Introduction
According to the most recent classification of the
World Health Organization in 2010, mixed carcinomas containing an
exocrine and a neuroendocrine component, with one component
exceeding 30%, are designated as mixed adenoneuroendocrine
carcinomas (MANECs) (1).
Neuroendocrine neoplasm of the stomach were divided into three
categories: Neuroendocrine tumor (NET), neuroendocrine carcinoma
(NEC) and mixed adenoneuroendocrine carcinoma (MANEC). In practice,
there is much variation in the percentage of components between
adenocarcinoma and neuroendocrine tumor in stomach neoplasm.
However, MANEC consists of both components with at least 30% of
either component. Those which fit in the criteria of MANEC are rare
and only case report can be found in the literature (2). The epidemiology of MANEC has not yet
been described. This kind of neoplasm usually has a poor prognosis
since both components are malignant. Here we report a patient with
a locally advanced unresectable gastric MANEC who received
multimodality treatment including chemotherapy, surgical resection
and radiotherapy, still remains alive with no evidence of
metastasis or recurrence at more than five years so far.
Case report
In May 2012, a 65-year old male was admitted to our
hospital, presented with anorexia, epigastric distention, abdominal
and lumbar pain and weight loss over a one-month period. His past
medical history included a chronic gastritis and he had undergone
appendectomy 31 years earlier. Physical examination showed no
abnormalities.
An ultrasound revealed a substantial mass in the
upper abdominal cavity. A subsequent contrast CT scan of the
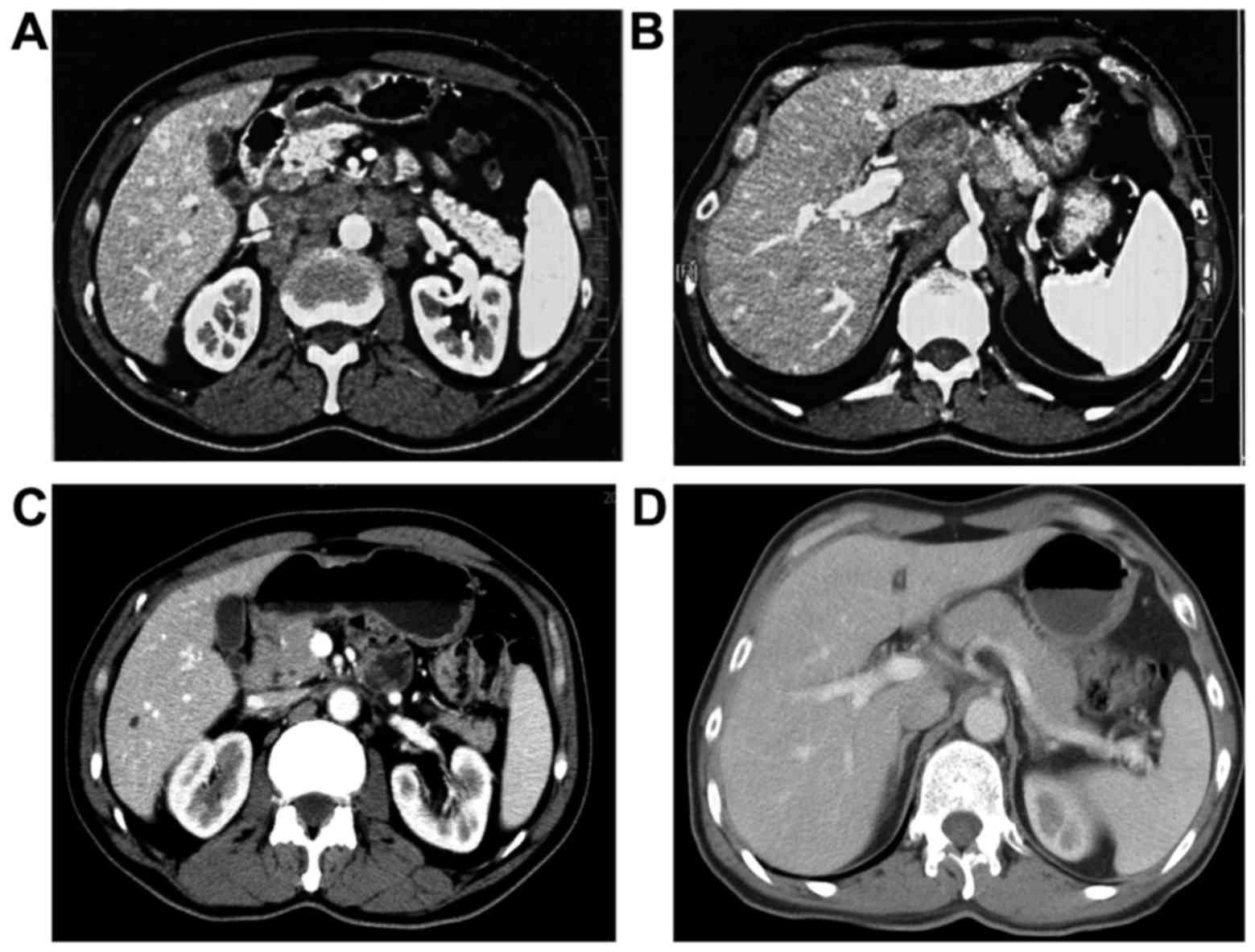
abdomen confirmed that multiple lesions located in the lesser
curvature of the stomach, hepatic hilar region, mesenteric root,
retroperitoneal region and above the head of the pancreas. All of
these lesions merged into a large mass, measuring approximately
5.5×4.8 cm (Fig. 1A and B). Upper
gastrointestinal endoscopy showed an ulcerous lesion at lesser
curvature of stomach with protruded ring dike-like mucous membrane.
