Introduction
Breast cancer (BC) is the most common cancer among
women worldwide with 1.67 million new cases in 2012, and the
incidence has until recently been continuously increasing (1). The 5-year relative survival has
improved over time and is now around 85% (2), which is partly explained by earlier
diagnosis and improved cancer treatment, including surgery,
chemotherapy, radiation, HER2-tageted treatment, and endocrine
therapy. In Denmark alone, ≥62,000 women live with the potential
side effects of breast cancer treatment (2). One important side effect is the
increased bone turnover, the so called cancer treatment-induced
bone loss (CTIBL) (3), which
potentially may cause osteoporosis in the long run.
Osteoporosis is defined as a skeletal disorder
compromising bone strength and leading to increased risk of
fracture (4). Microarchitecture
(quality) and bone density (quantity) are the two components of
bone strength. For practicality the diagnosis is not based on
microarchitecture but most often on measurements of the bone
mineral density (BMD) by DXA scanning. The criterion of
osteoporosis in postmenopausal women is a score of 2.5 SD below the
mean BMD of a young healthy woman (equivalent of a T-score
<-2.5) (4). The diagnosis is
based on the lowest BMD either at the lumbar spine or proximal
femur (4).
So far, the literature has shown the highest extent
of bone loss to take place within the first six months after start
of chemotherapy (5,6). The use of corticosteroids is a
well-known risk factor of osteoporosis when administered low-dose
over a long period of time (7). The
effect is more uncertain when given as pulse doses in supportive
care to prevent vomiting and nausea. Despite the large doses given
over a relatively short time period, a recent study found no
relation between prednisolone dose and BMD change (8). Actually, the BMD had increased four
months after adjuvant chemotherapy, contrary to what other studies
have shown (3,8–10).
Most studies on CTIBL address the effect in
postmenopausal women, where chemotherapy itself induces bone loss
(11,12). Furthermore, standard adjuvant
endocrine treatment in the form of aromatase inhibitors for five
years increases the bone turnover 2–3 fold in postmenopausal women
and decreases the BMD significantly due to the low levels of
systemic oestrogen (13,14). For premenopausal women
chemotherapy-induced premature ovarian dysfunction is a long term
risk factor of bone loss leading to increased risk of osteoporosis
and thereby fractures (5,10,13,15).
Also, premenopausal patients with oestrogen receptor (ER) positive
BC are likely to receive 10 years of treatment with tamoxifen
resulting in increased bone turnover. Tamoxifen is able to preserve
bone mass in postmenopausal women, whereas it may cause bone loss
in premenopausal women (16–18).
The current guideline in Denmark recommends
zoledronic acid as adjuvant therapy in postmenopausal women to
reduce the risk of skeletal recurrence and improve survival
(19). Zoledronic acid also reduces
the risk of fractures and improves bone health over time by
decreasing the osteoclast bone resorption activity and hence
limiting CTIBL (6,19). Since no effect of zoledronic acid on
survival in premenopausal patients has been shown, it is not
recommended in this subpopulation. Therefore the younger patients
may be more prone to later development of osteoporosis (19).
We aimed to quantify bone loss and identify risk
factors associated with increased bone loss during
neoadjuvant/adjuvant chemotherapy. Menopausal status and
neoadjuvant compared to adjuvant chemotherapy was of particular
interest to elucidate the influence of extended chemotherapy and
the amount of corticosteroids used in supportive care.
