Introduction
Small-cell lung cancer (SCLC) is an aggressive type
of lung cancer exhibiting rapid growth and widespread metastases,
with a poor prognosis (1). Unlike
therapy for lung adenocarcinoma (LADC), the treatment for SCLC has
not significantly advanced over the last three decades (2–4). To
understand the genomic landscape and identify candidate therapeutic
targets in SCLC, large-scale genomic analyses were performed, which
revealed that mutations in TP53 and RB1, and
amplifications of the MYC family members SOX2 and
SRSF1, have been recurrently identified (5–8).
Although it was reported that somatic genomic rearrangements of
TP73 contribute to SCLC tumorigenesis (5), druggable gene aberrations are rarely
identified. As there is no standard targeted therapy for SCLC,
platinum-based doublet chemotherapy is recommended as first-line
treatment for advanced SCLC; however, its effectiveness is limited
(2). Therefore, there is a need for
development of further treatment options for patients with
SCLC.
The blockade of immune checkpoints in cancer
immunotherapy has exhibited durable positive efficacy in
non-small-cell lung cancer (NSCLC), particularly LADC (9–11).
Recent clinical trials have further investigated the efficacy of
monotherapy with nivolumab (12) and
of combination therapy with platinum-based doublet regimens
(13). Due to these positive
treatment effects on NSCLC, the blockade of immune checkpoints is
also expected to be a useful therapy for SCLC. Although efforts
have been made to develop a biomarker to identify patients who may
benefit from immunotherapy (14),
such a biomarker has yet to be determined. Previous reports
demonstrated that the programmed death-ligand 1 (PD-L1) protein in
tumor cells is a potential predictive biomarker of response to
anti-PD-1/PD-L1 immunotherapy (10,15,16).
Furthermore, PD-L1 expression in tumors was reported to be
associated with improved efficacy of pembrolizumab (15) and with significantly longer
progression-free and overall survival (17). We recently revealed the mechanism
through which high expression of PD-L1 is caused by focal
amplification of CD274, encoding the PD-L1 protein in SCLC
(18). Although only a subset of
SCLC tumors highly express PD-L1, such SCLC tumors may be
particularly susceptible to immune checkpoint blockade therapy.
Therefore, the aim of the present study was to investigate PD-L1
expression in an independent cohort of SCLCs, in order to identify
a candidate responder to PD-L1 blockade therapy.
Delta-like protein 3 (DLL3) and enhancer of zeste
homologue 2 (EZH2) expression was also investigated in SCLC tumors.
DLL3 is highly expressed in the majority of SCLCs and inhibits the
Notch receptor pathway, promoting SCLC tumorigenesis (19). A recent clinical trial demonstrated
that rovalpituzumab tesirine (Rova-T), a DLL3-targeted
antibody-drug conjugate, exhibited marked antitumor activity and
durability in recurrent or refractory SCLC (20). EZH2 is also highly expressed in SCLC,
and its inhibition by EZH2 inhibitor enhances the effectiveness of
current standard chemotherapy (21).
Higher expression of DLL3 and EZH2 in SCLC was associated with a
higher rate of response to Rova-T and EZH2 inhibitor, respectively;
thus, DLL3 and EZH2 expression may be a candidate predictive
biomarker for SCLC treatment.
Patients and methods
Patients
The present study included 20 primary and two
metastatic tumors obtained from 20 SCLC patients at surgery or
autopsy performed between 1991 and 2013 at Akita University (Akita,
Japan). We retrospectively collected information regarding age,
sex, ethnicity, pathological TNM stage (22,23), and
smoking status. The present study was approved by the Ethics
Committee of Akita University (reference nos. 1241 and 1246) and
written informed consent was obtained from all patients.