Endoscopic ultrasonography revealed that the tumor was located at
the mucosal and submucosal layer. The histolopathological
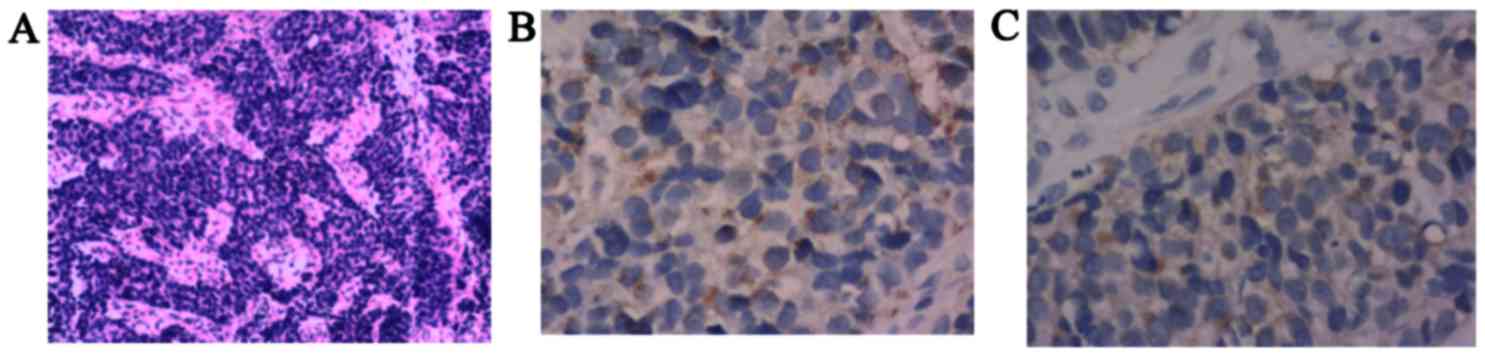
examination from the ulcerous lesion and ultrasound-guided fine
needle aspiration of one of the lesions revealed MANEC (Fig. 2A), and neuroendocrine markers for
chromogranin A (Fig. 2B) and
synaptophysin (Fig. 2C) were
positive in the neuroendocrine component only and negative in the
adenocarcinoma component. The staging chest X-ray showed no
thoracic disease.
On the basis of tumor biology and the disease stage,
the patient was not a candidate for surgery, and systemic cytotoxic
chemotherapy was scheduled from May 26 to August 14 in 2012, four
cycles of chemotherapy consisting of VP16, cisplatin and TS-1
(repeated every 21 days) were administered to the patient. Contrast
CT scan afterwards showed that the thick of gastric wall had little
change but the multiple enlarged lymph nodes shrunk rapidly and
significantly (Fig. 1C and D). The
main adverse effects of chemotherapy included grade 3 leukopenia,
grade 2 neutropenia, grade 2 anorexia, grade 1 nausea and grade 1
vomiting. Related guidance advocates that all gastric small cell
carcinoma should be treated with oncological resections without
considering grade and stage (3).