Patients and methods
Patients
This investigation was conducted as a retrospective
cohort study including 492 patients with early stage BC, who
received either adjuvant or neoadjuvant chemotherapy at the
Department of Oncology, Vejle Hospital, Denmark between January 1,
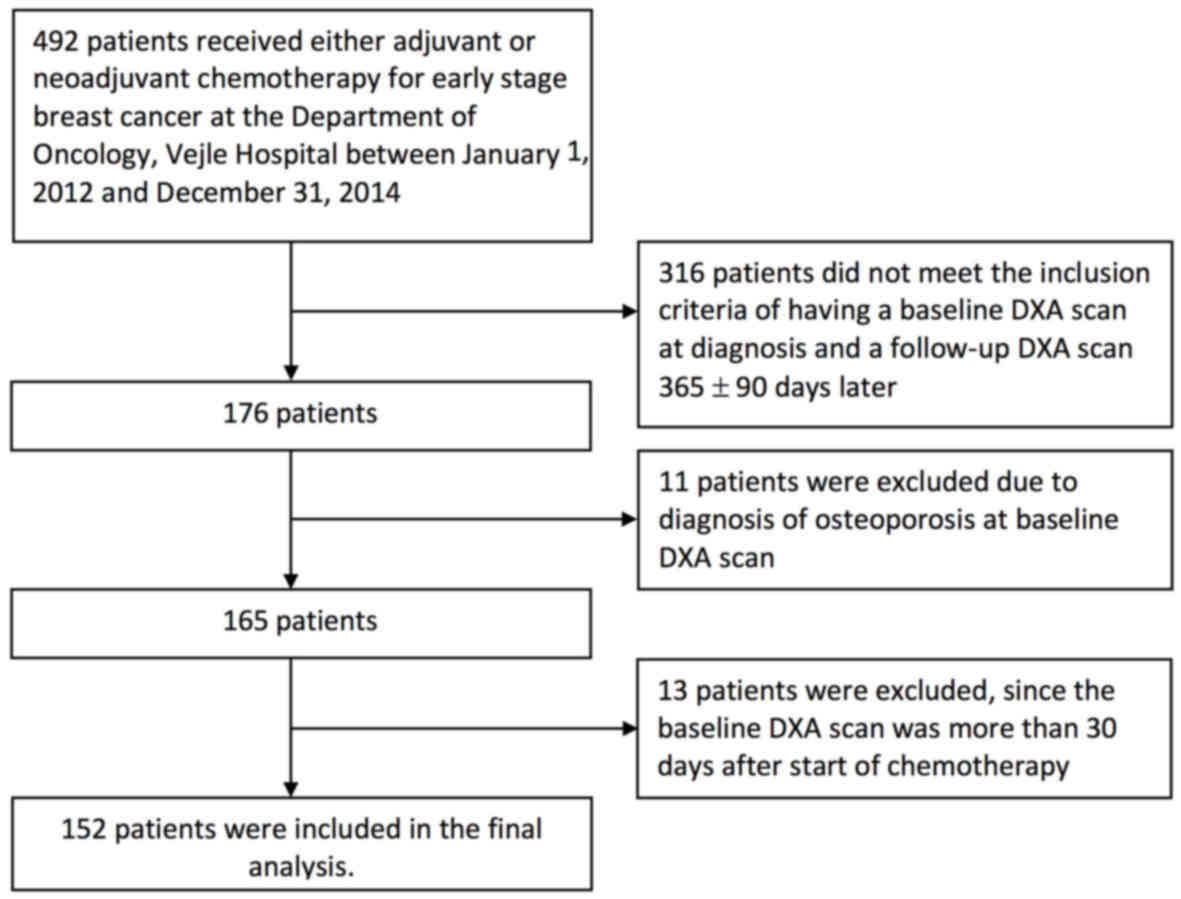
2012 and December 31, 2014. A total of 340 patients were excluded
from the final analysis as seen in the Consort diagram (Fig. 1). Most patients (316) were excluded
due to a missing baseline DXA scan before the first cycle of
chemotherapy or a missing follow-up DXA scan one year after the
first DXA scan ± 90 days. Eleven patients were diagnosed with
osteoporosis before or at the first DXA scan and were excluded to
avoid treatment directly influencing BMD. Thirteen patients had the
first DXA scan more than 30 days after the first day of
chemotherapy and were thus excluded. For the final analysis 152
patients were left.
Data was collected through the electronic patient
record system after approval by the Danish Health and Medicines
Authority and the Danish Data Protection Agency. According to
Danish law this study did not require approval by the Ethics
Committee (project-ID: S-20150139).
Treatment
The vast majority of the patients receiving adjuvant
chemotherapy (69 out of 71), had three cycles of epirubicin (90
mg/m2, q3w) and cyclophosphamide (600 mg/m2,
q3w) (EC) followed by three cycles of docetaxel (100
mg/m2, q3w). The remaining two patients had three and
five cycles of chemotherapy, respectively. Seventy-two of the 81
patients receiving neoadjuvant chemotherapy had four cycles of
paclitaxel (80 mg/m2, q1w, for 11 weeks) or docetaxel
and four cycles of EC. The majority of the nine remaining patients
received only six or seven cycles of chemotherapy due to side
effects. The dose of chemotherapy was given according to national
guidelines.
