|
1
|
Goel G, Makkar HP, Francis G and Becker K:
Phorbol esters: Structure, biological activity, and toxicity in
animals. Int J Toxicol. 26:279–288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weinstein IB: The origins of human cancer:
Molecular mechanisms of carcinogenesis and their implications for
cancer prevention and treatment-twenty-seventh G.H.A. Clowes
memorial award lecture. Cancer Res. 48:4135–4143. 1988.PubMed/NCBI
|
|
3
|
Kikkawa U, Takai Y, Tanaka Y, Miyake R and
Nishizuka Y: Protein kinase C as a possible receptor protein of
tumor-promoting phorbol esters. J Biol Chem. 258:11442–11445.
1983.PubMed/NCBI
|
|
4
|
Cooke M, Magimaidas A, Casado-Medrano V
and Kazanietz MG: Protein kinase C in cancer: The top five
unanswered questions. Mol Carcinog. 56:1531–1542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reid BJ, Culotti JG, Nash RS and Pringle
JR: Forty-five years of cell-cycle genetics. Mol Biol Cell.
26:4307–4312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yasutis KM and Kozminski KG: Cell cycle
checkpoint regulators reach a zillion. Cell Cycle. 12:1501–1509.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jackson PK: The hunt for cyclin. Cell.
134:199–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reed SI: G1 specific cyclins: In search
