Introduction
Cervical cancer is one of the most common
malignancies in females worldwide. There were an estimated 527,600
new cervical cancer cases and 265,700 deaths in 2012 according to
cancer statistics (1), despite the
mortality decreasing due to widespread screening programs and the
increased use of the human papillomavirus vaccine in Western
countries.
Although radical surgery and concurrent
chemoradiotherapy (CCRT) have been established as the standard
therapy for patients with localized cervical cancer and locally
advanced carcinoma, one third of patients experience recurrence and
the effect of treatment for recurrence remains far from
satisfactory, resulting in a 5-year survival rate after recurrence
of less than 5% (2,3). Furthermore, little is known about the
factors indicating the prognosis after recurrence. Tokunaga et
al analyzed the effect of chemotherapy on patients with
recurrent uterine cervical cancer (RUCC) after CCRT, and revealed
that the overall response and survival rates did not differ
significantly according to the recurrence site, post-CCRT interval,
or chemotherapy regimen (3).
Additionally, some studies reported the survival benefit of
surgical intervention for recurrent or persistent uterine and
cervical malignancies (4).
Treatments for RUCC mainly depend on the previous treatments and
sites of recurrence (5), and the
prognosis may also be affected by the treatment for recurrence. The
factors that affect the prognosis of RUCC patients remain
controversial.
The aim of this study was to evaluate the long-term
clinical outcome and elucidate the prognostic factors in patients
with RUCC.
Patients and methods
Patients
We retrospectively reviewed all the records of 740
patients with uterine cervical cancer who were initially treated in
our hospital from January 1998 to December 2014. This study was
approved by the Ethics Committee of Nagoya University. One hundred
and sixty-five patients experienced recurrence. Their clinical data
including age, pathological or clinical stage, histological
subtype, initial treatment, recurrence site, date of recurrence
diagnosis, and outcomes were collected. Nine patients with
insufficient information and five patients with stage IA were
excluded, and finally 151 patients were included in the present
analysis.
Methods
Primary treatments for each patient were determined
by several gynecologic oncologists in our hospital depending on
their age, performance status (PS), and International Federation of
Gynecology and Obstetrics (FIGO) stage. For example, early-stage
patients with good PS were indicated for radical hysterectomy with
or without adjuvant CCRT. Patients who were contraindicated for
radical surgery were mostly treated with primary CCRT,
chemotherapy, or radiotherapy (RT) alone. Cisplatin (70
mg/m2, on day 1) and 5-fluorouracil (700
mg/m2, 24-h continuous intravenous infusion, on days
1–4) combination chemotherapy was usually administered as the
initial chemotherapy. When creatinine clearance was <60 ml/min,
nedaplatin or carboplatin was considered instead of cisplatin. RT
involved a combination of external beam radiotherapy (ERBT) and
intra-cavity brachytherapy (ICBT). ERBT was performed at 1.8 Gy
once per day (total dose of 50.4 Gy in 28 fractions). ICBT was
performed during external beam radiation therapy using a remote
after loading the system with a Co 60 source. The total dose to
point A (a reference location 2 cm lateral and 2 cm superior to the
cervical os) was 18–24 Gy. The radiation field extended from the
space between L4 and L5 to the base of the obturator foramen.
At the end of the treatment, all the patients
underwent a strict follow-up consisting of clinical checkups such
as a pelvic examination, ultrasonographic scan, serological tumor
marker evaluation, and periodic computed tomographic scan/positron
emission tomography. Patients with radiologic recurrence were
defined as those who were found to have tumor recurrence based on
computed tomography, magnetic resonance imaging, positron emission
tomography, or ultrasound. Images were viewed by at least a
gynecologic oncologist and a radiologist. Post-recurrence survival
(PRS) was calculated from the date of recurrence diagnosis to the
date of death or that of the last follow-up. Post-recurrence
treatments such as surgical intervention, radiotherapy, and/or
chemotherapy were performed depending on the individual cases. Most
of the patients received several courses of chemotherapy such as
paclitaxel plus carboplatin, irinotecan, or nedaplatin. Some
patients underwent secondary radical surgery including pelvic
exenteration, and some patients received lobectomy for solitary
pulmonary metastasis. Patients who had PAN metastasis or pelvic
recurrence without a history of irradiation received radiotherapy.
