumour was renal clear
cell carcinoma that had been operated 3.5 years earlier. Following
pancreaticoduodenectomy, the patient is free of cancer for four
years now.
A total of 32 secondary tumours, including our own
case, have been reported to occur within the ampulla so far
(Table I). Mean age at diagnosis of
the secondary tumour is 56 years (median 55; range 27–81) (6–33). Renal
carcinoma (n=11; 34%), malignant melanoma (n=10; 31%) and breast
cancer (n=4, 13%) are the most common corresponding primary
tumours. These three entities account for approximately three
fourths of cases.
 | Table I.Secondary tumours of the ampulla of
Vater-literature overview and case report. |
Table I.
Secondary tumours of the ampulla of
Vater-literature overview and case report.
| Authors, year | No. | Sex | Age at diagnosis of
metastasis | Macroscopy of
ampullary lesion | Location of primary
tumour | Diagnosis of primary
tumour | Time interval between
diagnosis of primary tumour and metastasis | Symptomsa | Additional metastases
(at time of diagnosis of ampullary tumour) | Therapyb | Outcome | (Refs.) |
|---|
| England and Sarr,
1990 | 1 | M | 70 | NS | Skin: posterior
thorax | Malignant
melanoma | 3 years | 1, 3, 4 | Bone, liver | 3, 4, 5 | NS | (6) |
| Sans et al,
1996 | 2 | M | 51 | 1 | Skin (NS) | Malignant
melanoma | 3 months | 1, 3 | Brain | 2 | Succumbed after 3
months | (7) |
| Meyers et al,
1998 | 3 | M | 56 | 1, 3 | Skin: left upper
back | Malignant
melanoma | 3 years | 2, 3 | Brain, skin | 3 | Succumbed after 4,5
months | (8) |
| Caballero-Mendoza
et al, 1999 | 4 | M | 48 | NS | Skin: back | Malignant
melanoma | Synchronous | 1, 3, 4 | Lungs, stomach,
mediastinum, liver, spleen | 2, 4 | Succumbed after 4
months | (9) |
| Le Borgne et
al, 2000 | 5 | F | 62 | 3 | Skin (NS) | Malignant
melanoma | 8 years | 3 | No | 3, 4 | No evidence of
disease 12 months after resection | (10) |
| Le Borgne et
al, 2000 | 6 | F | 33 | 3 | Skin (NS) | Malignant
melanoma | NS | 3 | NS | 3 | Survived 2 months
after resection | (10) |
| van Bokhoven et
al, 2006 | 7 | F | 66 | 1 | Skin: left side of
the trunk | Malignant melanoma
(2 primary lesions) | 3 years | 1, 3 | No | 2 | NS | (11) |
| Marks et al,
2010 | 8 | F | 66 | 1, 3 | Skin: right
forearm | Malignant
melanoma | 4 years | 1, 3 | Right superior
eyelid, chest, gallbladder, abdomen, pelvis, superficial soft
tissue | 2, 4 | Succumbed after 15
months | (12) |
| Uiterwaal et
al, 2011 | 9 | F | 41 | NS | Skin: left
shoulder | Malignant
melanoma | 10 months | 1 | Brain | 4, 5 | Succumbed 8
months | (13) |
| Nakayama et
al, 2011 | 10 | F | 81 | 1 | Vagina | Malignant
melanoma | Synchronous | 2, 4 | Liver, the primary
lesion extended to the cervix of uterus and to the pelvis, behind
the bladder | 1 | Succumbed after 1
month | (14) |
| McKenna and
Kozarek, 1989 | 11 | M | 52 | 1 | Kidney (right) | Clear cell
carcinoma | 10 years | 3, 4 | Left kidney, right
lower pulmonary lobe | 2, 3 | NS | (15) |
| Robertson and
Gertler, 1990 | 12 | M | 70 | 1, 2, 3 | Kidney (left) | Clear cell
carcinoma | 12 years | 2, 4 | No | 3 | NS | (16) |
| Venu et al,
1991 | 13 | M | 64 | 1, 2 | Kidney (left) | Clear cell
carcinoma | 11 years | 2, 4 | Bone | 3, 4 | Succumbed