for an S-phase promoting factor. Trends Genet. 7:95–99. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pines J and Hunter T: Cyclin-dependent
kinases: A new cell cycle motif? Trends Cell Biol. 1:117–121. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kishimoto T: Entry into mitosis: A
solution to the decades-long enigma of MPF. Chromosoma.
124:417–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nasmyth K: Viewpoint: Putting the cell
cycle in order. Science. 274:1643–1645. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
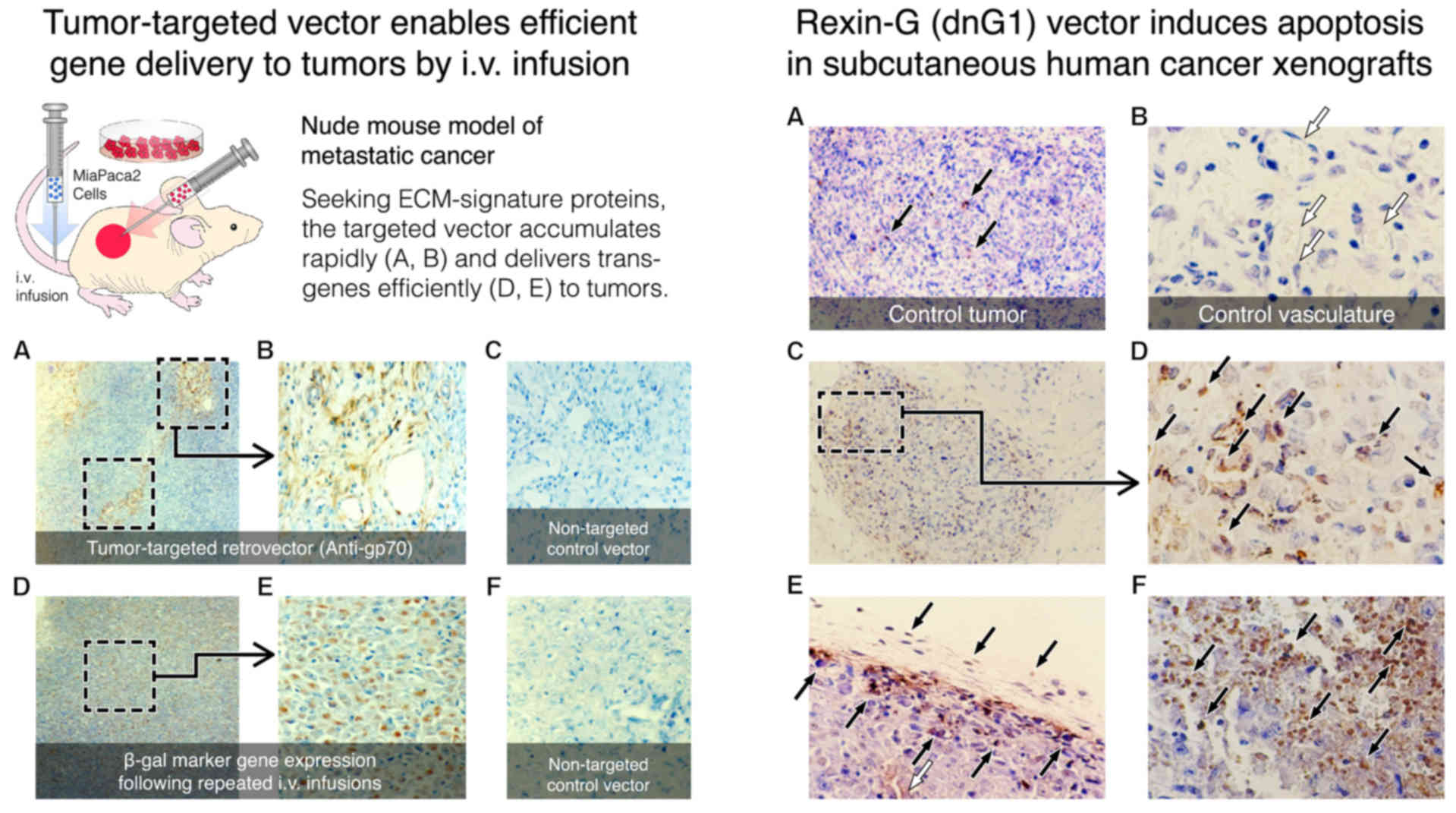
|
Strausfeld UP, Howell M, Descombes P,