In-field recurrence was defined as a recurrence where previous
radiotherapy had been conducted.
Statistical analysis
Statistical analysis was performed using JMP Pro 11.
Survival analysis was based on the Kaplan-Meier method, and
survival curves were compared using the log-rank test.
Multivariable analysis was performed with the Cox proportional
hazard model to evaluate independent factors possibly affecting
survival. P<0.05 was considered significant.
Results
Patient characteristics
Of the 740 cervical cancer patients, 165 patients
experienced recurrence (recurrence rate: 22.3%), and 83 patients
died within a median follow-up of 34.3 months. Of the 165
recurrence patients, 9 were excluded because sufficient information
was not available. In addition, five stage IA patients who
experienced recurrence were also excluded. As a consequence, 151
stage IB-IVB patients who experienced recurrence were included in
this study. Clinicopathologic characteristics of patients are
described in Table I. The median age
was 55 years (range, 20–88) years. The most common clinical stage
according to the FIGO staging system was Stage II (43.7%). Squamous
cell carcinomas (SCC) were the most frequently observed
histological type (63.6%) followed by adenocarcinomas (AC) (29.1%).
As an initial treatment, 70 (46.3%) patients underwent radical
surgery with or without adjuvant therapy, while 81 (53.7%) patients
were treated with primary CCRT or primary RT. Solitary recurrences
in the pelvis were observed in 54 patients (35.8%), and recurrences
in para-aortic lymph nodes (PAN) regardless of pelvic recurrence
were diagnosed in 32 patients (21.2%). Sixty-three (41.7%) patients
experienced recurrence in distant or mixed regions. Generally, in-
or out-field recurrence was observed in 43 and 88 patients,
respectively.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | N | % |
|---|
| Total | 151 |
|
| Median age | 55 |
|
| (Range) | 20–88 |
|
| Stage |
|
|
| IB | 34 | 22.5 |
| II | 66 | 43.7 |
| III | 15 | 9.9 |
| IV | 36 | 23.8 |