Immunohistochemical (IHC) staining and
evaluation
IHC staining for PD-L1 was performed as previously
described (18). In addition, IHC
staining for DLL3 and EZH2 were performed. Briefly, IHC staining
was performed on 4-µm paraffin-embedded histological sections that
had been fixed in 10% buffered formalin using a polymer peroxidase
method (EnVision+/HRP; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA). Following deparaffinization with xylene and
rehydration using a descending alcohol series, the tissue sections
were treated with 0.3% hydrogen peroxide in methanol for 30 min at
room temperature to block endogenous peroxidase activity. Following
rinsing in PBS, the sections were incubated with rabbit monoclonal
anti-PD-L1 (1:400; cat. no. 13684, E1L3N; Cell Signaling
Technology, Danvers, MA, USA), rabbit polyclonal anti-DLL3 (1:100;
ab103102, Abcam, Cambridge, MA, USA), and rabbit monoclonal
anti-EZH2 (1:100; cat. no. 12408, D2C9, Cell Signaling Technology)
at 4°C overnight. An additional wash in PBS was followed by
treatment with a ready-to-use peroxidase-labeled polymer conjugated
to goat anti-rabbit immunoglobulins (catalog no. SM801; EnVision+
kit; Dako; Agilent Technologies, Inc.) as the secondary antibody
for 30 min at room temperature. The staining was visualized with
diaminobenzidine, followed by counterstaining with hematoxylin.
IHC staining for PD-L1, DLL3 and EZH2 was evaluated
by two independent observers (M.S. and A.G.), including an expert
pathologist (A.G.). For the evaluation of PD-L1 and EZH2, the
H-score method was used (18).
Briefly, staining percentages (0–100%) and the intensity (0,
negative; 1, very weak; 2, moderate; and 3, strong expression) in
tumor cells were evaluated, and immunostained slides were scored
ranging from 0 to 300 by multiplying the percentage of tumor area.
For the evaluation of PD-L1, the score was divided into two
intensity levels (positive, ≥3; and negative, 0–2) due to antibody
specificity (18). For the
evaluation of EZH2, the score was divided into two intensity levels
(positive, >100; and negative, ≤100), according to the previous
study (24).
For the evaluation of DLL3, cytoplasmic or
membranous staining at any intensity in the tumor cells was scored.
The staining percentage (0–100%) was then evaluated and divided
into two intensity levels (high, ≥50%; and low, 0–49%), according
