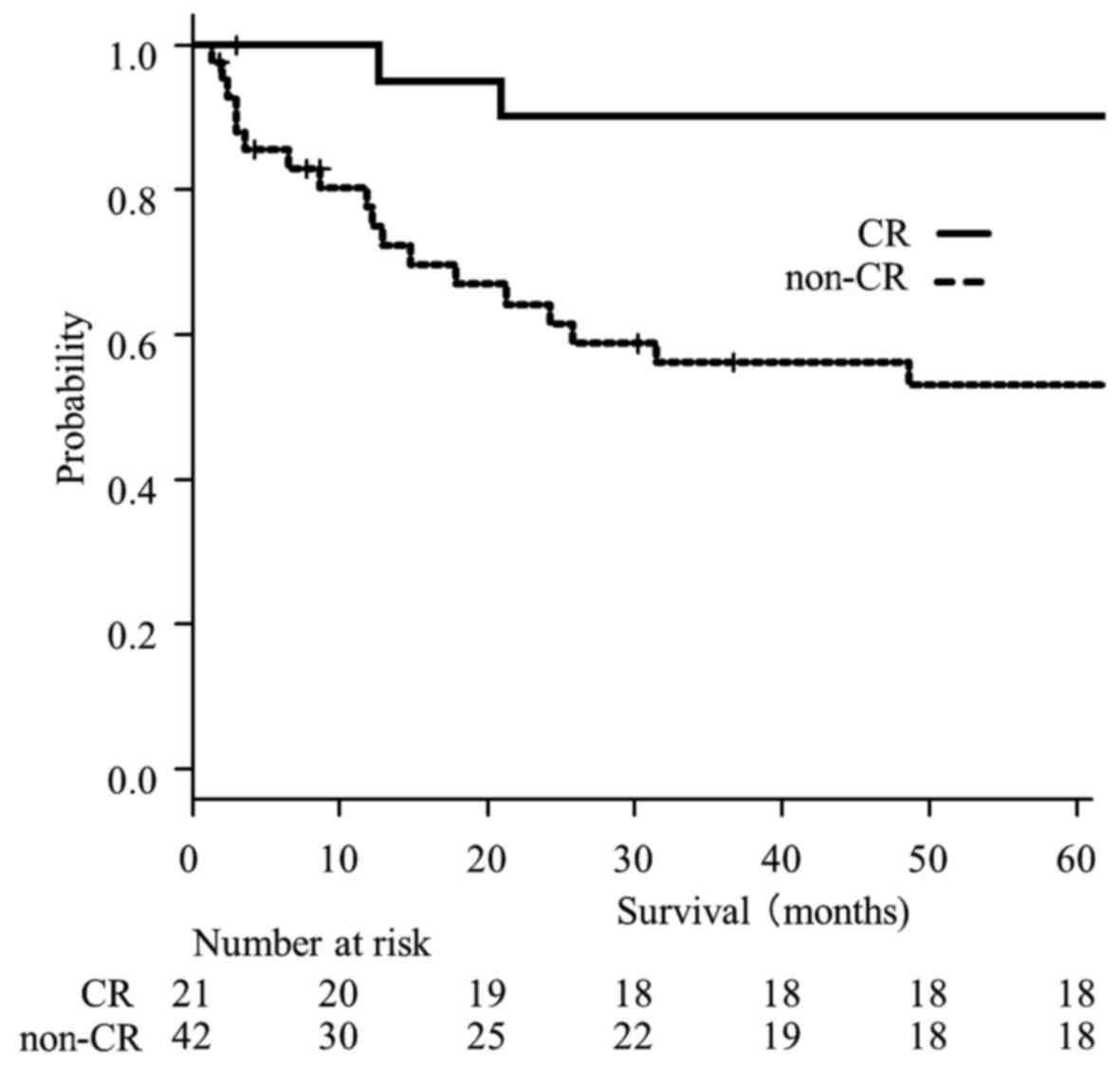
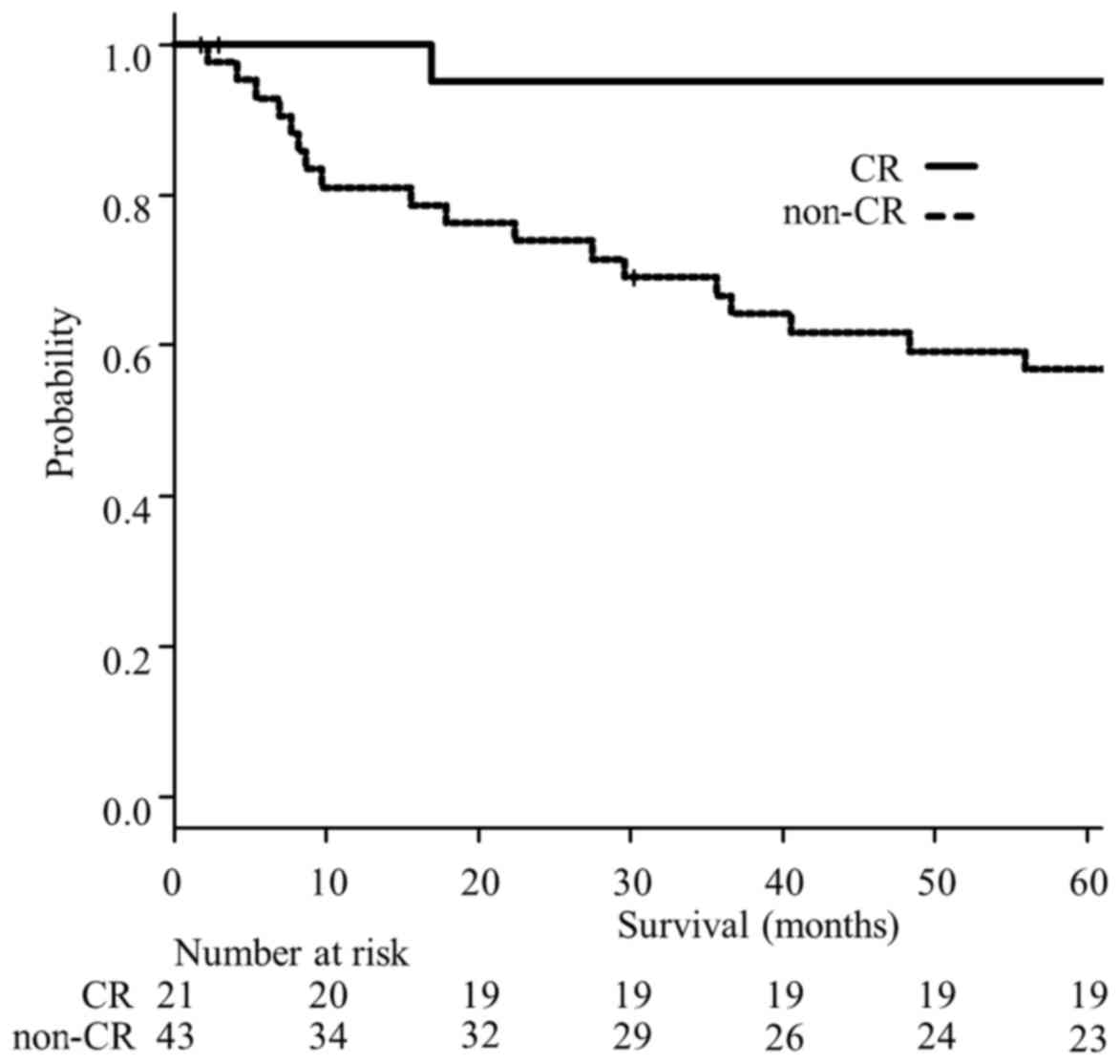
|
1
|
Lefebvre JL, Chevalier D, Luboinski B,
Kirkpatrick A and Collette L; Sahmoud: Larynx preservation in
pyriform sinus cancer, : Preliminary results of a European
Organization for Research and Treatment of Cancer phase III trial.
EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst.
88:890–899. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paccagnella A, Orlando A, Marchiori C,
Zorat PL, Cavaniglia G, Sileni VC, Jirillo A, Tomio L, Fila G, Fede
A, et al: Phase III trial of initial chemotherapy in stage III or
IV head and neck cancers: A study by the Gruppo di Studio sui
Tumori della Testa e del Collo. J Natl Cancer Inst. 86:265–272.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hasegawa Y, Goto M, Hanai N, Ijichi K,
Terada A, Hyodo I, Ogawa T and Fukushima M: Prediction of
chemosensitivity using multigene analysis in head and neck squamous
cell carcinoma. Oncology. 73:104–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oze I, Shimada S, Nagasaki H, Akiyama Y,
Watanabe M, Yatabe Y, Matsuo K and Yuasa Y: Plasma microRNA-103,
microRNA-107 and microRNA-194 levels are not biomarkers for human
diffuse gastric cancer. J Cancer Res Clin Oncol. 143:551–554. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Posner MR, Hershock DM, Blajman CR,
Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM,
Cullen K, Ervin TJ, et al: Cisplatin and fluorouracil alone or with
docetaxel in head and neck cancer. N Engl J Med. 357:1705–1715.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: Cisplatin, fluorouracil, and docetaxel in unresectable
head and neck cancer. N Engl J Med. 357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kodera Y, Ito S, Fujiwara M, Mochizuki Y,
Nakayama G, Ohashi N, Koike M, Yamamura Y and Nakao A: Gene
expression of 5-fluorouracil metabolic enzymes in primary gastric
cancer: Correlation with drug sensitivity against 5-fluorouracil.
Cancer Lett. 252:307–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sameshima S, Tomozawa S, Horikoshi H,
Motegi K, Hirayama I, Koketsu S, Okada T, Kojima M, Kon Y and
Sawada T: 5-Fluorouracil-related gene expression in hepatic artery
infusion-treated patients with hepatic metastases from colorectal
carcinomas. Anticancer Res. 28:389–393. 2008.PubMed/NCBI
|
|
11
|
Etienne MC, Chéradame S, Fischel JL,
Formento P, Dassonville O, Renée N, Schneider M, Thyss A, Demard F
and Milano G: Response to fluorouracil therapy in cancer patients:
The role of tumoral dihydropyrimidine dehydrogenase activity. J
Clin Oncol. 13:1663–1670. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salonga D, Danenberg KD, Johnson M,
Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman
L, Diasio RB, et al: Colorectal tumors responding to 5-fluorouracil
have low gene expression levels of dihydropyrimidine dehydrogenase,
thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res.
6:1322–1327. 2000.PubMed/NCBI
|
|
13
|
Kobayashi H, Koike T, Nakatsuka A, Kurita
H, Sagara J, Taniguchi S and Kurashina K: Dihydropyrimidine
dehydrogenase expression predicts survival outcome and
chemosensitivity to 5-fluorouracil in patients with oral squamous
cell carcinoma. Oral Oncol. 41:38–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calascibetta A, Contino F, Feo S, Gulotta
G, Cajozzo M, Antona A, Sanguedolce G and Sanguedolce R: Analysis
of the thymidylate synthase gene structure in colorectal cancer
patients and its possible relation with the 5-Fluorouracil drug
response. J Nucleic Acids. 2010:pii: 3067542010. View Article : Google Scholar
|
|
15
|
Martin LP, Hamilton TC and Schilder RJ:
Platinum resistance: The role of DNA repair pathways. Clin Cancer
Res. 14:1291–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kartalou M and Essigmann JM: Mechanisms of
resistance to cisplatin. Mutat Res. 478:23–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crul M, Schellens JH, Beijnen JH and
Maliepaard M: Cisplatin resistance and DNA repair. Cancer Treat
Rev. 23:341–366. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozols RF: Chemotherapy for advanced
epithelial ovarian cancer. Hematol Oncol Clin North Am. 6:879–894.