postoperatively due to massive pulmonary embolism | (17) |
| Bolkier et
al, 1991 | 14 | F | 55 | 1 | Kidney (left) | Clear cell
carcinoma | NS | 3 | Left breast,
brain | 2 | Succumbed after 2
months | (18) |
| Leslie et
al, 1996 | 15 | F | 78 | 1, 3 | Kidney (left) | Clear cell
carcinoma | 10 years | 3, 4 | No | 3 | No evidence of
disease 2.5 years after resection | (19) |
| Leslie et
al, 1996 | 16 | M | 53 | 1, 3 | Kidney (right) | Clear cell
carcinoma | 8 years | 2, 4 | Mesenteric
nodulation, retroperitoneal mass | 3, 4 | Alive with residual
disease 6.5 years after resection | (19) |
| Janzen et
al, 1998 | 17 | M | 75 | 1, 3 | Kidney (left) | Clear cell
carcinoma | 17.5 years | 2, 3 | Spleen | 3 | NS | (20) |
| Mendoza et
al, 2001 | 18 | M | 49 | 1, 2, 3 | Kidney (right) | Clear cell
carcinoma | 2.5 years | 1, 2 | No | 3 | Succumbed after 3
months | (21) |
| Hata et al,
2013 | 19 | F | 50 | 1, 2, 3 | Kidney (left) | Clear cell
carcinoma | 13 years | 2 | Bone, spleen | 3 | Alive with residual
disease 1 year after resection | (22) |
| – | 20 | M | 57 | 1, 2, 3 | Kidney (left) | Clear cell
carcinoma | 3.5 years | 2 | No | 3 | No evidence of
disease 4 years after resection | Present case |
| Haidong et
al, 2014 | 21 | M | 50 | NS | Kidney (right) | Clear cell
carcinoma | Synchronous | 3 | No | 3,4 | No evidence of
disease 5 years after resection | (32) |
| Titus et al,
1997 | 22 | F | 50 | 1, 3 | Breast (left) | Invasive carcinoma
of no special type | 4 years | 3 | No | 3, 4 | alive after 4
months | (23) |
| Rego et al,
2009 | 23 | F | 66 | NS | Breast (left) | Invasive carcinoma
of no special type | 2 years | 4 | No | 4 | NS | (24) |
| Rego et al,
2009 | 24 | F | 39 | 1 | Breast (NS) | Invasive lobular
carcinoma | NS | 1 | NS | 3 | NS | (24) |
| Bastos et
al, 2014 | 25 | F | 63 | 1 | Breast | Invasive lobular
carcinoma | 1.5 years | 1,3 | Lumbar spine | 2 | NS | (33) |
| Lee et al,
2005 | 26 | F | 50 | 1, 2 | Uterine cervix | Squamous cell
carcinoma | 2 years | 1 | No | 4 | NS | (25) |
| Sreenarasimhaiah
and Hoang, 2005 | 27 | M | 62 | 1 | Oesophagus | Squamous cell
carcinoma | Synchronous | NS | No | 4, 5 | NS | (26) |
| Buyukcelik et
al, 2003 | 28 | M | 71 | 1, 2, 3 | Larynx | Squamous cell
carcinoma | 5 years | 1, 3 | Right adrenal
gland, multiple pulmonary nodules | 2, 4 | Succumbed after 1
year | (27) |
| Kamusella et
al, 2007 | 29 | M | 41 | 1, 2 | Lung (right
superior lobe) | Adenocarcinoma | Synchronous | NS | No | 2 | NS | (28) |
| Silva et al,
1996 | 30 | F | 53 | NS | Ovary and
uterus | Endometrioid
adenocarcinoma (both ovaries and endometrium) | 5 years | 1, 2, 3 | NS | 2, 4 | No evidence of
disease 9 months after resection | (29) |
| Green et al,
2008 | 31 | M | 54 | 1 | Bladder | Urothelial
carcinoma | 3 years | 1, 3 | No | 2, 4 | NS | (30) |
| Kadakia et
al, 1992 | 32 | M | 27 | 1, 2 | Right femur | Osteosarcoma | 4 years | 1, 2, 4 | NS | NS | NS | (31) |
The mean time interval (known for 29 patients)
between the diagnosis of the primary tumour and the ampullary
metastasis is 4.8 years (median 3.5; range 0–17.5). However,
lesions may also be detected synchronously with the primary tumour
(n=5). Of note, time intervals for metastatic renal cancer lesions
are particularly long (mean 8.8 years, median 10 years; range