to the previous study (20).
Results
PD-L1 expression in SCLC
We previously reported that a subset (4/210, 1.9%)
of SCLC cases exhibited high expression of PD-L1 caused by
high-level amplification of CD274 (18). As PD-L1-positive cases are rarely
identified in SCLC, we further expanded the investigation to
include 20 Japanese patients with SCLC (Table I). We performed IHC analyses of PD-L1
protein expression with the same anti-PD-L1 antibody used in our
previous study (18), and identified
one case (1/20, 5.0%) with positive PD-L1 expression (Fig. 1). This patient (AK014) had liver and
lymph node metastases, and the metastatic tumors were positive for
PD-L1 expression.
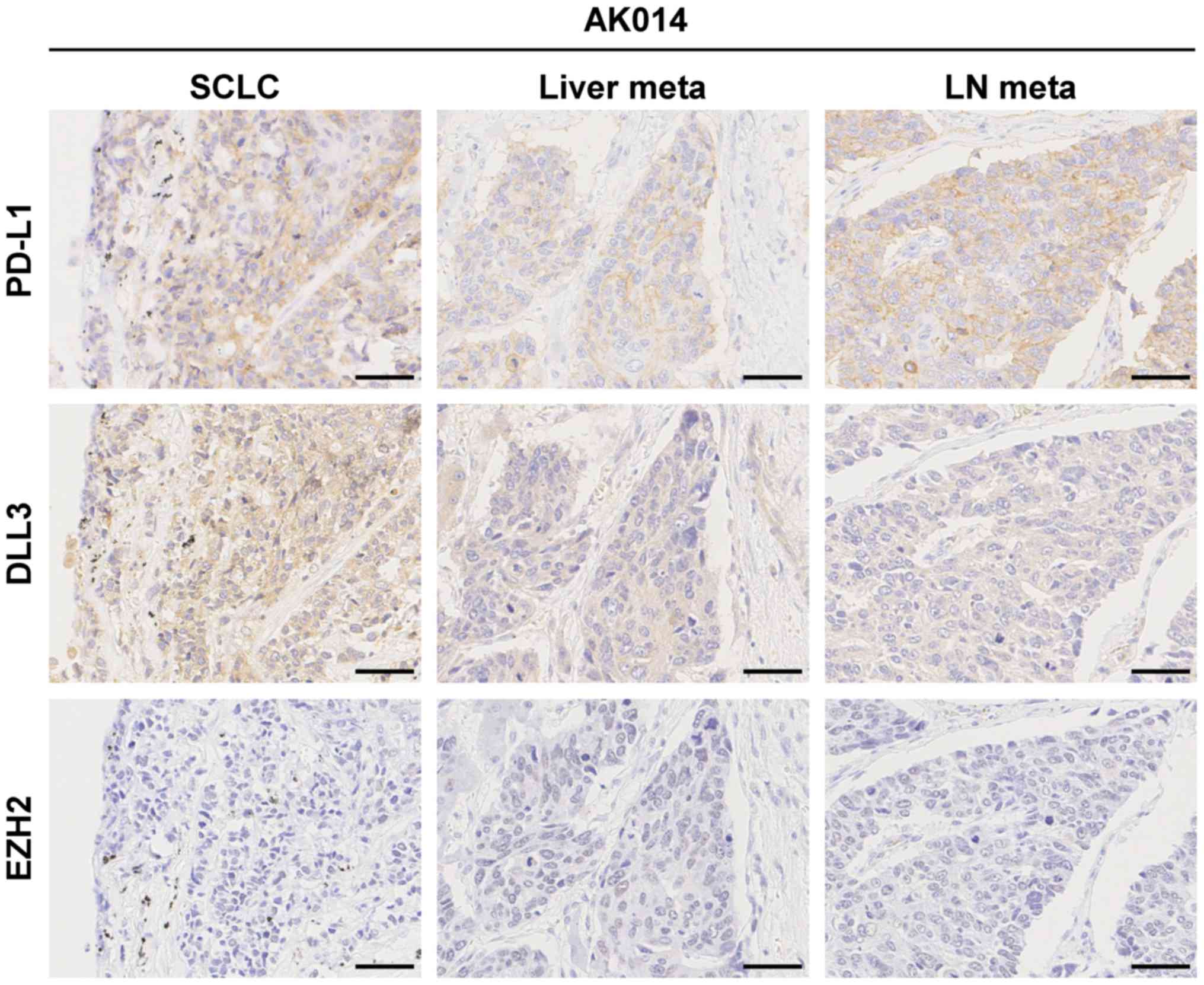 | Figure 1.Immunohistochemical staining of PD-L1,
DLL3, and EZH2 in SCLC. Positive expression for PD-L1, high
expression for DLL3, and negative expression for EZH2 in SCLC and
metastatic liver tumor and lymph node in a patient (AK014). Scale
bars, 50 µm. SCLC, small-cell lung cancer; PD-L1, programmed
death-ligand 1; DLL3, delta-like protein 3; EZH2, enhancer of zeste
homologue 2; meta, metastasis. |
 | Table I.Patient characteristics of this study
cohort (n=20). |
Table I.
Patient characteristics of this study
cohort (n=20).
| Characteristics | No. (%) |
|---|
| Age, years |
|
| Mean
(range) | 68.0 (45–82) |
| Sex |
|
| Male | 20 (100) |
|
Female | 0 (0) |
| Ethnicity (%) |
|
|
Asian | 20 (100) |
|
Caucasian | 0 (0) |
| African
american | 0 (0) |
| Smoking status |
|
| Never
smoker | 1 (5) |
| Current
or former smoker (pack years) | 19 (95) |
|
<20 | 17 (85) |
| ≥20 | 0 (0) |
|
Unknown | 2 (10) |
| TNM stage |
|
| I | 6 (30) |
| II | 1 (5) |
| III | 8 (40) |
| IV | 5 (25) |
DLL3 and EZH2 expression in SCLC
IHC staining was next performed for DLL3 and EZH2 to
identify candidate responders for Rova-T and EZH2 inhibitor therapy
(Figs. 1 and 2). DLL3 expression of ≥1% was observed in
18/20 cases (90%) and of ≥50% in 14/20 cases (70%). The staining
intensity of DLL3 was consistent with a previous report from the
USA (Table II) (20). EZH2 was positively expressed in 17/20
patients (85%) in our cohort. A case with positive PD-L1 expression
exhibited high DLL3 and negative EZH2 expression in a metastatic
liver tumor and a lymph node metastasis, as well as in a primary
SCLC tumor (Fig. 1).