Chevalier S, Rempel RE, Adamczewski J, Maller JL, Hunt T and Blow
JJ: Both cyclin A and cyclin E have S-phase promoting (SPF)
activity in Xenopus egg extracts. J Cell Sci. 109:1555–1563.
1996.PubMed/NCBI
|
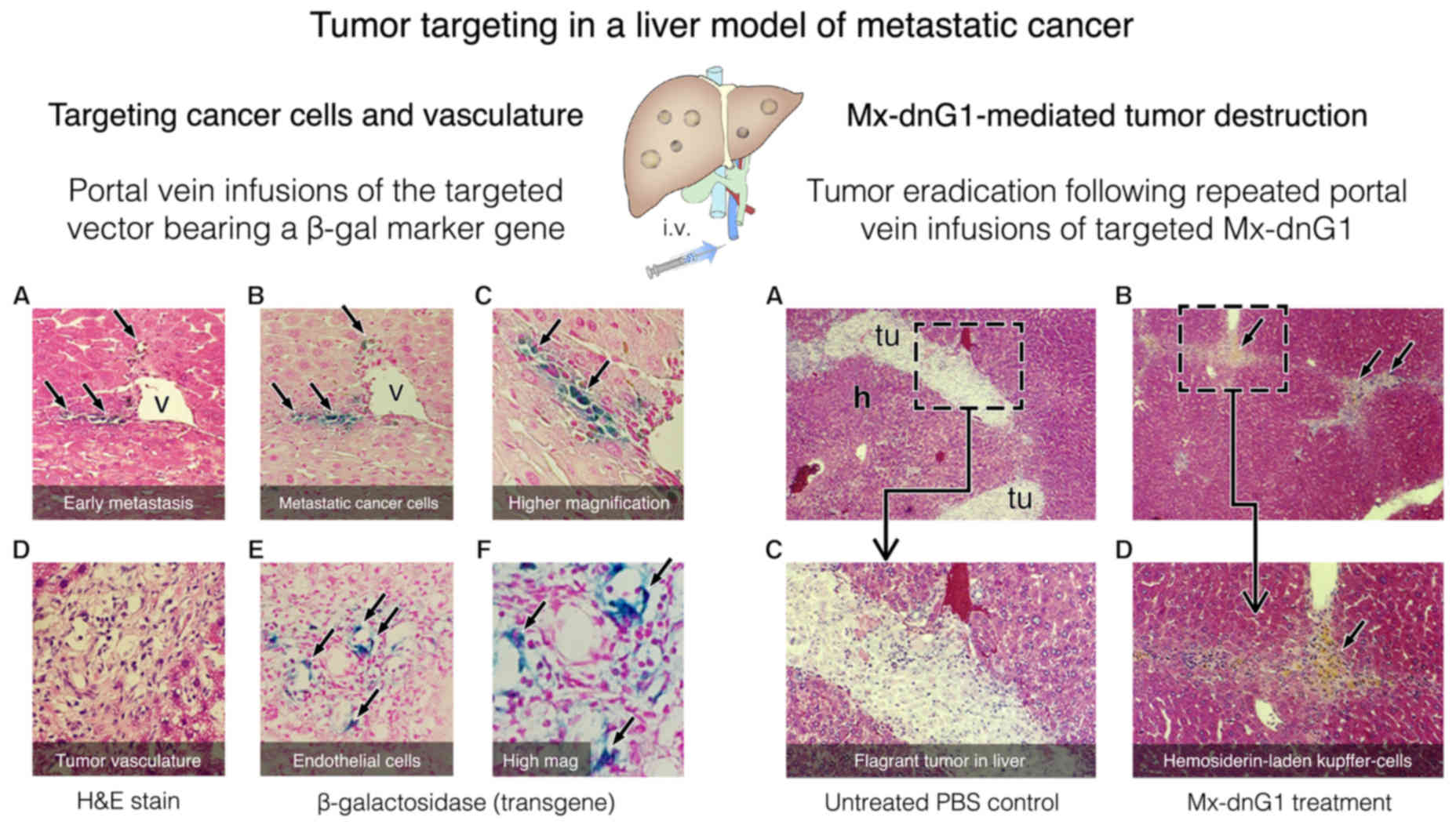
|
13
|
Vulliet PR, Hall FL, Mitchell JP and
Hardie DG: Identification of a novel proline-directed
serine/threonine protein kinase in rat pheochromocytoma. J Biol
Chem. 264:16292–16298. 1989.PubMed/NCBI
|
|
14
|
Hall FL, Mitchell JP and Vulliet PR:
Phosphorylation of synapsin I at a novel site by proline-directed
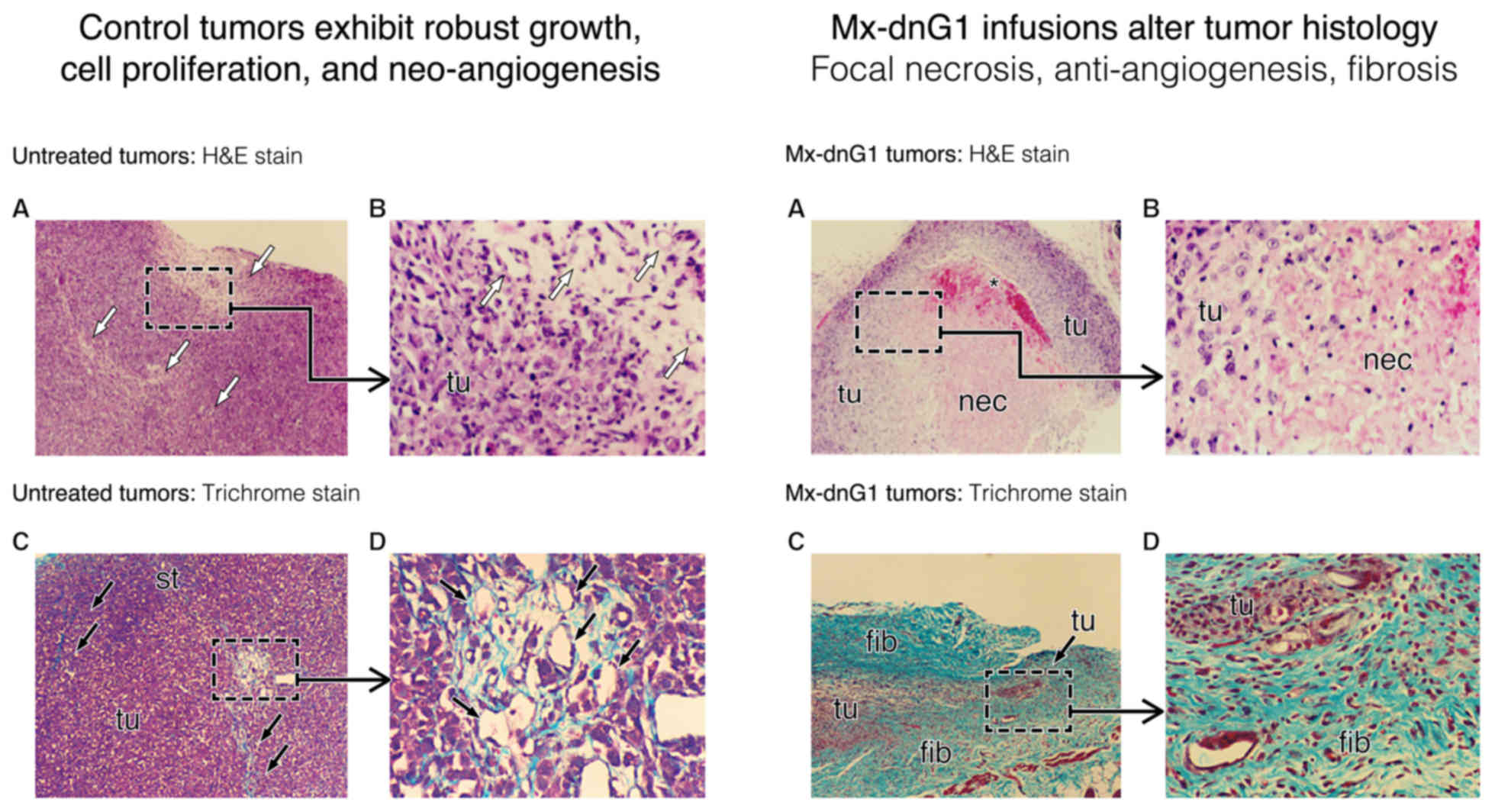
protein kinase. J Biol Chem. 265:6944–6948. 1990.PubMed/NCBI