0–17.5). In only one third of patients (n=12, 38%), as in the
presented case, the ampullary metastasis was the only metastatic
lesion, while in the remaining patients cancer spread to one or
more organs was observed, most commonly to brain, liver, lungs, and
bone. Notably, the majority of malignant melanomas showed
involvement of several distant organs (Table I).
Clinical symptoms of secondary ampullary tumours are
unspecific and similar to the presentation of primary tumours.
Affected individuals may present with abdominal discomfort (n=13,
41%), jaundice and related symptoms (n=18, 56%), such as pruritus
or alterations in stool and urine or with upper gastrointestinal
bleeding (n=11, 34%).
Likewise, endoscopic presentation is
indistinguishable from that of primary tumours apart from
metastatic melanomas which occasionally appear as pigmented lesions
(34,35). According to the data retrieved from
literature, the majority (n=24/26, 92%) of lesions are polypoid and
irregular, soft and friable masses which can also be ulcerated. In
addition to endoscopy, several imaging methods, such as
transpapillary intraductal ultrasonography and computed tomography,
may be applied to detect small lesions of the papilla and to
provide a proper staging (36).
However, criteria to differentiate primary from secondary ampullary
tumours have not been developed for these techniques. Definitive
diagnosis requires clinical data and standard biopsies:
cyto-architectural appearance and immunohistochemical profile are
analysed to exclude primary neoplasms or less common lesions, e.g.,
sarcomas, GISTs and malignant lymphomas (37). In the evaluation of primary ampullary
tumours, the diagnostic accuracy of forceps biopsies is known to
range from 47 to 95% (37). False
negative results may result from submucosal localisation of the
tumour, and it is expected that the diagnostic accuracy for
secondary tumours is lower, as these usually do not originate for
the mucosa, but affect the luminal surface in a secondary manner
(37).
As the prognosis of patients with secondary
ampullary tumours is poor and a surgical approach bears a
considerable risk of postoperative morbidity and mortality surgical
intervention should be planned carefully (38). Outcome data was available for 19
patients. Three patients (16%), including our own patient, remained
free of cancer after surgery for more than 2.5 years (2.5, 4, 5
years, respectively) and one patient is alive with residual disease
6.5 years following resection (Table
I). Notably, all four of them were patients with metastatic
renal cell carcinoma.
Data regarding therapeutic strategies were available
in 31 (97%) patients and one half of patients underwent a surgical
procedure (n=16), including Whipple resection (n=10). A total of 15
(48%) patients received chemotherapy, either in addition to surgery
or alone. Drainage and/or stenting were applied in 10 (31%)
patients, in five patients combined with chemotherapy. For small
lesions, endoscopic (or surgical) ampullectomy appears to be an
alternative with reduced length of inpatient stay, lower morbidity
and mortality (38). However,
endoscopic ampullectomy is considered a ‘high-risk’ procedure due
to potential complications (39).