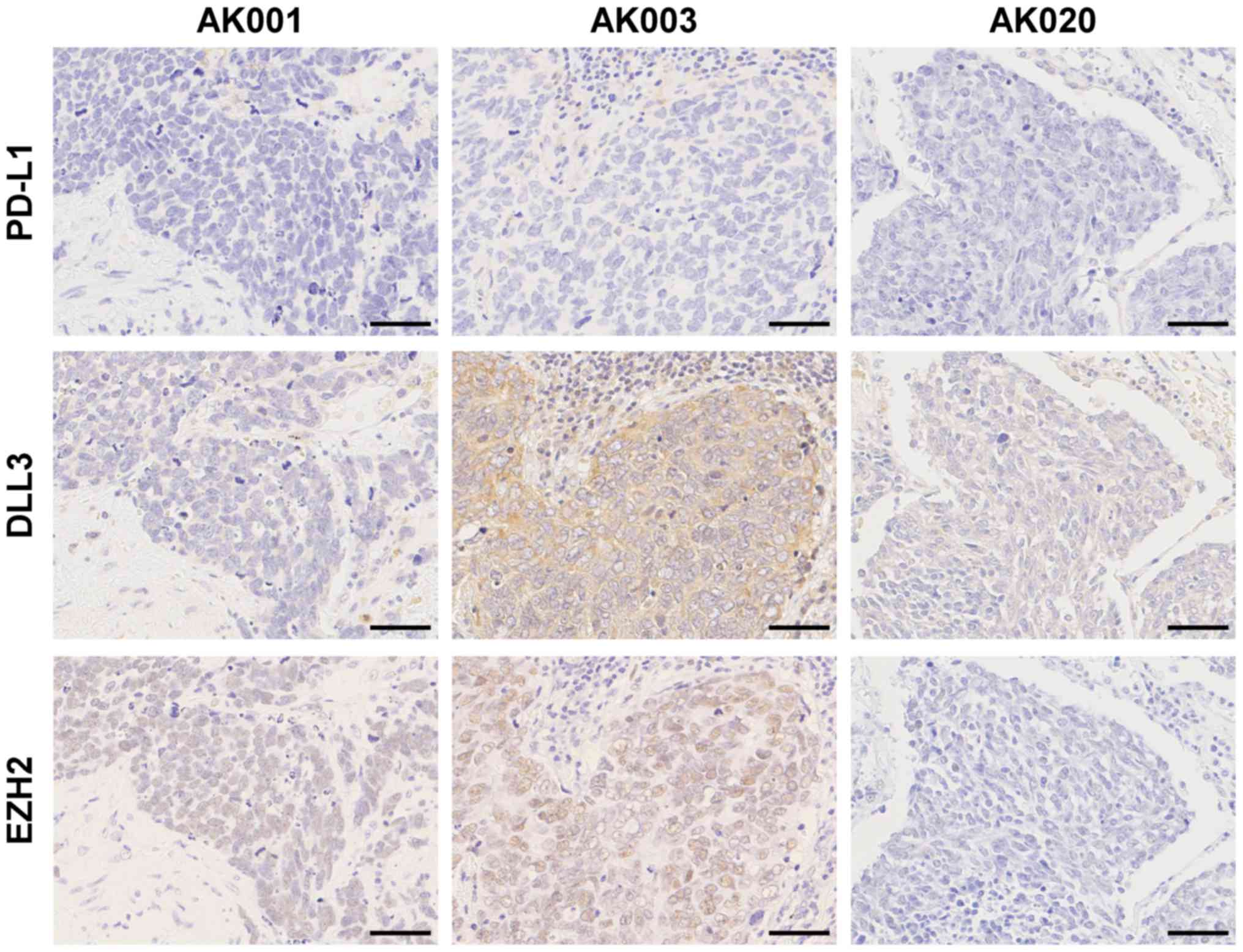 | Figure 2.Immunohistochemical staining of PD-L1,
DLL3, and EZH2 in SCLC. Negative expression for PD-L1, low
expression of DLL3, and positive expression for EZH2 in a patient
(AK001). Negative expression for PD-L1, high expression of DLL3,
and positive expression for EZH2 in a patient (AK003). Negative
expression for PD-L1, low expression of DLL3, and negative
expression for EZH2 in a patient (AK020). Scale bars, 50 µm. SCLC,
small-cell lung cancer; PD-L1, programmed death-ligand 1; DLL3,
delta-like protein 3; EZH2, enhancer of zeste homologue 2. |
 | Table II.Comparison of delta-like protein 3
expression in small-cell lung cancer. |
Table II.