Prophylactic prednisolone against nausea, vomiting,
and anaphylaxis was given in standard doses: 150 mg prednisolone
per cycle with EC, 300 mg per cycle with docetaxel, and 100 mg
weekly with paclitaxel. In total, 1,350 mg of prednisolone was
given during standard adjuvant chemotherapy and 1,700 mg during
standard neoadjuvant chemotherapy.
Following neoadjuvant/adjuvant chemotherapy patients
with ER-positive tumours started either tamoxifen or AI depending
on the menopausal status at diagnosis. Adjuvant zoledronic acid was
implemented in the guidelines at Vejle Hospital early 2014 and
subsequently, postmenopausal patients were offered zoledronic acid
4 mg every six months for a period of 4 years.
Clinical and histological data
Data on age, height, weight, smoking status, date of
referral, type of surgery, chemotherapy, endocrine treatment,
HER2-targeted treatment, and concurrent medicine were obtained from
the patients medical record. Pathology data, including histology
type, HER2-status (defined as positive with immunohistochemistry 3+
or 2+ combined with fluorescence in situ hybridization ratio
≥2.0), ER-status (defined positive in case of 1% or more ER
positive tumour cells), and nodal status, were obtained from the
patients pathology records. BMI was calculated as weight in kg
divided by the height in meters squared. BMI was categorized as
less than 25 (underweight/normal), 25 to 30 (overweight) and more
than 30 (obese) according to the World Health Organization
criteria.
Outcome
T-score and BMD (g/cm2) based on the DXA
scans were obtained from the scan report. The vast majority of the
DXA scans were performed with the same scanner at Vejle
Hospital.
Statistical analyses
Categorical data were described with frequency and
percentage. Continuous data were described using mean and standard
deviations.
For the uni- and multivariate analyses all exposure
variables were transformed into dichotomous data: ER-status
(negative vs. positive), BMI (<25 vs. >25), smoking status
(current smokers vs. former/never/unknown.), zoledronic acid (no
vs. yes), endocrine treatment (AI treatment vs. tamoxifen/none).
The time from the last cycle of chemotherapy to the follow-up DXA
scan was dichotomised by separating the observations at the median
to evaluate whether those with a late follow-up DXA scan lost more
BMD than the group with an early DXA scan.
The BMD measures in the lumbar spine and hip before
chemotherapy and one year after as well as the difference between
the first and second DXA scan of the hip and lumbar spine (∆BMD hip
and ∆BMD spine) were tested for normality using QQ plots, which
showed normality to be a reasonable approximation for the
distributions. The paired t-test was used to test the
null-hypothesis: No difference in BMD between the first and second
DXA-scan of hip and lumbar spine, respectively.
In the univariate analyses, we used linear
regression to test the association between ∆BMD hip and ∆BMD spine
by different variables. When the association showed a P-value
<0.2, the variable was introduced into the multiple linear
regression analysis, which was performed to study the association
between a variable and the BMD change, having controlled for the
effects of other variables.
The analyses described were all planned in advance.
All statistical analyses were carried out using STATA 14.2 for Mac
(StataCorp Stata 14.2, College Station, TX, USA).
Results
Patient characteristics
The final analysis included 152 patients (Fig. 1). Patient characteristics are
outlined in Table I showing that 79
patients (52%) were premenopausal and 73 patients (48%)
postmenopausal. The mean age was 53 years (range 30 to 84 years). A
total of 81 patients (53%) had neoadjuvant chemotherapy and 71
patients (47%) had adjuvant chemotherapy. At baseline 58 patients
(38%) had osteopenia and the remaining had a normal T-score
(>-1). The mean BMD at baseline was 0.933 g/cm2 (95%
CI: 0.913; 0.952) and 1.014 g/cm2 (95% CI: 0.992; 1.036)
for hip and lumbar spine, respectively. Thirty-one postmenopausal
patients (20%) had zoledronic acid treatment, 28 of which were
treated once and three had zoledronic acid twice in the observation
period.
 | Table I.Characteristics of the study
population. |
Table I.