These can be classified as early (pancreatitis, bleeding,
perforation, and ampullectomy reported from large, tertiary care
referral centres varies between 8 and 35% (39). So far, this technique has not been
applied for secondary ampullary lesions, but it could be the best
approach in cases presenting with mechanical cholestasis or even
cholangitis and poor prognosis due to progress of the systemic
malignant disease.
As seen in our case, ampullary metastases from renal
cell cancer seem to behave differently both in terms of time
intervals until the development of secondary tumours and overall
prognosis. Similarly, in renal cell carcinoma metastatic to the
stomach the mean time interval until the detection of a secondary
tumour to the ampulla was around ten years (40). When complete resection of all
metastatic lesions can be achieved patient show improved survival
(41). Thus, Whipple resection with
or without pylorus preservation which is the standard treatment for
primary ampullary cancer may be also performed in these cases when
all metastatic sites are amenable to wide resection (42,43).
In conclusion, secondary tumours of the ampulla of
Vater are uncommon and malignant melanoma, renal and breast cancer
are the most frequent primaries. Patients may present with jaundice
or upper gastrointestinal bleeding. The time interval between the
diagnosis of the primary tumour and the ampullary metastasis is
highly variable and may be >10 years, particularly in patients
with renal cancer. The management of metastatic ampullary lesions
requires a multi-modal approach, including endoscopic, surgical,
and oncological procedures.
References
|
1
|
Horiguchi S and Kamisawa T: Major duodenal
papilla and its normal anatomy. Dig Surg. 27:90–93. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adsay V, Ohike N, Tajiri T, Kim GE,
Krasinskas A, Balci S, Bagci P, Basturk O, Bandyopadhyay S, Jang
KT, et al: Ampullary region carcinomas: Definition and site
specific classification with delineation of four
clinicopathologically and prognostically distinct subsets in an
analysis of 249 cases. Am J Surg Pathol. 36:1592–1608. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perysinakis I, Margaris I and Kouraklis G:
Ampullary cancer-a separate clinical entity? Histopathology.
64:759–768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albores-Saavedra JHR, Klimstra DS and
Zamboni G: Invasive adenocarcinoma of the ampullary region 2WHO
Classification of Tumours of the Digestive System. Bosman FTCF,
Hruban RH and Theise ND: IARC Press; pp. 87–91. 2010
|
|
5
|
Wei SC, Su WC, Chang MC, Chang YT, Wang CY
and Wong JM: Incidence, endoscopic morphology and distribution of
metastatic lesions in the gastrointestinal tract. J Gastroenterol
Hepatol. 22:827–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
England MD and Sarr MG: Metastatic
melanoma: An unusual cause of obstructive jaundice. Surgery.
107:595–596. 1990.PubMed/NCBI
|
|
7
|
Sans M, Llach J, Bordas JM, Andreu V,
Campo A, Castells A, Mondelo E, Terés J and Rodés J: Metastatic
malignant melanoma of the papilla of Vater: An unusual case of
obstructive cholestasis treated with biliary prostheses. Endoscopy.
28:791–792. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meyers MO, Frey DJ and Levine EA:
Pancreaticoduodenectomy for melanoma metastatic to the duodenum: A
case report and review of the literature. Am Surg. 64:1174–1176.
1998.PubMed/NCBI
|
|
9
|
Caballero-Mendoza E, Gallo-Reynoso S,
Arista-Nasr J and Angeles-Angeles A: Obstructive jaundice as the
first clinical manifestation of a metastatic malignant melanoma in
the ampulla of vater. J Clin Gastroenterol. 29:188–189. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Le Borgne J, Partensky C, Glemain P, Dupas
B and de Kerviller B: Pancreaticoduodenectomy for metastatic
ampullary and pancreatic tumors. Hepatogastroenterology.
47:540–544. 2000.PubMed/NCBI
|
|
11
|
van Bokhoven MM, Aarntzen EH and Tan AC:
Metastatic melanoma of the common bile duct and ampulla of Vater.