Comparison of delta-like protein 3
expression in small-cell lung cancer.
| Positively stained
tumor cells (%) | Present study,
n/total (%) (n=20) | Rudin et al
(20), n/total (%) (n=48) |
|---|
| ≥1 | 18/20 (90) | 42/48 (88) |
| ≥50 | 14/20 (70) | 32/48 (67) |
Therapeutic possibilities for
SCLC
The clinicopathological characteristics, treatment
history and results of IHC staining for PD-L1, DLL3 and EZH2 in the
20 cases are summarized in Table
III. The cases with high DLL3 or positive EZH2 expression did
not have any specific characteristics associated with age, sex,
smoking, stage or treatment (Table
III). Among the 20 cases, 1 exhibited positive PD-L1
expression, 14 exhibited high DLL3 expression, and 17 exhibited
positive EZH2 expression. This result suggested that PD-L1 blockade
therapy, Rova-T therapy, or EZH2 inhibitor therapy, may be used in
19/20 cases (95%).
 | Table III.Clinicopathological characteristics
and IHC staining results of SCLC. |
Table III.
Clinicopathological characteristics
and IHC staining results of SCLC.
|
|
|
|
|
|
|
| IHC staining |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Patient ID | Age, years | Sex | Smoking (pack
years) | pStage | 1st treatment | 2nd treatment | PD-L1 | DLL3 | EZH2 |
|---|
| AK001 | 66 | Male | 46 | I | Surgery |
| Negative | Low | Positive |
| AK002 | 80 | Male | 65 | III | Surgery |
| Negative | High | Positive |
| AK003 | 82 | Male | 58 | I | Surgery |
| Negative | High | Positive |
| AK004 | 79 | Male | 40 | I | Surgery |
| Negative | High | Positive |
| AK005 | 79 | Male | 87 | I | Surgery |
| Negative | Low | Positive |
| AK006 | 45 | Male | 25 | II | Chemotherapy | Surgery | Negative | High | Positive |
| AK007 | 77 | Male | N/A | III | Surgery |
| Negative | Low | Positive |
| AK008 | 60 | Male | 40 | I | Surgery |
| Negative | High | Positive |
| AK009 | 77 | Male | 53 | I | Surgery |
| Negative | High | Positive |
| AK010 | 71 | Male | 25 | III | Surgery |
| Negative | Low | Positive |
| AK011 | 74 | Male | 120 | IV |
Chemotherapy/radiation |
| Negative | High | Positive |
| AK012 | 59 | Male | 50 | IV | Chemotherapy |
| Negative | High | Positive |
| AK013 | 63 | Male | 75 | III | Chemotherapy |
| Negative | High | Positive |
| AK014 | 68 | Male | 0 | IV | Radiation |
| Positive | High | Negative |
| AK015 | 74 | Male | 100 | III | Chemotherapy |
| Negative | High | Positive |
| AK016 | 68 | Male | 23 | IV |
Chemotherapy/radiation |
| Negative | High | Positive |
| AK017 | 64 | Male | 50 | III |
Chemotherapy/radiation |
| Negative | High | Positive |
| AK018 | 47 | Male | 60 | III |
Chemotherapy/radiation |
| Negative | Low | Positive |
| AK019 | 59 | Male | 40 | IV |
Chemotherapy/radiation |
| Negative | High | Negative |
| AK020 | 68 | Male | N/A | III | Chemotherapy |
| Negative | Low | Negative |
Discussion
In the present study, it was reconfirmed that tumor
expression of PD-L1 is rarely found to be upregulated at the
protein level in SCLC. IHC analyses of PD-L1 protein expression was
performed, employing the PD-L1 antibody E1L3 N, which was used in
our previous study (18). The
specificity of this antibody for PD-L1 was considered as high,
since one case with high-level focal CD274 amplification and
high transcript levels only exhibited positive PD-L1 protein
expression in our previous study (18). Therefore, we concluded that focal
CD274 amplification is associated with high PD-L1 antigen
expression (18). The present study
evaluated PD-L1 expression in cases with SCLC; however, the
amplification of CD274 was not investigated due to the lack
of frozen tissue samples.