Characteristics of the study
population.
|
| Study cohort
n=152 |
|---|
| Age at first DXA
scan, years | Number (%) |
|
30–39 | 16 (11) |
|
40–49 | 46 (30) |
|
50–59 | 51 (34) |
|
60–69 | 30 (20) |
|
>70 | 9 (6) |
| Type of surgery | |
|
Lumpectomy | 107 (70) |
|
Mastectomy | 45 (30) |
| Type of
carcinoma | |
|
Ductal | 121 (80) |
|
Lobular | 11 (7) |
|
Other/unknown | 20 (13) |
| Oestrogen receptor
status | |
|
Negative | 37 (24) |
|
Positive | 115 (76) |
| HER2 status | |
|
Positive | 32 (21) |
|
Negative | 119 (78) |
|
Unknown | 1 (1) |
| Nodal status | |
| 0 | 91 (60) |
| 1–4 | 44 (29) |
|
>4 | 17(11) |
| Menopausal
status | |
|
Premenopausal | 79 (52) |
|
Postmenopausal | 73 (48) |
| BMI | |
|
<25 | 78 (51) |
|
25–30 | 42 (28) |
|
>30 | 32 (21) |
| Smoking status | |
|
Never | 79 (52) |
| Former
smoker | 39 (26) |
| Current
smoker | 33 (22) |
|
Unknown | 1 (1) |
| Zoledronic
acid |
|
|
Yes | 31 (20) |
| No | 121 (80) |
| Endocrine
treatment |
|
|
Tamoxifen | 62(41) |
|
Aromatase inhibitor | 54 (36) |
|
None | 36 (24) |
| Chemotherapy | |
|
Neoadjuvant | 81 (53) |
|
Adjuvant | 71 (47) |
Overall changes in BMD
Patients receiving neoadjuvant/adjuvant chemotherapy
had a significant loss in mean BMD. The mean change in BMD was
−0.0124 g/cm2 (95% CI −0.018; −0.007 P<0.001) in the
hip and −0.029 g/cm2 (95% CI: −0.036; −0.023 P<0.001)
in the lumbar spine corresponding to a reduction in BMD of 1.3 and
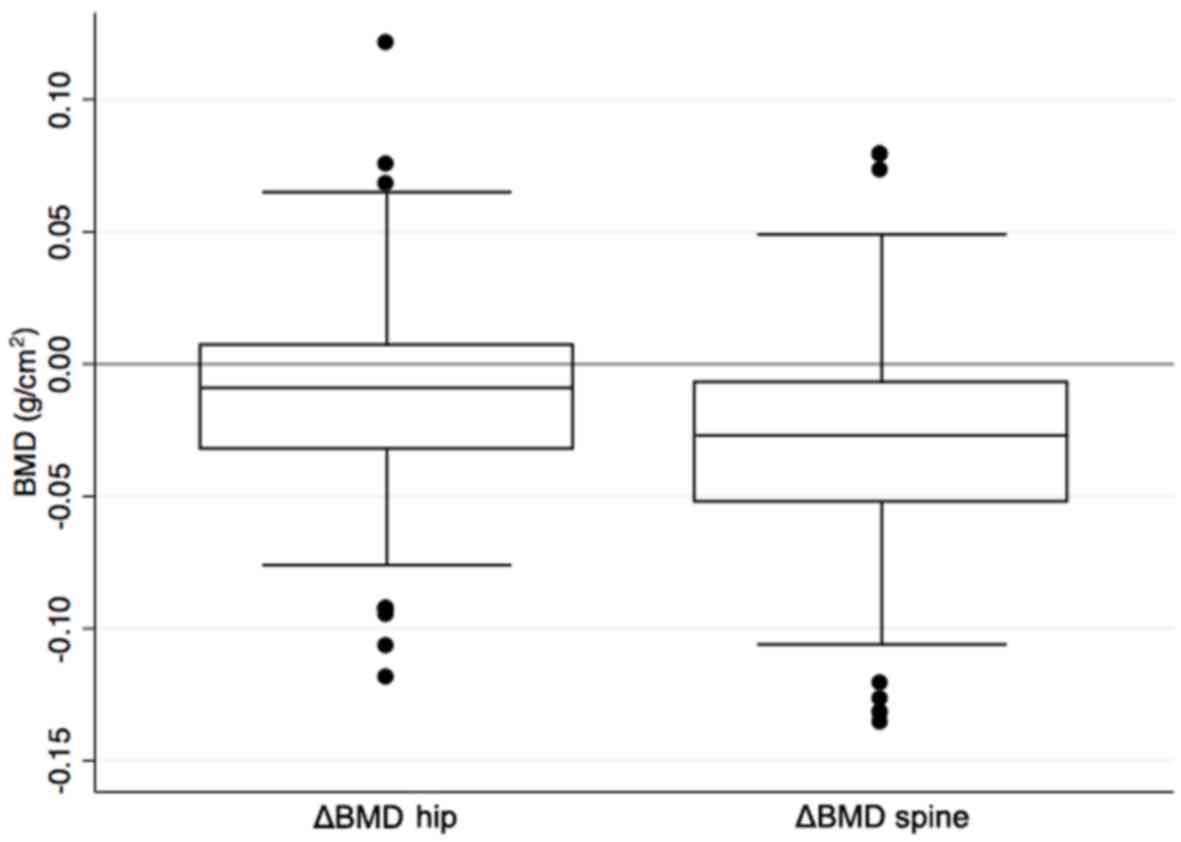
2.9% for the hip and lumbar spine, respectively. Range and
interquartile for BMD changes in spine and hip are shown in
Fig. 2.
Univariate and multivariate analysis
of factors affecting BMD
The results of the univariate and multivariate
analyses of the lumbar spine BMD values are shown in Table II. In the univariate analysis the
premenopausal patients had a substantial BMD loss of 0.045
g/cm2 equivalent to 4.3%. This was significantly higher
than the loss seen in postmenopausal women (P<0.001).
Furthermore, ER-positive patients had a lower BMD loss than
ER-negative patients (P=0.048) as did current smokers compared to
former smokers/unknown (P=0.021). Patients receiving zoledronic
acid had a lower BMD loss than those who did not have zolendronic
acid (P<0.001), and the BMD loss of patients treated with AI was
lower than those treated with tamoxifen or having no endocrine
treatment (P<0.001).
 | Table II.Uni-and multivariate analysis of