| Histological
type |
|
|
| SCC | 96 | 63.6 |
| AC | 44 | 29.1 |
| AS | 7 | 4.6 |
| Small
cell | 4 | 2.6 |
| Initial
treatment |
|
|
|
NAC-surgery | 2 | 1.3 |
|
NACCRT-surgery | 50 | 33.1 |
|
Surgery | 18 | 11.9 |
| Primary
CCRT | 66 | 43.7 |
| Primary
RT | 15 | 9.9 |
| Recurrence site |
|
|
|
Out-field | 88 | 58.3 |
|
In-field | 43 | 28.5 |
| w/o
RT | 20 | 13.2 |
Kaplan-Meier method
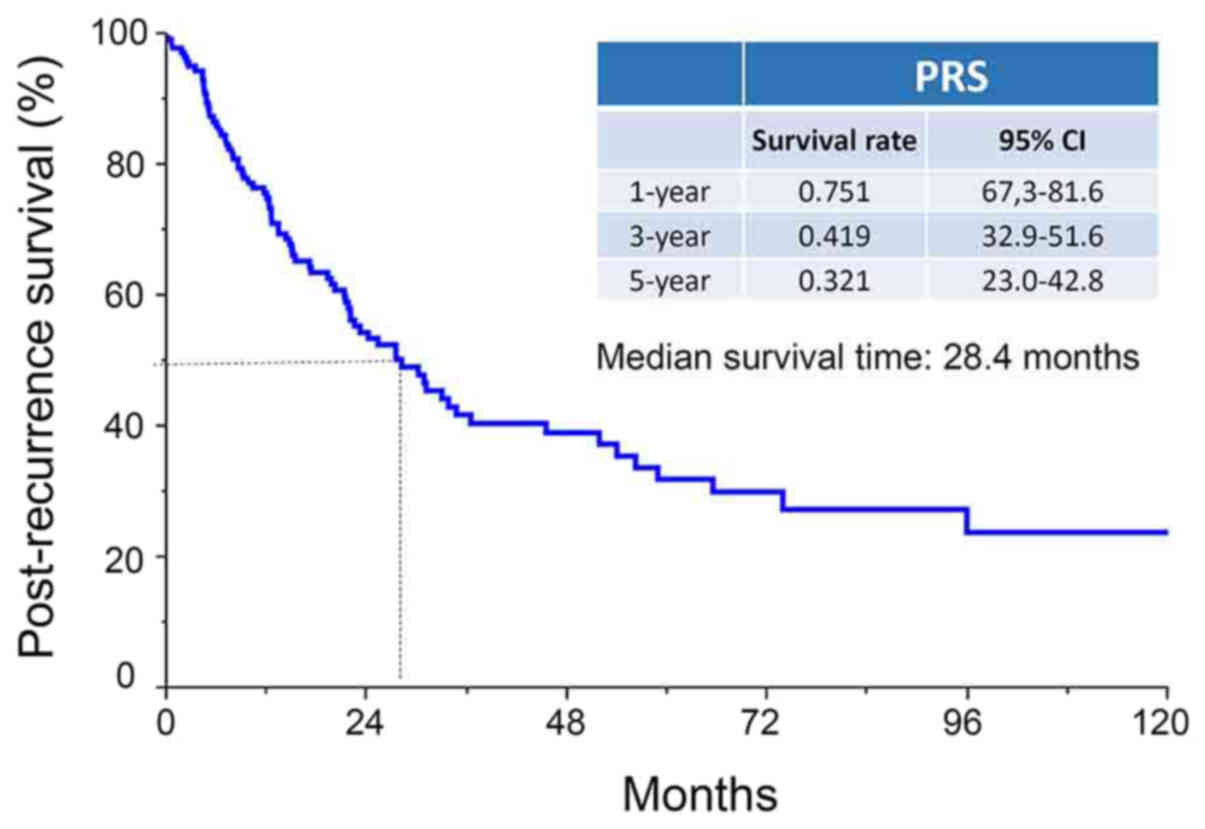
The median PRS period was 28.4 months (range,
0–154.7 months), and the 1-, 3-, and 5-year PRS rates of patients
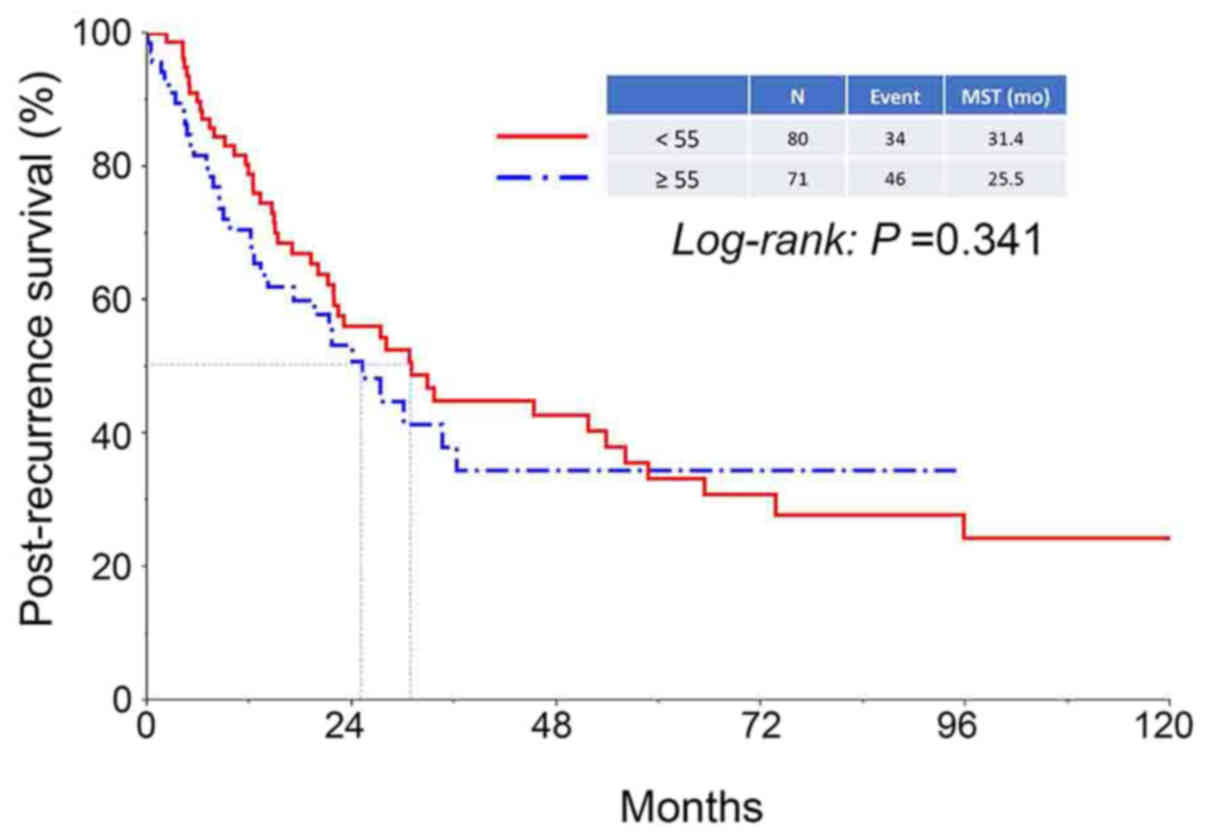
were 75.1, 41.9, and 32.1%, respectively (Fig. 1). There was no difference in PRS of
patients between the two age categories (<55 vs. ≥55 years)
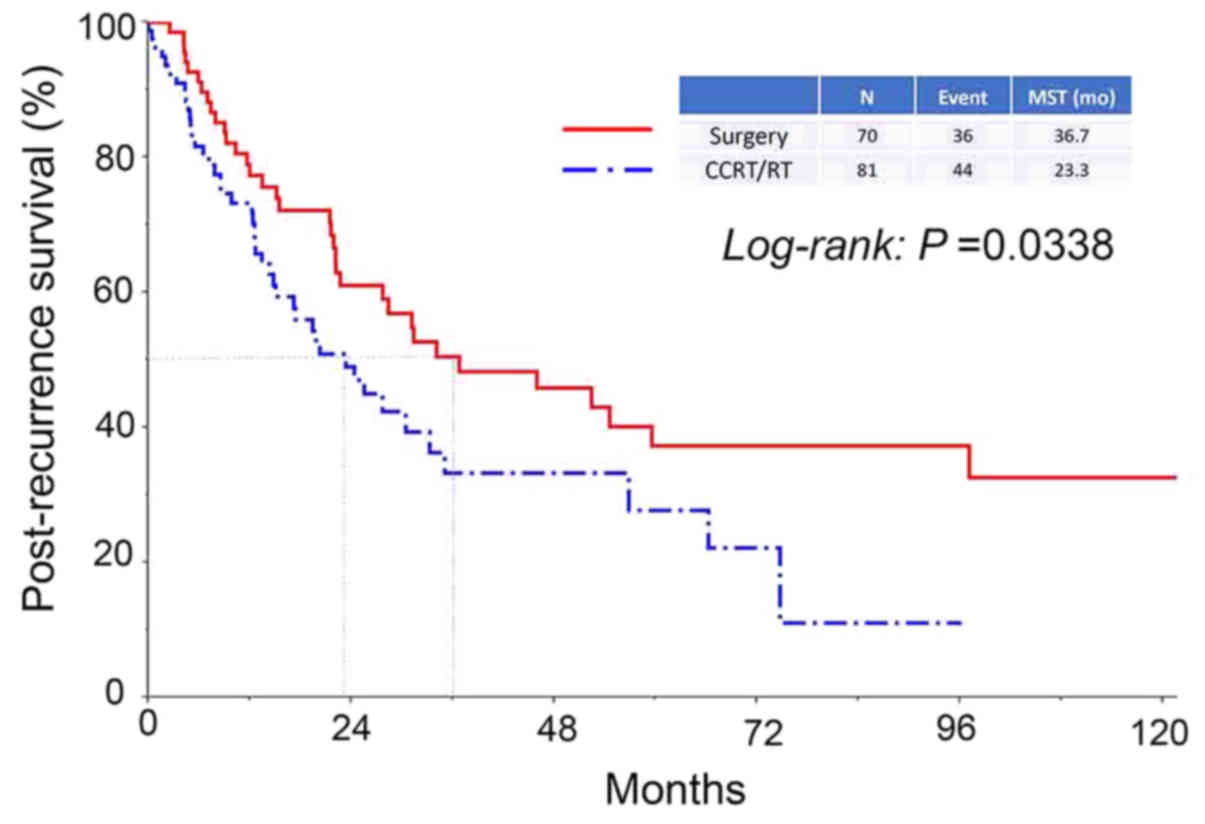
(Fig. 2). In addition, the median
survival period in patients who had received surgery as an initial
treatment was significantly longer compared with that in patients
who had never undergone surgery (36.7 vs. 23.3 months,
respectively, P=0.0338) (Fig. 3).