Based on the excellent response, on September 10, 2012, the patient
undergone open distal gastrectomy with D2 lymphadenectomy. But the
number 16 lymph nodes were not dissected. Gross examination of the
surgical specimen revealed an ulcerous lesion measuring 0.9×0.6×0.3
cm located at anterior of antrum near lesser curvature.
Histolopathological examination of the surgical specimen showed
that there was no neuroendocrine component. Only a little
degenerated adenocarcinoma cells remained in the mucosa, which
invaded muscularis mucosae and were infiltrated with large amount
of inflammatory cells. No metastasis was revealed among the checked
28 lymph nodes.
Four weeks after surgical resection, the patient
received additional two cycles of postoperative chemotherapy with
the same regimen. The tolerability was well. The main adverse
effects included grade 1 neutropenia, grade 1 decline of hemoglobin
and grade 1 nausea.
Considering the high rate of local recurrence,
combined treatment with radiation therapy was adopted. From
December 24 to January 25 in 2013, concurrent chemo-radiotherapy
was administered. Lymph node stations in the radiation fields
included the retroperitoneal lymph nodes with No. 7, 8, 12, 13, 14
and 16 lymph nodes. Especially, the radiation focus was on No. 16
lymph nodes to give clinical target volume. The planning target
volume dosage was 45 Gy, which was delivered in 25 fractions within
35 days. PTV1 was 56 Gy/25 f/35 d with concurrent oral
capecitabine. The main adverse effects included grade 1 decline of
hemoglobin, grade 2 anorexia, grade 1 nausea and grade 1
fatigue.
After completion of all above therapy, the patient
undergone regular follow-up every 4 to 6 months, including CT scan
of chest, abdomen, pelvis and blood chemistry. Until the last
follow up in May of 2017, the patient has been alive for 5 years
since diagnosis without recurrence or metastasis.
Discussion
There is a wide spectrum of combinations of exocrine
and neuroendocrine components in the stomach neoplasm. However,
according to the 2010 WHO grading system, MANECs are characterized
by only those neoplasms in which each component represents at least
30% of the lesion. But the 30% cut-off is not based on enough data
on the prognostic significance of the neuroendocrine component. In
Park et al study (4), 10%
cut-off may be more clinically useful and may serve as an
informative parameter for predicting patient outcome. All eligible
patients had received gastrectomy and were staged I–III. The 5-year
OS rates for NEC, MANEC and gastric carcinoma with neuroendocrine
differentiation were 59.1, 53.5 and 16.7%, respectively. However,
the 5-year OS in patients with gastric carcinomas without
neuroendocrine morphology is 85.2%. Although the early detection
and treatment of gastric tumors has increased in accordance with
the progression of endoscopic techniques, the early detection of
MANEC is extremely rare. Even in the early stage of the disease,
the neuroendocrine carcinoma component is detected in the deeper
portion of the mucosal or submucosal layers, and can only be
diagnosed after resection of the tumor.
Until now, the research for MANEC prognosis and
treatment is limited, also because MANEC in the stomach is
extremely rare, there is no recommended therapeutic treatment
strategy.
Surgical resection is indicated in patients without
metastasis. However, curative surgery is probably possible in
<30% of all NET patients and recurrence is common (5). When curative surgery is not possible,
locoregional resective surgery was associated with increased
survival on crude and multivariate analysis (6).
As the high risk of metastasis and recurrence rate
of MANEC, chemotherapy has been reported to be the treatment of
choice, which had improved the median survival to a range of 6 to
12 months, with occasional long-term survival (7). So far, the chemotherapy regimen of
MANECs is not standardized. Owing to the effective chemotherapeutic
regimen with a combination of etoposide and cisplatin in treating
small cell lung cancer, the first study explored infusional
cisplatin and etoposide among 45 patients with metastatic NECs (of
whom 18 had PD tumors) between 1987 and 1990. In this study, a
response rate of 67% was reported in patients with PD NECs, with
response duration of 8 months and a median survival of 19 months
(8). Alternative regimens
substituting carboplatin for cisplatin or irinotecan for etoposide
have been validated in metastatic small-cell lung cancer and are
therefore thought to be acceptable options for management of PD
NECs (9). Based on the treatment
paradigm for limited-stage small-cell lung cancer, first-line
systemic chemotherapy with a platinum agen and etoposide (cisplatin
or carboplatin and etoposide for 4–6 cycles) is recommended for
most patients with metastatic-stage disease (10). Especially, chemotherapy combined with
radiation can be considered patients with MANECs, particularly when
surgical resection is difficult.