factors affecting BMD of the lumbar spine. |
Table II.
Uni-and multivariate analysis of
factors affecting BMD of the lumbar spine.
|
|
|
| Univariate linear
regression | Multiple linear
regression |
|---|
|
|
|
|
|
|
|---|
| Variables | ΔBMD | CI | Coef. | 95% CI | P-value | Coef. | 95% CI | P-value |
|---|
| Menopausal
status |
|
|
|
|
|
|
|
|
|
Premenopausal | −0.045 |
(−0.053;-0.037) | Reference |
|
| Reference |
|
|
|
Postmenopausal | −0.013 |
(−0.022;-0.003) | 0.032 | (0.020;0.044) |
<0.001 | 0.018 | (−0.001;0.038) | 0.060 |
| ER status |
|
|
|
|
|
|
|
|
|
ER=0% | −0.041 |
(−0.053;-0.030) | Reference |
|
| Reference |
|
|
|
ER≥1% | −0.026 |
(−0.034;-0.018) | 0.015 | (0.000;0.031) | 0.048 | 0.007 | (−0.010;0.024) | 0.419 |
| Current smoking
status |
|
|
|
|
|
|
|
|
|
Non-smoker/unknown | −0.033 |
(−0.041;-0.026) | Reference |
|
| Reference |
|
|
|
Smoker | −0.015 |
(−0.026;-0.004) | 0.019 | (0.003;0.034) | 0.021 | 0.071 | (0.002;0.032) | 0.022 |
| BMI |
|
|
|
|
|
|
|
|
|
<25 | −0.031 |
(−0.024;-0.023) | Reference |
|
|
|
|
|
|
>25 | −0.028 |
(−0.038;-0.017) | 0.004 | (−0.010;0.017) | 0.583 |
|
|
|
| Time from last
cycle of chemotherapy to follow up DXA scan |
|
|
|
|
|
|
|
|
| ≤237
days | −0.033 |
(−0.041;-0.024) | Reference |
|
|
|
|
|
|
>237 days | −0.026 |
(−0.036;-0.016) | 0.007 | (−0.007;0.020) | 0.334 |
|
|
|
| Zoledronic acid
treatment |
|
|
|
|
|
|
|
|
|
No | −0.036 |
(−0.044;-0.029) | Reference |
|
| Reference |
|
|
|
Yes | −0.002 | (−0.015;0.011) | 0.034 | (0.019;0.050) |
<0.001 | 0.011 | (−0.010;0.030) | 0.266 |
| Endocrine
therapy |
|
|
|
|
|
|
|
|
|
Tamoxifen or none | −0.043 |
(−0.049;-0.035) | Reference |
|
| Reference |
|
|
|
Aromatase
inhibitor | −0.006 | (−0.017;0.006) |
0.037 | (0.024;0.049) |
<0.001 | 0.014 | (−0.010;0.038) | 0.247 |
| Chemotherapy |
|
|
|
|
|
|
|
|
|
Adjuvant | −0.025 |
(−0.035;-0.014) | Reference |
|
| Reference |
|
|
|
Neoadjuvant | −0.034 |
(−0.042;-0.023) | −0.009 | (−0.224;0.004) | 0.176 | −0.007 | (−0.019;0.006) | 0.280 |
The multiple linear regression showed a lower BMD
loss in current smokers than in non-smokers/unknown (P=0.022). No
other variables in relation to the spine were statistically
significant. There was a trend of higher BMD loss in premenopausal
women compared to postmenopausal women (P=0.060).
In the univariate analysis of the BMD values from