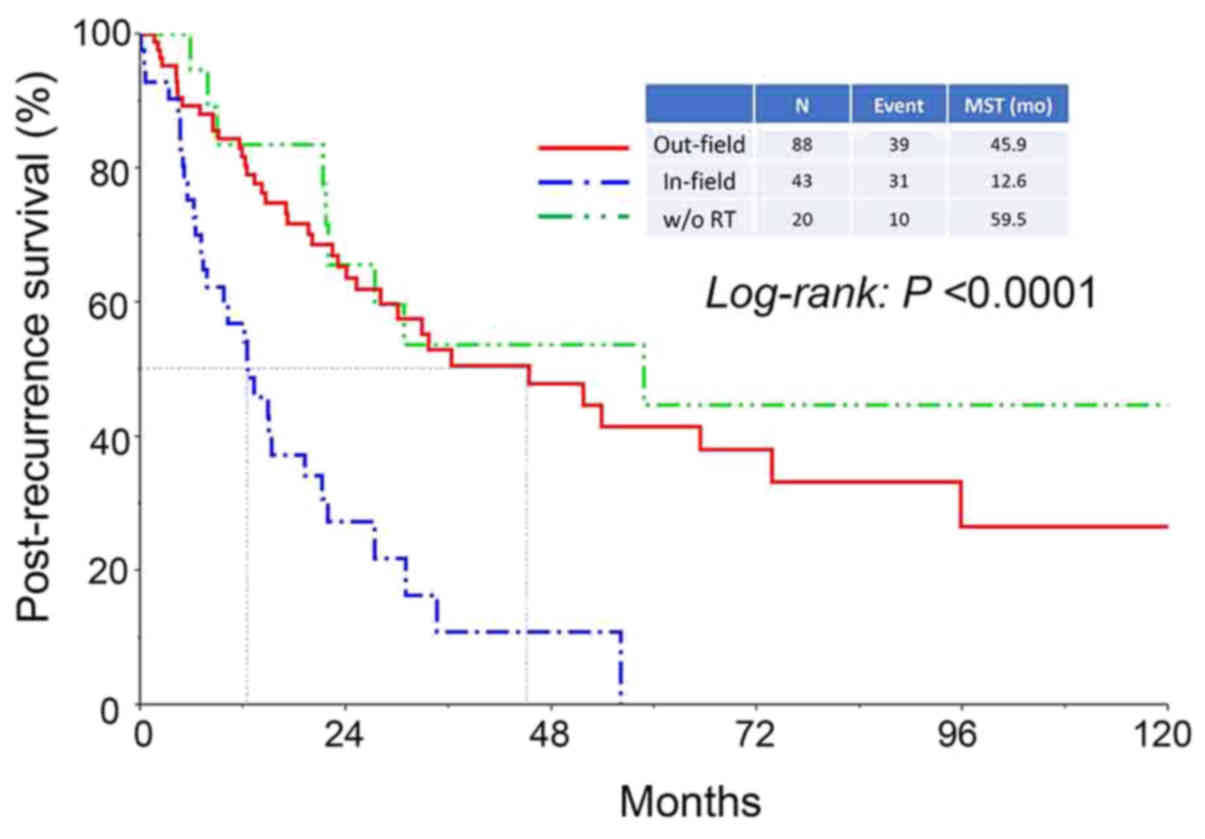
Furthermore, the median survival period in patients who had
experienced in-field recurrence was significantly shorter compared
with that in patients who had experienced out-field or
radiotherapy-free recurrence (in-field vs. out-field: 12.6 vs. 45.9
months, respectively, P<0.0001) (Fig.