|
|
15
|
Hall FL and Vulliet PR: Proline-directed
protein phosphorylation and cell cycle regulation. Curr Opin Cell
Biol. 3:176–184. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
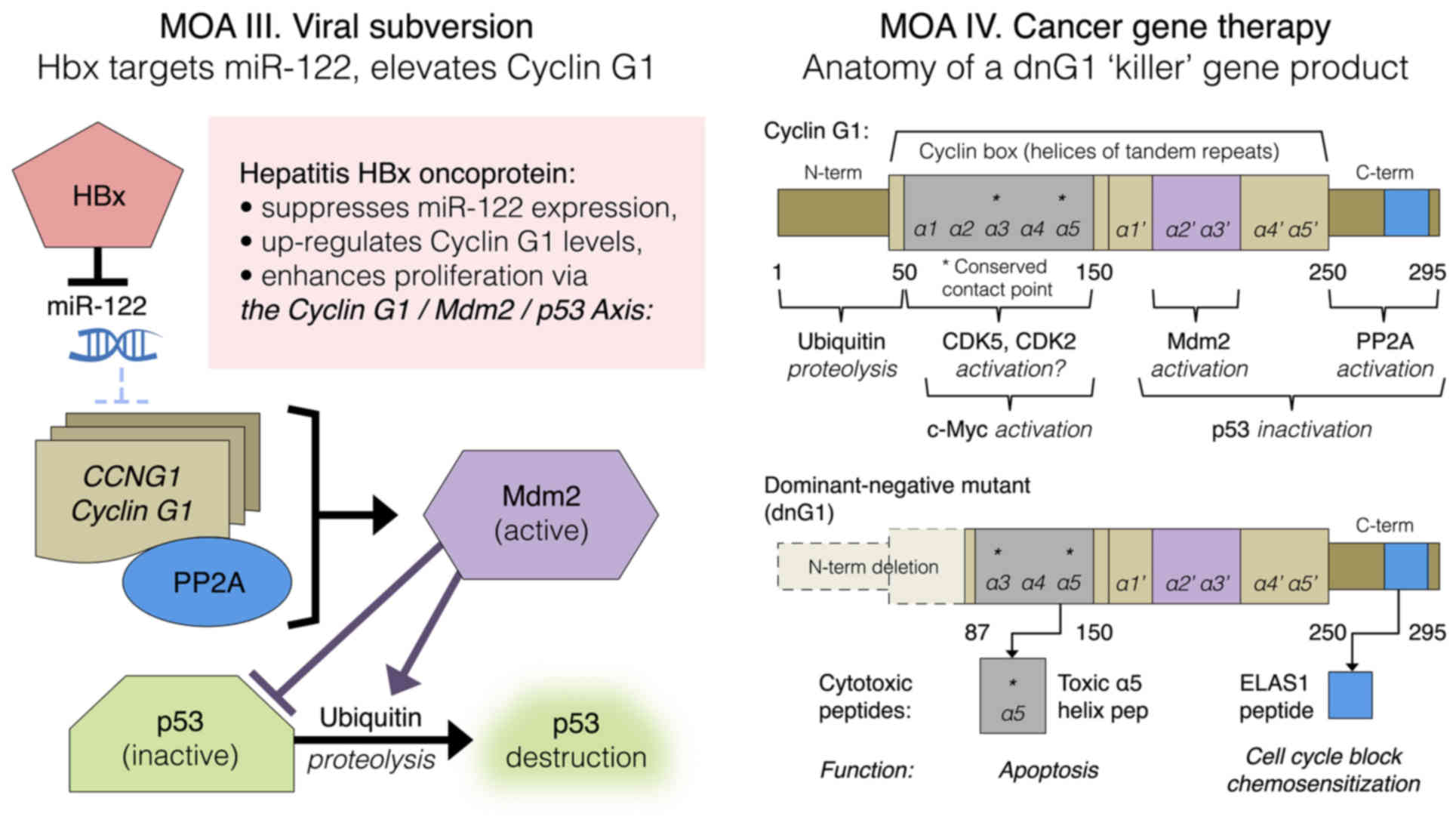
|
Suzuki M: SPXX, a frequent sequence motif
in gene regulatory proteins. J Mol Biol. 207:61–84. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hall FL, Braun RK, Mihara K, Fung YT,
Berndt N, Carbonaro-Hall DA and Vulliet PR: Characterization of the
cytoplasmic proline-directed protein kinase in proliferative cells
and tissues as a heterodimer comprised of p34cdc2 and p58cyclin A.