Gastrointest Endosc. 63:873–874. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marks JA, Rao AS, Loren D, Witkiewicz A,
Mastrangelo MJ and Berger AC: Malignant melanoma presenting as
obstructive jaundice secondary to metastasis to the Ampulla of
Vater. JOP. 11:173–175. 2010.PubMed/NCBI
|
|
13
|
Uiterwaal MT, Mooi WJ and Van Weyenberg
SJ: Metastatic melanoma of the ampulla of Vater. Dig Liver Dis.
43:e82011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakayama H, Miyazaki S, Kikuchi H, Saito
N, Shimada H, Sakai S, Suzuki M and Kimura K: Malignant vaginal
melanoma with metastases to the papilla of Vater in a dialysis
patient: A case report. Intern Med. 50:345–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McKenna JI and Kozarek RA: Metastatic
hypernephroma to the ampulla of Vater: An unusual cause of
malabsorption diagnosed at endoscopic sphincterotomy. Am J
Gastroenterol. 84:81–83. 1989.PubMed/NCBI
|
|
16
|
Robertson GS and Gertler SL: Late
presentation of metastatic renal cell carcinoma as a bleeding
ampullary mass. Gastrointest Endosc. 36:304–306. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Venu RP, Rolny P, Geenen JE, Hogan WJ,
Komorowski RA and Ferstenberg R: Ampullary tumor caused by
metastatic renal cell carcinoma. Dig Dis Sci. 36:376–378. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bolkier M, Ginesin Y, Moskovitz B,
Munichor M and Levin DR: Obstructive jaundice caused by metastatic
renal cell carcinoma. Eur Urol. 19:87–88. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leslie KA, Tsao JI, Rossi RL and Braasch
JW: Metastatic renal cell carcinoma to ampulla of Vater: An unusual
lesion amenable to surgical resection. Surgery. 119:349–351. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Janzen RM, Ramj AS, Flint JD, Scudamore CH
and Yoshida EM: Obscure gastrointestinal bleeding from an ampullary
tumour in a patient with a remote history of renal cell carcinoma:
A diagnostic conundrum. Can J Gastroenterol. 12:75–78. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mendoza JL, Lana R, Defarges V, Meroño E,
Candía A and Loscos JM: Late metastasis of hypernephroma of the
Vater's ampulla. Rev Esp Enferm Dig. 93:606–608. 2001.PubMed/NCBI
|
|
22
|
Hata T, Sakata N, Aoki T, Yoshida H, Kanno
A, Fujishima F, Motoi F, Masamune A, Shimosegawa T and Unno M:
Repeated pancreatectomy for metachronous duodenal and pancreatic
metastases of renal cell carcinoma. Case Rep Gastroenterol.
7:442–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Titus AS, Baron TH, Listinsky CM and
Vickers SM: Solitary breast metastasis to the ampulla and distal
common bile duct. Am Surg. 63:512–515. 1997.PubMed/NCBI
|
|
24
|
Rego RF, Atiq M, Velchala N, Nevin D,
McElreath DP, McKnight WD and Aduli F: Ampullary metastasis from
breast cancer: An unusual finding. Endoscopy. 41 Suppl 2:E278–E279.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee TH, Park SH, Lee CK, Lee SH, Chung IK,
Kim SJ and Kim SW: Ampulla of Vater metastasis from recurrent
uterine cervix carcinoma presenting as groove pancreatitis.
Gastrointest Endosc. 73:362–363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sreenarasimhaiah J and Hoang MP:
Esophageal squamous cell carcinoma with metastasis to the ampulla.
Gastrointest Endosc. 62:310–311, discussion 311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Büyükçelik A, Ensari A, Sarioğlu M,
Işikdogan A and Içli F: Squamous cell carcinoma of the larynx
metastasized to the ampulla of Vater. Report of a case. Tumori.