4).
Univartiate and multivariate
analyses
Uni- and multivariate analyses of PRS according to
the patients' characteristics and recurrent features were
performed. Age (≤55 vs. >55), FIGO stage (I vs. II/III/IV),
histological type (SCC vs. non-SCC), initial treatment (surgery vs.
CCRT/RT), and recurrence site (in-field vs. out-field vs. RT-free)
were analyzed. The results of multivariate analysis are shown in
Table II. No significant
differences were found in the age, FIGO Stage, histological type,
or modality of initial treatment. However, the in-field recurrence
had an impact on PRS after the multivariate analysis [in-field vs.
out-field: HR (95% CI): 2.848 (1.707–4.738), P<0.0001].
 | Table II.Uni- and multivariable analyses of
clinicopathological parameters in relation to post-recurrence
survival of patients enrolled.a |
Table II.
Uni- and multivariable analyses of
clinicopathological parameters in relation to post-recurrence
survival of patients enrolled.a
|
| Univariable
analysis | Multivariable
analysis |
|---|
|
|
|
|
|---|
| Characteristic | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
|
| <
55 | Referent |
|
| Referent |
|
|
|
| ≥ 55 | 1.245 | 0.511–1.270 | 0.345 | 1.076 | 0.650–1.776 | 0.772 |
| FIGO Stage |
|
|
|
|
|
|
| I | Referent |
|
| Referent |
|
|
|
| II / III
/ IV | 1.367 | 0.824–2.385 | 0.232 | 1.101 | 0.603–2.077 | 0.758 |
| Histological
type |
|
|
|
|
|
|
| SCC | Referent |
|
| Referent |
|
|
|
|
Non-SCC | 0.890 | 0.569–1.412 | 0.616 | 1.202 | 0.725–1.970 | 0.469 |
| Initial
treatment |
|
|
|
|
|
|
|
Surgery | Referent |
|
| Referent |
|
|
|
|
CCRT/RT | 1.627 | 1.036–2.578 | 0.0344 | 1.259 | 0.712–2.255 | 0.429 |
| Recurrence
site |
|
|
|
|
|
|
|
Out-field | Referent |
|
| Referent |
|
|
|
|
In-field | 3.043 | 1.856–4.962 | <0.0001 | 2.848 | 1.707–4.738 | <0.0001 |
| w/o
RT | 0.766 | 0.347–1.515 | 0.461 | 0.854 | 0.359–1.877 | 0.705 |
Discussion
The present study involved a large-scale
retrospective analysis to evaluate the post-recurrence clinical
outcomes of RUCC patients. Although much supporting evidence of
initial treatment including CCRT has been established and the
survival rate has been improved (6,7), little
is known regarding the optimal treatment for patients with RUCC,
and the prognosis of RUCC patients is poor. In this study, we
analyzed clinical outcomes of patients with RUCC in our institute
and evaluated prognostic factors concerning PRS instead of overall
survival (OS) or progression-free survival (PFS). The recurrence
rate of all the patients was 22.3%, which was consistent with
previous reports (8–12). As expected, patients who experienced
recurrence had an extremely poor prognosis (median PRS of 28.4
months and 5-year PRS rate of 32.1%). We identified two prognostic
factors affecting PRS. The in-field recurrence and ineligibility
for primary radical surgery were significantly correlated with a
poor prognosis.
We showed the significant impact of initial surgery
on PRS. According to the findings of Rungruang et al,
patients with stage IB2 cervical cancer, and patients who received
primary surgery showed a longer survival than those who received
primary radiotherapy regardless of chemotherapy. Although the
details of chemotherapy were not clear, it was a large-scale study
using the Surveillance, Epidemiology, and End Results (SEER)
database (13). In addition, Derks
et al suggested that radical surgery may be a good treatment
option for patients with stage IB2/IIA2 cervical cancer because
patients who received surgery-based treatment showed relatively
longer survival (14). Therefore,
surgical intervention is considered a favorable treatment for local
cervical carcinoma. Furthermore, some reports showed an impact of
the surgical procedure on advanced or recurrent cervical cancer
(8,15).