J Biol Chem. 266:17430–17440. 1991.PubMed/NCBI
|
|
18
|
Elledge SJ, Richman R, Hall FL, Williams
RT, Lodgson N and Harper JW: CDK2 encodes a 33-kDa cyclin
A-associated protein kinase and is expressed before CDC2 in the
cell cycle. Proc Natl Acad Sci USA. 89:2907–2911. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peeper DS, Parker LL, Ewen ME, Toebes M,
Hall FL, Xu M, Zantema A, van der Eb AJ and Piwnica-Worms H: A- and
B-type cyclins differentially modulate substrate specificity of
cyclin-cdk complexes. EMBO J. 12:1947–1954. 1995.
|
|
20
|
Giacinti C and Giordano A: RB and cell
cycle progression. Oncogene. 25:5220–5227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bertoli C, Skotheim JM and de Bruin RA:
Control of cell cycle transcription during G1 and S phases. Nat Rev
Mol Cell Biol. 14:518–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Foster DA, Yellen P, Xu L and Saqcena M:
Regulation of G1 cell cycle progression: Distinguishing the
restriction point from a nutrient-sensing cell growth
checkpoint(s). Genes Cancer. 1:1124–1131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Csikász-Nagy A, Kapuy O, Tóth A, Pál C,
Jensen LJ, Uhlmann F, Tyson JJ and Novák B: Cell cycle regulation
by feed-forward loops coupling transcription and phosphorylation.
Mol Syst Biol. 5:2362009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weinberg RA: The biology of cancer, 2nd
edition, Chapter 9: p53 and apoptosis: Master guardian and
executioner. Garland Sci; New York: 2014
|
|
25
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deshpande A, Sicinski P and Hinds PW:
Cyclins and cdks in development and cancer: A perspective.
Oncogene. 24:2909–2915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Malumbres M: Cyclin-dependent kinases.
Genome Biol. 15:1222014. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sherr CJ and McCormick F: The RB and p53
pathways in cancer. Cancer Cell. 2:103–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giordano A, McCall C, Whyte P and Franza
BR Jr: Human cyclin A and the retinoblastoma protein interact with
similar but distinguishable sequences in the adenovirus E1A gene
product. Oncogene. 6:481–485. 1991.PubMed/NCBI
|
|
30
|
Wang J, Chenivesse X, Henglein B and
Bréchot C: Hepatitis B virus integration in a cyclin A gene in a
hepatocellular carcinoma. Nature. 343:555–557. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Zindy F, Chenivesse X, Lamas E,
Henglein B and Bréchot C: Modification of cyclin A expression by
hepatitis B virus DNA integration in a hepatocellular carcinoma.
Oncogene. 7:1653–1656. 1992.PubMed/NCBI
|
|
32
|
Bréchot C: Oncogenic activation of cyclin
A. Curr Opin Genet Dev. 3:11–18. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bodey B, Williams RT, Carbonaro-Hall DA,
Horvath A, Tolo VT, Luck JV Jr, Taylor CR and Hall FL:
Immunocytochemical detection of cyclin A and cyclin D in
formalin-fixed, paraffin-embedded tissues: Novel, pertinent markers
of cell proliferation. Mod Pathol. 7:846–852. 1994.PubMed/NCBI
|
|
34
|
Motokura T and Arnold A: Cyclin D and
oncogenesis. Curr Opin Genet Dev. 3:5–10. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hunter T and Pines J: Cyclins and Cancer
II: Cyclin D and CDK inhibitors come of age. Cell. 79:573–582.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Santamaria D, Barrière C, Cerqueira A,
Hunt S, Tardy C, Newton K, Cáceres JF, Dubus P, Malumbres M and
Barbacid M: Cdk1 is sufficient to drive the mammalian cell cycle.
Nature. 448:811–815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Blagosklonny MV and Pardee AB: The
restriction point of the cell cycle. Cell Cycle. 1:103–110. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hwang HC and Clurman BE: Cyclin E in
normal and neoplastic cell cycles. Oncogene. 24:2776–2786. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Narasimha AM, Kaulich M, Shapiro GS, Choi
YJ, Sicinski P and Dowdy SF: Cyclin D activates the Rb tumor
suppressor by mono-phosphorylation. Elife. 3:2014.doi:
10.7554/eLife.02872. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
El-Deiry WS: p21(WAF1) mediates cell cycle
inhibition, relevant to cancer suppression and therapy. Cancer Res.