89:199–201. 2003.PubMed/NCBI
|
|
28
|
Kamusella P, Wissgott C and Steinkamp HJ:
[Extrahepatic biliary obstruction caused by papillary metastasis of
pulmonary adenocarcinoma]. RoFo Fortschr Geb Rontgenstr Nuklearmed.
179:1272–1273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Silva R, Paiva ME and Santos CC:
Obstructive jaundice caused by ampullary metastases of an
endometrioid adenocarcinoma. Gastrointest Endosc. 44:195–197. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Green D, Rowsell C and Cohen L: Metastatic
bladder carcinoma to ampulla of Vater. Gastrointest Endosc.
68:199–200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kadakia SCPA, Parker A and Canales L:
Metastatic tumors to the upper gastrointestinal tract: Endoscopic
experience. Am J Gastroenterol. 87:1418–1423. 1992.PubMed/NCBI
|
|
32
|
Haidong W, Jianwei W, Guizhong L, Ning L,
Feng H and Libo M: Ampullary tumor caused by metastatic renal cell
carcinoma and literature review. Urol J. 11:1504–1507.
2014.PubMed/NCBI
|
|
33
|
Bastos T, Souza TF, Otoch JP, Grecco E,
Àvila F and Artifon EL: Metastasis of breast cancer to major
duodenal papilla. Rev Gastroenterol Peru. 34:149–150.
2014.PubMed/NCBI
|
|
34
|
Buissin D, Sterle A, Schmiegelow P,
Wassenberg D and Ambe PC: Primary anorectal malignant melanoma: a
rare but aggressive tumor: report of a case. World J Surg Oncol.
13:122015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weigt J and Malfertheiner P: Metastatic
Disease in the Stomach. Gastrointest Tumors. 2:61–64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ito K, Fujita N, Noda Y, Kobayashi G and
Horaguchi J: Diagnosis of ampullary cancer. Dig Surg. 27:115–118.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wiech T, Walch A and Werner M:
Histopathological classification of nonneoplastic and neoplastic
gastrointestinal submucosal lesions. Endoscopy. 37:630–634. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ceppa EP, Burbridge RA, Rialon KL,
Omotosho PA, Emick D, Jowell PS, Branch MS and Pappas TN:
Endoscopic versus surgical ampullectomy: An algorithm to treat
disease of the ampulla of Vater. Ann Surg. 257:315–322. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
De Palma GD, Masone S, Rega M, Simeoli I,
Donisi M, Addeo P, Iannone L, Pilone V and Persico G: Metastatic
tumors to the stomach: Clinical and endoscopic features. World J
Gastroenterol. 12:7326–7328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pollheimer MJ, Hinterleitner TA,
Pollheimer VS, Schlemmer A and Langner C: Renal cell carcinoma
metastatic to the stomach: Single-centre experience and literature
review. BJU Int. 102:315–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Leibovich BCCJ, Cheville JC, Lohse CM,
Zincke H, Frank I, Kwon ED, Merchan JR and Blute ML: A scoring
algorithm to predict survival for patients with metastatic clear
cell renal cell carcinoma: A stratification tool for prospective
clinical trials. J Urol. 174:1759–1763, discussion 1763. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hornick JR, Johnston FM, Simon PO, Younkin
M, Chamberlin M, Mitchem JB, Azar RR, Linehan DC, Strasberg SM,
Edmundowicz SA, et al: A single-institution review of 157 patients
presenting with benign and malignant tumors of the ampulla of
Vater: Management and outcomes. Surgery. 150:169–176. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Klein F, Jacob D, Bahra M, Pelzer U, Puhl
G, Krannich A, Andreou A, Gül S and Guckelberger O: Prognostic
factors for long-term survival in patients with ampullary
carcinoma: The results of a 15-year observation period after
pancreaticoduodenectomy. HPB Surg. 2014:9702342014. View Article : Google Scholar : PubMed/NCBI
|