Anti-PD-1/PD-L1 immunotherapy, alone or in
combination with other treatment modalities, exhibits a significant
efficacy for patients with various malignant tumors, including
NSCLC. Predictive biomarkers, enabling the selection of patients
who will benefit the most from PD-1/PD-L1-targeted therapy and the
prevention of adverse events, are required to further increase
positive outcomes. Although several studies have attempted to
develop a predictive biomarker using IHC, including staining for
PD-L1, there is currently no reliable predictive biomarker of this
immunotherapy due to cancer immune complexity. In addition, PD-L1
antibodies used in IHC staining differ among various studies and
different cut-off values of PD-L1 positivity are used, making it
difficult to compare results across studies. Indeed, different
companion antibodies of PD-L1 made by various pharmaceutical
manufacturers have been used in clinical trials (25). Although PD-L1 expression was
evaluated by IHC staining of SCLC cells in the present study, it
has not been established whether PD-L1 expression is correlated
with clinical response and outcome.
DLL3 expression was also evaluated in SCLC tumors.
DLL3 is a novel druggable target of Rova-T. Rova-T is a
first-in-class antibody-drug conjugate directed against DLL3, and
early-phase clinical trials of Rova-T assessed DLL3 expression by
IHC staining. While objective responses were recorded in patients
with high DLL3 expression, those with low DLL3 expression had no
recorded objective responses (20).
Therefore, DLL3 expression may be a therapeutic biomarker, as well
as a therapeutic target. In fact, it was reported that DLL3 was
expressed on the surface of tumor cells in ~85% of SCLC patients
(19,20). Consistently, DLL3 was highly
expressed in our Japanese SCLC patients, and may be expected to be
a candidate responder to Rova-T therapy. In addition to DLL3, EZH2
expression was evaluated in SCLC tumors. EZH2 is also expected to
be a candidate therapeutic target for SCLC. However, no clinical
trials targeting EZH2 in SCLC patients are currently underway.
The early success of Rova-T is accentuating the
significance of targeted therapy for improving the prognosis of
patients with SCLC. Thus, EZH2 inhibition, as well as Rova-T
therapy, may be an option for patients with recurrent SCLC. In
cases where first-line treatment results in failure, monitoring the
status of PD-L1, DLL3, or EZH2 in recurrent or metastatic tumors
may serve as the next regimen.
In conclusion, PD-L1, DLL3 and EZH2 expression was
evaluated in additional SCLC patients following our previous study,
to investigate the adoption of precision medicine. In addition to
anti-PD-1/PD-L1 immunotherapy, Rova-T therapy or other DLL3- and
EZH2-targeted drugs may be expected to be proven useful for the
treatment of SCLC patients.
Acknowledgements
The present study was supported by JSPS KAKENHI
(grant no. 15K10275).
References
|
1
|
Govindan R, Page N, Morgensztern D, Read
W, Tierney R, Vlahiotis A, Spitznagel EL and Piccirillo J: Changing
epidemiology of small-cell lung cancer in the United States over
the last 30 years: Analysis of the surveillance, epidemiologic and
end results database. J Clin Oncol. 24:4539–4544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sabari JK, Lok BH, Laird JH, Poirier JT
and Rudin CM: Unravelling the biology of SCLC: Implications for
therapy. Nat Rev Clin Oncol. 14:549–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koinis F, Kotsakis A and Georgoulias V:
Small cell lung cancer (SCLC): No treatment advances in recent
years. Transl Lung Cancer Res. 5:39–50. 2016.PubMed/NCBI
|
|
4
|
Saito M, Suzuki H, Kono K, Takenoshita S
and Kohno T: Treatment of lung adenocarcinoma by molecular-targeted
therapy and immunotherapy. Surg Today. 2017.
|
|
5
|
George J, Lim JS, Jang SJ, Cun Y, Ozretić
L, Kong G, Leenders F, Lu X, Fernández-Cuesta L, Bosco G, et al:
Comprehensive genomic profiles of small cell lung cancer. Nature.