We showed that pelvic recurrence was significantly
correlated with a poor prognosis. Legge et al reported that
the median survival of patients with visceral (e.g., lungs, bones,
liver, brain) or lymph nodal metastatic relapse was significantly
better compared with that in patients with pelvic recurrence
(8). By contrast, other reports
concluded that pelvic recurrence could be salvaged with CCRT in
patients who were not initially treated with radiation therapy, and
that central pelvic recurrence might be salvaged with pelvic
exenteration (16–18). Additionally, it has been considered
that vaginal recurrence after radical surgery was related to a good
prognosis (12), while distant
metastases have been considered incurable except for isolated
pulmonary metastasis (16,17). Concerning PAN metastasis, the
prognosis of those patients may not be worse than expected because
CCRT contributed to longer survival (5,12,19). A
previous report suggested that there was no difference in the
survival rate between patients with recurrence in a previously
irradiated field and those with recurrence in an extra-irradiated
field (3). However, the unfavorable
outcome of in-field recurrence in our study is due to the fact that
tumor recurred in the irradiated field may have a more aggressive
biological behavior with more radio- and/or chemo-resistant
hallmark. In the actual clinical situation, treatment options for
such tumors are extremely limited.
Finally, considering our two negative prognostic
factors, local control of carcinoma by radical surgery may be
important for survival. In addition, another study reported that
both OS and PFS were improved with neoadjuvant chemotherapy
followed by radical surgery for patients with early-stage or
locally-advanced cervical cancer (20).
There are several limitations to the present study
because of the retrospective nature based on our clinical records.
First, detailed information including the extent of the disease,
performance status, and intraoperative findings was unclear.
Second, because our cases were accumulated over a long time, the
salvage chemotherapy for RUCC was not necessarily homogenous.
Furthermore, information on salvage cytoreductive surgery was
lacking. Tumor debulking for solitary mass of RUCC appears to be
effective. We hope to verify this in a future study.
In conclusion, the performance of radical surgery as
an initial treatment and out-field/RT-free recurrence are good
prognostic factors after the recurrence of cervical cancer.
Particularly, in-field recurrence was an independent indicator for
poorer post-recurrence survival. Coping with this type of
recurrence is major critical issue for the improvement of patient
prognosis. If a tumor is limited in the central part of pelvis
without dense adhesion to the pelvic wall, we should not hesitate
to carry out the pelvic exenteration. However, if we encounter a
patient who experience in-field recurrence, with chemoresistance,
earlier induction of palliative care needs to be considered rather
than continuing chemotherapy. To assess the further appropriateness
of in-field recurrence, we would like to accumulate more cases and
reconfirm the current results in the future.
Glossary
Abbreviations
Abbreviations:
|
RUCC
|
recurrent uterine cervical
carcinoma
|
|
PRS
|
post-recurrence survival
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global Cancer Statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allemani C, Weir HK, Carreira H, Harewood
R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A,
et al: Global surveillance of cancer survival 1995–2009: Analysis
of individual data for 25,676,887 patients from 279
population-based registries in 67 countries (CONCORD-2). Lancet.
385:977–1010. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tokunaga H, Nakanishi T, Iwata T, Aoki D,
Saito T, Nagase S, Takahashi F, Yaegashi N and Watanabe Y: Effects
of chemotherapy on patients with recurrent cervical cancer
previously treated with concurrent chemoradiotherapy: A
retrospective multicenter survey in Japan. Int J Clin Oncol.