76:5189–5191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Roussel MF: The INK4 family of cell cycle
inhibitors in cancer. Oncogene. 18:5311–5317. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Serrano M, Lee H, Chin L, Cordon-Cardo C,
Beach D and DePinho RA: Role of the INK4a locus in tumor
suppression and cell mortality. Cell. 85:27–37. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shapiro GI and Harper JW: Anticancer drug
targets: Cell cycle and checkpoint control. J Clin Invest.
104:1645–1653. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Casimiro MC, Velasco-Velázquez M,
Aguirre-Alvarado C and Pestell RG: Overview of cyclins D1 function
in cancer and the CDK inhibitor landscape: Past and present. Expert
Opin Investig Drugs. 23:295–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peyressatre M, Prével C, Pellerano M and
Morris MC: Targeting cyclin-dependent kinases in human cancers:
From small molecules to Peptide inhibitors. Cancers (Basel).
7:179–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Asghar U, Witkiewicz AK, Turner NC and
Knudsen ES: The history and future of targeting cyclin-dependent
kinases in cancer therapy. Nat Rev Drug Discov. 14:130–146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sherr CJ, Beach D and Shapiro GI:
Targeting CDK4 and CDK6: From discovery to therapy. Cancer Discov.
6:353–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Horne MC, Goolsby GL, Donaldson KL, Tran
D, Neubauer M and Wahl AF: Cyclin G1 and Cyclin G2 comprise a new
family of cyclins with contrasting tissue-specific and cell
cycle-regulated expression. J Biol Chem. 271:6050–6061. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu L, Liu L, Yee A, Carbonarohall D, Tolo
V and Hall F: Molecular-cloning of the human CYCG1 gene encoding a
G-type cyclin-overexpression in human osteosarcoma cells. Oncol
Rep. 1:705–711. 1994.PubMed/NCBI
|
|
51
|
Okamoto K and Beach D: Cyclin G is a
transcriptional target of the p53 tumor suppressor protein. EMBO J.
13:4816–4822. 1994.PubMed/NCBI
|
|
52
|
Efeyan A and Serrano M: p53: Guardian of
the genome and policeman of the oncogenes. Cell Cycle. 6:1006–1010.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Smith ML, Kontny HU, Bortnick R and
Fornace AJ Jr: The p53-regulated cyclin G gene promotes cell
growth: p53 downstream effectors cyclin G and Gadd45 exert
different effects on cisplatin chemosensitivity. Exp Cell Res.
230:61–68. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Skotzko M, Wu L, Anderson WF, Gordon EM
and Hall FL: Retroviral vector-mediated gene transfer of antisense
Cyclin G1 (CYCG1) inhibits proliferation of human osteogenic
sarcoma cells. Cancer Res. 55:5493–5498. 1995.PubMed/NCBI
|
|
55
|
Chen DS, Zhu NL, Hung G, Skotzko MJ,
Hinton DR, Tolo V, Hall FL, Anderson WF and Gordon EM: Retroviral
vector-mediated transfer of an antisense cyclin G1 construct
inhibits osteosarcoma tumor growth in nude mice. Hum Gene Ther.
8:1667–1674. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hung G, Skotzko MJ, Chang M, Zhu NL,
Parekh D, Hall FL, Gordon EM and Anderson WF: Intratumoral
injection of an antisense cyclin G1 retroviral vector inhibits
growth of undifferentiated carcinoma xenografts in nude mice.
Pediatr Hematol Oncol. 4:317–325. 1997.
|
|
57
|
Piette J, Neel H and Maréchal V: Mdm2:
Keeping p53 under control. Oncogene. 15:1001–1010. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Momand J, Jung D, Wilczynski S and Niland
J: The MDM2 gene amplification database. Nucleic Acids Res.