524:47–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iwakawa R, Kohno T, Totoki Y, Shibata T,
Tsuchihara K, Mimaki S, Tsuta K, Narita Y, Nishikawa R, Noguchi M,
et al: Expression and clinical significance of genes frequently
mutated in small cell lung cancers defined by whole exome/RNA
sequencing. Carcinogenesis. 36:616–621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iwakawa R, Takenaka M, Kohno T, Shimada Y,
Totoki Y, Shibata T, Tsuta K, Nishikawa R, Noguchi M, Sato-Otsubo
A, et al: Genome-wide identification of genes with amplification
and/or fusion in small cell lung cancer. Genes Chromosomes Cancer.
52:802–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Semenova EA, Nagel R and Berns A: Origins,
genetic landscape and emerging therapies of small cell lung cancer.
Genes Dev. 29:1447–1462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity and immune correlates
of anti-PD-1 antibody in cancer. N Engl J Med. 366:2443–2454. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gettinger S, Rizvi NA, Chow LQ, Borghaei
H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman
JW, et al: Nivolumab monotherapy for first-line treatment of
advanced non-small-cell lung cancer. J Clin Oncol. 34:2980–2987.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rizvi NA, Hellmann MD, Brahmer JR,
Juergens RA, Borghaei H, Gettinger S, Chow LQ, Gerber DE, Laurie
SA, Goldman JW, et al: Nivolumab in combination with platinum-based
doublet chemotherapy for first-line treatment of advanced
non-small-cell lung cancer. J Clin Oncol. 34:2969–2979. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: KEYNOTE-001 Investigators: Pembrolizumab for the treatment
of non-small-cell lung cancer. N Engl J Med. 372:2018–2028. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taube JM, Klein A, Brahmer JR, Xu H, Pan
X, Kim JH, Chen L, Pardoll DM, Topalian SL and Anders RA:
Association of PD-1, PD-1 ligands and other features of the tumor
immune microenvironment with response to anti-PD-1 therapy. Clin
Cancer Res. 20:5064–5074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: KEYNOTE-024 Investigators: Pembrolizumab versus
chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl
J Med. 375:1823–1833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
George J, Saito M, Tsuta K, Iwakawa R,
Shiraishi K, Scheel AH, Uchida S, Watanabe SI, Nishikawa R, Noguchi
M, et al: Genomic amplification of CD274 (PD-L1) in small-cell lung
cancer. Clin Cancer Res. 23:1220–1226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saunders LR, Bankovich AJ, Anderson WC,
Aujay MA, Bheddah S, Black K, Desai R, Escarpe PA, Hampl J, Laysang
A, et al: A DLL3-targeted antibody-drug conjugate eradicates
high-grade pulmonary neuroendocrine tumor-initiating cells in vivo.
Sci Transl Med. 7:302ra1362015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rudin CM, Pietanza MC, Bauer TM, Ready N,
Morgensztern D, Glisson BS, Byers LA, Johnson ML, Burris HA III,
Robert F, et al: Rovalpituzumab tesirine, a DLL3-targeted
antibody-drug conjugate, in recurrent small-cell lung cancer: A
first-in-human, first-in-class, open-label, phase 1 study. Lancet
Oncol. 18:42–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gardner EE, Lok BH, Schneeberger VE,
Desmeules P, Miles LA, Arnold PK, Ni A, Khodos I, de Stanchina E,
Nguyen T, et al: Chemosensitive relapse in small cell lung cancer
proceeds through an EZH2-SLFN11 axis. Cancer Cell. 31:286–299.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sobin LH and Compton CC: TNM seventh
edition: What's new, what's changed: Communication from the
international union against cancer and the American joint committee
on cancer. Cancer. 116:5336–5339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sobin LH, Gospodarowicz M, Wittekind C and
Wittekind Ch: International Union Against Cancer (UICC) TNM
Classification of Malignant Tumors. 7th. Wiley-Blackwell; Oxford
UK: 2009
|
|
24
|
Lu C, Han HD, Mangala LS, Ali-Fehmi R,
Newton CS, Ozbun L, Armaiz-Pena GN, Hu W, Stone RL, Munkarah A, et
al: Regulation of tumor angiogenesis by EZH2. Cancer Cell.
18:185–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guan J, Lim KS, Mekhail T and Chang CC:
Programmed death ligand-1 (PD-L1) expression in the programmed
death receptor-1 (PD-1)/PD-L1 blockade: A key player against
various cancers. Arch Pathol Lab Med. 141:851–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
