20:561–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andikyan V, Khoury-Collado F, Sonoda Y,
Gerst SR, Alektiar KM, Sandhu JS, Bochner BH, Barakat RR, Boland PJ
and Chi DS: Extended pelvic resections for recurrent or persistent
uterine and cervical malignancies: An update on out of the box
surgery. Gynecol Oncol. 125:404–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gadducci A, Tana R, Cosio S and Cionini L:
Treatment options in recurrent cervical cancer (Review). Oncol
Lett. 1:3–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morris M, Eifel PJ, Lu J, Grigsby PW,
Levenback C, Stevens RE, Rotman M, Gershenson DM and Mutch DG:
Pelvic radiation with concurrent chemotherapy compared with pelvic
and para-aortic radiation for high-risk cervical cancer. N Engl J
Med. 340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Green JA, Kirwan JM, Tierney JF, Symonds
P, Fresco L, Collingwood M and Williams CJ: Survival and recurrence
after concomitant chemotherapy and radiotherapy for cancer of the
uterine cervix: A systematic review and meta-analysis. Lancet.
358:781–786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Legge F, Chiantera V, Macchia G, Fagotti
A, Fanfani F, Ercoli A, Gallotta V, Morganti AG, Valentini V,
Scambia G and Ferrandina G: Clinical outcome of recurrent locally
advanced cervical cancer (LACC) submitted to primary multimodality
therapies. Gynecol Oncol. 138:83–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gadducci A, Sartori E, Maggino T, Zola P,
Cosio S, Zizioli V, Lapresa M, Piovano E and Landoni F:
Pathological response on surgical samples is an independent
prognostic variable for patients with Stage Ib2-IIb cervical cancer
treated with neoadjuvant chemotherapy and radical hysterectomy: An
Italian multicenter retrospective study (CTF Study). Gynecol Oncol.
131:640–644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh AK, Grigsby PW, Rader JS, Mutch DG
and Powell MA: Cervix carcinoma, concurrent chemoradiotherapy, and
salvage of isolated paraaortic lymph node recurrence. Int J Radiat
Oncol Biol Phys. 61:450–455. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rose PG, Java J, Whitney CW, Stehman FB,
Lanciano R, Thomas GM and DiSilvestro PA: Nomograms predicting
progression-free survival, overall survival, and pelvic recurrence
in locally advanced cervical cancer developed from an analysis of
identifiable prognostic factors in patients from NRG
Oncology/Gynecologic oncology group randomized trials of
chemoradiotherapy. J Clin Oncol. 33:2136–2142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiu JT, Abdullah NA, Chou HH, Lin CT, Jung
SM, Wang CC, Chen MY, Huang KG, Chang TC and Lai CH: Outcomes and
prognosis of patients with recurrent cervical cancer after radical
hysterectomy. Gynecol Oncol. 127:472–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rungruang B, Courtney-Brooks M, Beriwal S,
Zorn KK, Richard SD, Olawaiye AB, Krivak TC and Sukumvanich P:
Surgery versus radiation therapy for stage IB2 cervical carcinoma:
A population-based analysis. Int J Gynecol Cancer. 22:484–489.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Derks M, Biewenga P, van der Velden J,
Kenter GG, Stalpers LJA and Buist MR: Results of radical surgery in
women with stage IB2/IIA2 cervical cancer. Acta Obstet Gynecol
Scand. 95:166–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmidt AM, Imesch P, Fink D and Egger H:
Indications and long-term clinical outcomes in 282 patients with
pelvic exenteration for advanced or recurrent cervical cancer.
Gynecol Oncol. 125:604–609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elit L and Reade CJ: Recommendations for
Follow-up care for gynecologic cancer survivors. Obstet Gynecol.
126:1207–1214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mabuchi S, Isohashi F, Yoshioka Y, Temma
K, Takeda T, Yamamoto T, Enomoto T, Morishige K, Inoue T and Kimura
T: Prognostic factors for survival in patients with recurrent
cervical cancer previously treated with radiotherapy. Int J Gynecol
Cancer. 20:834–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jang H, Chun M, Cho O, Heo JS, Ryu HS and
Chang SJ: Prognostic factors and treatment outcome after
radiotherapy in cervical cancer patients with isolated para-aortic
lymph node metastases. J Gynecol Oncol. 24:229–235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rydzewska L, Tierney J, Vale CL and
Symonds PR: Neoadjuvant chemotherapy plus surgery versus surgery
for cervical cancer. Cochrane Database Syst Rev.
12:CD0074062012.PubMed/NCBI
|