1992.PubMed/NCBI
|
|
19
|
Rabik CA and Dolan ME: Molecular
mechanisms of resistance and toxicity associated with platinating
agents. Cancer Treat Rev. 33:9–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McGurk CJ, Cummings M, Köberle B, Hartley
JA, Oliver RT and Masters JR: Regulation of DNA repair gene
expression in human cancer cell lines. J Cell Biochem.
97:1121–1136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Orelli B, McClendon TB, Tsodikov OV,
Ellenberger T, Niedernhofer LJ and Schärer OD: The XPA-binding
domain of ERCC1 is required for nucleotide excision repair but not
other DNA repair pathways. J Biol Chem. 285:3705–3712. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Welsh C, Day R, McGurk C, Masters JR, Wood
RD and Köberle B: Reduced levels of XPA ERCC1 and XPF DNA repair
proteins in testis tumor cell lines. Int J Cancer. 110:352–361.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dabholkar M, Bostick-Bruton F, Weber C,
Bohr VA, Egwuagu C and Reed E: ERCC1 and ERCC2 expression in
malignant tissues from ovarian cancer patients. J Natl Cancer Inst.
84:1512–1517. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shirota Y, Stoehlmacher J, Brabender J,
Xiong YP, Uetake H, Danenberg KD, Groshen S, Tsao-Wei DD, Danenberg
PV and Lenz HJ: ERCC1 and thymidylate synthase mRNA levels predict
survival for colorectal cancer patients receiving combination
oxaliplatin and fluorouracil chemotherapy. J Clin Oncol.
19:4298–4304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lord RV, Brabender J, Gandara D, Alberola
V, Camps C, Domine M, Cardenal F, Sánchez JM, Gumerlock PH, Tarón
M, et al: Low ERCC1 expression correlates with prolonged survival
after cisplatin plus gemcitabine chemotherapy in non-small cell
lung cancer. Clin Cancer Res. 8:2286–2291. 2002.PubMed/NCBI
|
|
26
|
Kim JS, Kim MA, Kim TM, Lee SH, Kim DW, Im
SA, Kim TY, Kim WH, Yang HK, Heo DS, et al: Biomarker analysis in
stage III–IV (M0) gastric cancer patients who received curative
surgery followed by adjuvant 5-fluorouracil and cisplatin
chemotherapy: Epidermal growth factor receptor (EGFR) associated
with favourable survival. Br J Cancer. 100:732–738. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee KH, Min HS, Han SW, Oh DY, Lee SH, Kim
DW, Im SA, Chung DH, Kim YT, Kim TY, et al: ERCC1 expression by
immunohistochemistry and EGFR mutations in resected non-small cell
lung cancer. Lung Cancer. 60:401–407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei Q, Cheng L, Hong WK and Spitz MR:
Reduced DNA repair capacity in lung cancer patients. Cancer Res.
56:4103–4107. 1996.PubMed/NCBI
|
|
29
|
Xuelei M, Jingwen H, Wei D, Hongyu Z, Jing
Z, Changle S and Lei L: ERCC1 plays an important role in predicting
survival outcomes and treatment response for patients with HNSCC: A
meta-analysis. Oral Oncol. 51:483–492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moon C, Oh Y and Roth JA: Current status
of gene therapy for lung cancer and head and neck cancer. Clin
Cancer Res. 9:5055–5067. 2003.PubMed/NCBI
|
|
31
|
Bradford CR, Wolf GT, Carey TE, Zhu S,
Beals TF, Truelson JM, McClatchey KD and Fisher SG: Predictive
markers for response to chemotherapy, organ preservation, and
survival in patients with advanced laryngeal carcinoma. Otolaryngol
Head Neck Surg. 121:534–538. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bradford CR, Zhu S, Wolf GT, Poore J,
Fisher SG, Beals T, McClatchey KD and Carey TE: Overexpression of
p53 predicts organ preservation using induction chemotherapy and
radiation in patients with advanced laryngeal cancer. Department of
Veterans Affairs Laryngeal Cancer Study Group. Otolaryngol Head
Neck Surg. 113:408–412. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yip HT, Chopra R, Chakrabarti R, Veena MS,
Ramamurthy B, Srivatsan ES and Wang MB: Cisplatin-induced growth
arrest of head and neck cancer cells correlates with increased
expression of p16 and p53. Arch Otolaryngol Head Neck Surg.