the hip (data not shown) a significantly larger BMD loss was found
in the group with a BMI <25 in comparison to those with a BMI
>25 (P=0.034). No other variables in this context showed
statistical significance. When controlling for other variables in
the multiple linear regression the higher extent of bone loss in
the group with BMI <25 compared to BMI >25 persisted in the
analysis of the BMD values on the hip (P=0.048). Zoledronic acid
prevented bone loss in the lumbar spine in the univariate analysis
(P<0.001), but the effect did not persist in the multivariate
analysis (P=0.266). No significant effect of zoledronic acid was
observed in the hip (univariate: P=0.162, multivariate:
P=0.826).
There was no significant difference in BMD loss
between patients having adjuvant and neoadjuvant treatment in
neither hip (univariate: P=0.667) nor lumbar spine (univariate:
P=0.176, multivariate: P=0.280).
Discussion
The main finding in this study is that early stage
BC patients treated with chemotherapy had a significant loss of BMD
in both hip (−1.3%) and lumbar spine (−2.9%) (P<0.0001 for hip
as well as lumbar spine), which is in line with other studies
(10,20). Furthermore, in the univariate
analysis premenopausal women had a significantly larger loss of BMD
in the lumbar spine than postmenopausal women (P<0.001), though
a trend only persisted in the multiple linear regression
(P=0.060).
Other studies on premenopausal patients have found a
range of BMD loss similar to ours (21). A bone loss of approximately 0.1
g/cm2 corresponds to one standard deviation, and a loss
of that size in the lumbar spine increases the risk of vertebral
fracture by a factor of 2.3 (22,23).
Looking at the subgroup of premenopausal women with a BMD loss of
0.045 g/cm2 after one year, this can translate into a
considerably increased risk of fracture, if not countered with
prophylaxis medication. These findings are important because the
majority of premenopausal women develops premature ovarian
dysfunction as a direct consequence of chemotherapy, leading to a
decreased systemic oestrogen level and thereby further bone loss in
the years to come (18,21,24).
Corticosteroids are known to induce bone loss, but
patients receiving neoadjuvant chemotherapy did not show a larger
BMD loss than those receiving adjuvant chemotherapy, although they
had two more cycles of chemotherapy and a higher total dose of
corticosteroids.