Although the biological similarity between small
cell carcinoma of the lung and gastric neuroendocrine carcinoma is
controversial, the EP regimen (combination of etoposide and
cisplatin), which is used for small cell carcinoma of the lung, is
the most widely used treatment in neuroendocrine carcinoma. For
this case, we selected TS-1, cisplatin, etoposide and achieved a
good partial response. The postoperative pathology showed that only
little adenocarcinoma component remained. Although the role of
radiation for MANEC is unclear, as with small cell lung cancer, we
think MANEC is sensitive to radiation, so we give the patient local
radiation to reduce the risk of local recurrence. Several trials
and meta-analyses have shown that radiotherapy combined with
chemotherapy could improve the curative effects, provide regional
control and do help to subsequent long term survival in isolated
cases (11).
After surgery resection with 6 cycles of
perioperative chemotherapy of EP regimen and consolidated
radiotherapy to the tumor remained region, the patient has a long
term disease free survival. We suggest that multimodality therapy
can be beneficial and necessary to the MANEC.
Acknowledgements
Not applicable.
Consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MANEC
|
mixed adenoneuroendocrine
carcinoma
|
|
NET
|
neuroendocrine tumor
|
|
NEC
|
neuroendocrine carcinoma
|
References
|
1
|
Kitajima T, Kaida S, Lee S, Haruta S,
Shinohara H, Ueno M, Suyama K, Oota Y, Fujii T and Udagawa H: Mixed
adeno(neuro)endocrine carcinoma arising from the ectopic gastric
mucosa of the upper thoracic esophagus. World J Surg Oncol.
11:2182013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gurzu S, Kadar Z, Bara T, Bara T Jr,
Tamasi A, Azamfirei L and Jung I: Mixed adenoneuroendocrine
carcinoma of gastrointestinal tract: Report of two cases. World J
Gastroenterol. 21:1329–1333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Basuroy R, Srirajaskanthan R, Prachalias
A, Quaglia A and Ramage JK: Review article: The investigation and
management of gastric neuroendocrine tumours. Aliment Pharmacol
Ther. 39:1071–1084. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JY, Ryu MH, Park YS, Park HJ, Ryoo
BY, Kim MG, Yook JH, Kim BS and Kang YK: Prognostic significance of
neuroendocrine components in gastric carcinomas. Eur J Cancer.
50:2802–2809. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Janson ET, Sorbye H, Welin S, Federspiel
B, Grønbæk H, Hellman P, Ladekarl M, Langer SW, Mortensen J,
Schalin-Jäntti C, et al: Nordic guidelines 2014 for diagnosis and
treatment of gastroenteropancreatic neuroendocrine neoplasms. Acta
Oncol. 53:1284–1297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Norlén O, Stålberg P, Öberg K, Eriksson J,
Hedberg J, Hessman O, Janson ET, Hellman P and Åkerström G:
Long-term results of surgery for small intestinal neuroendocrine
tumors at a tertiary referral center. World J Surg. 36:1419–1431.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Richards D, Davis D, Yan P and Guha S:
Unusual case of small cell gastric carcinoma: Case report and
literature review. Dig Dis Sci. 56:951–957. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moertel CG, Kvols LK, O'Connell MJ and
Rubin J: Treatment of neuroendocrine carcinomas with combined
etoposide and cisplatin. Evidence of major therapeutic activity in
the anaplastic variants of these neoplasms. Cancer. 68:227–232.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanna N, Bunn PA Jr, Langer C, Einhorn L,
Guthrie T Jr, Beck T, Ansari R, Ellis P, Byrne M, Morrison M, et
al: Randomized phase III trial comparing irinotecan/cisplatin with
etoposide/cisplatin in patients with previously untreated
extensive-stage disease small-cell lung cancer. J Clin Oncol.
24:2038–2043. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Strosberg JR, Coppola D, Klimstra DS, Phan
AT, Kulke MH, Wiseman GA and Kvols LK: North American
Neuroendocrine Tumor Society (NANETS): The NANETS consensus
guidelines for the diagnosis and management of poorly
differentiated (high-grade) extrapulmonary neuroendocrine
carcinomas. Pancreas. 39:799–800. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brenner B, Tang LH, Shia J, Klimstra DS
and Kelsen DP: Small cell carcinomas of the gastrointestinal tract:
Clinicopathological features and treatment approach. Semin Oncol.
34:43–50. 2007. View Article : Google Scholar : PubMed/NCBI
|