132:317–326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Friedman M, Grey P, Venkatesan TK, Bloch
I, Chawla P, Caldarelli DD and Coon JS: Prognostic significance of
Bcl-2 expression in localized squamous cell carcinoma of the head
and neck. Ann Otol Rhinol Laryngol. 106:445–450. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Strasser A, Harris AW, Jacks T and Cory S:
DNA damage can induce apoptosis in proliferating lymphoid cells via
p53-independent mechanisms inhibitable by Bcl-2. Cell. 79:329–339.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wilson GD, Grover R, Richman PI, Daley FM,
Saunders MI and Dische S: Bcl-2 expression correlates with
favourable outcome in head and neck cancer treated by accelerated
radiotherapy. Anticancer Res. 16:2403–2408. 1996.PubMed/NCBI
|
|
37
|
Hotz MA, Bosq J, Zbaeren P, Reed J, Schwab
G, Krajewski S, Brousset P and Borner MM: Spontaneous apoptosis and
the expression of p53 and Bcl-2 family proteins in locally advanced
head and neck cancer. Arch Otolaryngol Head Neck Surg. 125:417–422.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
van Delft MF and Huang DC: How the Bcl-2
family of proteins interact to regulate apoptosis. Cell Res.
16:203–213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zeitlin BD, Zeitlin IJ and Nör JE:
Expanding circle of inhibition: Small-molecule inhibitors of Bcl-2
as anticancer cell and antiangiogenic agents. J Clin Oncol.
26:4180–4188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pidgeon GP, Barr MP, Harmey JH, Foley DA
and Bouchier-Hayes DJ: Vascular endothelial growth factor (VEGF)
upregulates BCL-2 and inhibits apoptosis in human and murine
mammary adenocarcinoma cells. Br J Cancer. 85:273–278. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kaneko T, Zhang Z, Mantellini MG, Karl E,
Zeitlin B, Verhaegen M, Soengas MS, Lingen M, Strieter RM, Nunez G
and Nör JE: Bcl-2 orchestrates a cross-talk between endothelial and
tumor cells that promotes tumor growth. Cancer Res. 67:9685–9693.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takara K, Tsujimoto M, Kokufu M, Ohnishi N
and Yokoyama T: Up-regulation of MDR1 function and expression by
cisplatin in LLC-PK1 cells. Biol Pharm Bull. 26:205–209. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hoffmann AC, Wild P, Leicht C, Bertz S,
Danenberg KD, Danenberg PV, Stöhr R, Stöckle M, Lehmann J, Schuler
M and Hartmann A: MDR1 and ERCC1 expression predict outcome of
patients with locally advanced bladder cancer receiving adjuvant
chemotherapy. Neoplasia. 12:628–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Warmann S, Hunger M, Teichmann B, Flemming
P, Gratz KF and Fuchs J: The role of the MDR1 gene in the
development of multidrug resistance in human hepatoblastoma:
Clinical course and in vivo model. Cancer. 95:1795–1801. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bišof V, Zajc Petranović M, Rakušić Z,
Samardžić KR and Juretić A: The prognostic and predictive value of
excision repair cross-complementation group 1 (ERCC1) protein in
1288 patients with head and neck squamous cell carcinoma treated
with platinum-based therapy: A meta-analysis. Eur Arch
Otorhinolaryngol. 273:2305–2317. 2016. View Article : Google Scholar : PubMed/NCBI
|