The strength of this study is the relatively large
number of patients undergoing very uniform antineoplastic treatment
and the precise data on BMD change, since each patient was her own
control. On the other hand, the retrospective design is a
limitation, as it makes room for selection bias. The lack of
detailed information on smoking, alcohol, physical activity, and
level of vitamin D, which are all known to influence bone turnover,
may be potential confounders. DXA scans measure the bone density
but do not reflect bone quality.
Thirty-one patients received zoledronic acid as part
of their anti-cancer treatment, which, however, did not change
their outcome significantly in comparison to those, who did not
receive zoledronic acid. This is probably due to the fact that the
first dose was often given just before the follow-up DXA scan, and
therefore, the inhibitory effect on osteoclasts resorption in the
bone tissue did not have sufficient time to make a difference. In
contrast, a randomized study from 2008, where 4 mg zoledronic acid
was given every three months vs. placebo, showed that zoledronic
acid prevented BMD loss one year after chemotherapy (25).
The follow-up time in our study was between 9 and 15
months, and the loss of BMD did not change significantly with the
length of follow-up. This indicates the majority of BMD loss to
take place well before the time of follow-up.
Postmenopausal patients are now routinely offered
zoledronic acid as part of the adjuvant treatment, since it has
been shown to improve survival and reduce the risk of recurrence
and bone fractures (19). Our
results indicate that premenopausal women are likely to have
accelerated bone loss, and it is therefore worth considering
whether zoledronic acid should also be given to premenopausal women
to reduce potential, long term side-effects.
In conclusion, this study confirms that
neoadjuvant/adjuvant chemotherapy is associated with significant
BMD loss in both the hip and lumbar spine. Furthermore, a
pronounced loss of BMD in the lumbar spine in premenopausal women
is suggested. Further studies with long term follow-up on bone loss
and fracture risk evaluating prophylactic zoledronic acid in
premenopausal patients are warranted.
Acknowledgements
The authors would like to thank René Depont
Christensen, RD Statistics, for statistical assistance.
Funding
No funding received.
Availability of data and materials
The analysed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors contributions
The study was planned in collaboration between all
authors. CA collected the data from the source. The primary
analysis was made by TB and CA and the results were interpreted by
CA, AJ, EJ and TB in collaboration. CA wrote the first draft for
the manuscript. All authors revised it critically for important
intellectual content and made changes in the iterative process that
followed. This final manuscript has been approved by all
authors.
Ethics approval and consent to
participate
Data was collected through the electronic patient
record system after approval by the Danish Health and Medicines
Authority and the Danish Data Protection Agency. According to
Danish law this study did not require approval by the Ethics
Committee (project-ID: S-20150139).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Engholm G, Ferlay J, Christensen N, Kejs
AMT, Hertzum-Larsen R, Johannesen TB, Khan S, Leinonen MK,
Ólafsdóttir E, Petersen T, et al: 2016 NORDCAN: Cancer Incidence,
Mortality, Prevalence and Survival in the Nordic Countries, Version
7.3 (08.07.2016). Association of the Nordic Cancer Registries.
Danish Cancer Society. http://www.ancr.nuMarch 19–2017
|
|
3
|
Hadji P: Cancer treatment-induced bone
loss in women with breast cancer. Bonekey Rep. 4:6922015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hernlund E, Svedbom A, Ivergård M,
Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B and Kanis
JA: Osteoporosis in the European Union: Medical management,
epidemiology and economic burden. A report prepared in
collaboration with the International Osteoporosis Foundation (IOF)
and the European Federation of Pharmaceutical Industry Associations
(EFPIA). Arch Osteoporos. 8:1362013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cameron DA, Douglas S, Brown JE and
Anderson RA: Bone mineral density loss during adjuvant chemotherapy
in pre-menopausal women with early breast cancer: Is it dependent
on oestrogen deficiency? Breast Cancer Res Treat. 123:805–814.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hershman DL, McMahon DJ, Crew KD, Shao T,
Cremers S, Brafman L, Awad D and Shane E: Prevention of bone loss
by zoledronic acid in premenopausal women undergoing adjuvant
chemotherapy persist up to one year following discontinuing
treatment. J Clin Endocrinol Metab. 95:559–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Canalis E, Mazziotti G, Giustina A and
Bilezikian JP: Glucocorticoid-induced osteoporosis: Pathophysiology
and therapy. Osteoporos Int. 18:1319–1328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Christensen CØ, Cronin-Fenton D, Frøslev
T, Hermann AP and Ewertz M: Change in bone mineral density during
adjuvant chemotherapy for early-stage breast cancer. Support Care
Cancer. 24:4229–4236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalder M and Hadji P: Breast cancer and
osteoporosis-management of cancer treatment-induced bone loss in
postmenopausal women with breast cancer. Breast Care (Basel).
9:312–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Greep NC, Giuliano AE, Hansen NM, Taketani
T, Wang HJ and Singer FR: The effects of adjuvant chemotherapy on
bone density in postmenopausal women with early breast cancer. Am J
Med. 114:653–659. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bjarnason NH, Hitz M, Jorgensen NR and
Vestergaard P: Adverse bone effects during pharmacological breast
cancer therapy. Acta Oncol. 47:747–754. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Xu G and Yang F: Effect of
neoadjuvant chemotherapy on the serum levels of bone turnover
markers in women with early-stage breast cancer. PLoS One.
10:e01260532015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hadji P: Aromatase inhibitor-associated
bone loss in breast cancer patients is distinct from postmenopausal
osteoporosis. Crit Rev Oncol Hematol. 69:73–82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eastell R, Adams JE, Coleman RE, Howell A,
Hannon RA, Cuzick J, Mackey JR, Beckmann MW and Clack G: Effect of
anastrozole on bone mineral density: 5-year results from the
anastrozole, tamoxifen, alone or in combination trial 18233230. J
Clin Oncol. 26:1051–1057. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanis JA, McCloskey EV, Powles T, Paterson
AH, Ashley S and Spector T: A high incidence of vertebral fracture
in women with breast cancer. Br J Cancer. 79:1179–1181. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Love RR, Mazess RB, Barden HS, Epstein S,
Newcomb PA, Jordan VC, Carbone PP and DeMets DL: Effects of
tamoxifen on bone mineral density in postmenopausal women with
breast cancer. N Engl J Med. 326:852–856. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoneda K, Tanji Y, Ikeda N, Miyoshi Y,
Taguchi T, Tamaki Y and Noguchi S: Influence of adjuvant tamoxifen
treatment on bone mineral density and bone turnover markers in
postmenopausal breast cancer patients in Japan. Cancer Lett.
186:223–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vehmanen L, Elomaa I, Blomqvist C and
Saarto T: Tamoxifen treatment after adjuvant chemotherapy has
opposite effects on bone mineral density in premenopausal patients
depending on menstrual status. J Clin Oncol. 24:675–680. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Early Breast Cancer Trialists
Collaborative Group (EBCTCG): Adjuvant bisphosphonate treatment in
early breast cancer: Meta-analyses of individual patient data from
randomised trials. Lancet. 386:1353–1361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shapiro CL, Manola J and Leboff M: Ovarian
failure after adjuvant chemotherapy is associated with rapid bone
loss in women with early-stage breast cancer. J Clin Oncol.
19:3306–3311. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hadji P, Gnant M, Body JJ, Bundred NJ,
Brufsky A, Coleman RE, Guise TA, Lipton A and Aapro MS: Cancer
treatment-induced bone loss in premenopausal women: A need for
therapeutic intervention? Cancer Treat Rev. 38:798–806. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garg MK and Kharb S: Dual energy X-ray
absorptiometry: Pitfalls in measurement and interpretation of bone
mineral density. Indian J Endocrinol Metab. 17:203–210. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marshall D, Johnell O and Wedel H:
Meta-analysis of how well measures of bone mineral density predict
occurrence of osteoporotic fractures. BMJ. 312:1254–1259. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riggs BL, Khosla S and Melton LJ III: Sex
steroids and the construction and conservation of the adult
skeleton. Endocr Rev. 23:279–302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hershman DL, McMahon DJ, Crew KD, Cremers
S, Irani D, Cucchiara G, Brafman L and Shane E: Zoledronic acid
prevents bone loss in premenopausal women undergoing adjuvant
chemotherapy for early-stage breast cancer. J Clin Oncol.
26:4739–4745. 2008. View Article : Google Scholar : PubMed/NCBI
|