26:3453–3459. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Haupt S, Vijayakumaran R, Miranda PJ,
Burgess A, Lim E and Haupt Y: The role of MDM2 and MDM4 in breast
cancer development and prevention. J Mol Cell Biol. 9:53–61.
2017.PubMed/NCBI
|
|
60
|
Momand J, Zambetti GP, Olson DC, George D
and Levine AJ: The mdm-2 oncogene product forms a complex with the
p53 protein and inhibits p53-mediated transactivation. Cell.
69:1237–1245. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Iwakuma T and Lozano G: DM2, an
introduction. Mol Cancer Res. 1:993–1000. 2003.PubMed/NCBI
|
|
62
|
Shi D and Gu W: Dual roles of MDM2 in the
regulation of p53: Ubiquitination dependent and ubiquitination
independent mechanisms of MDM2 repression of p53 activity. Genes
Cancer. 3:240–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shangary S and Wang S: Targeting the
MDM2-p53 interaction for cancer therapy. Clin Cancer Res.
14:5318–5324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tisato V, Voltan R, Gonelli A, Secchiero P
and Zauli G: MDM2/X inhibitors under clinical evaluation:
Perspectives for the management of hematological malignancies and
pediatric cancer. J Hematol Oncol. 10:1332017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Estrada-Ortiza N, Neochoritisa CG and
Dömlinga A: How to design a successful p53-MDM2/X interaction
inhibitor: A thorough overview based on crystal structures. Chem
Med Chem. 11:757–772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Meek DW and Knippschild U:
Posttranslational modification of MDM2. Mol Cancer Res.
1:1017–1026. 2003.PubMed/NCBI
|
|
67
|
Okamoto K, Kamibayashi C, Serrano M,
Prives C, Mumby MC and Beach D: p53-dependent association between
cyclin G and the B' subunit of protein phosphatase 2A. Mol Cell
Biol. 16:6593–6602. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Okamoto K, Li H, Jensen MR, Zhang T, Taya
Y, Thorgeirsson SS and Prives C: Cyclin G recruits PP2A to
dephosphorylate Mdm2. Mol Cell. 9:761–771. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Westermarck J and Hahn WC: Multiple
pathways regulated by the tumor suppressor PP2A in transformation.
Trends Mol Med. 14:152–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kimura SH and Nojima H: Cyclin G1
associates with MDM2 and regulates accumulation and degradation of
p53 protein. Genes Cells. 7:869–880. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Giono LE and Manfredi JJ: The p53 tumor
suppressor participates in multiple cell cycle checkpoints. J Cell
Physiol. 209:13–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen X: Cyclin G: A regulator of the
p53-Mdm2 network. Dev Cell. 2:518–519. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jensen MR, Factor VM, Fantozzi A, Helin K,
Huh CG and Thorgeirsson SS: Reduced hepatic tumor incidence in
cyclin G1-deficient mice. Hepatology. 37:862–870. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhu NL, Wu L, Liu PX, Gordon EM, Anderson
WF, Starnes VA and Hall FL: Down-regulation of cyclin G1 expression
by retrovirus-mediated antisense gene transfer inhibits vascular
smooth muscle cell proliferation and neointima formation.
Circulation. 96:628–635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kampmeier J, Behrens A, Wang Y, Yee A,
Anderson WF, Hall FL, Gordon EM and McDonnell PJ: Inhibition of
rabbit keratocyte and human fetal lens epithelial cell
proliferation by retroviral-mediated transfer of antisense cyclin
G1 and antisense MAT1 constructs. Hum Gene Ther. 11:1–8. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jensen MR, Factor VM and Thorgeirsson SS:
Regulation of Cyclin G1 during murine hepatic regeneration
following Dipin-induced DNA damage. Hepatology. 28:537–546. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xu F, Prescott MF, Liu PX, Chen ZH, Liau
G, Gordon EM and Hall FL: Long term inhibition of neointima
formation in balloon-injured rat arteries by intraluminal
instillation of a matrix-targeted retroviral vector bearing an
improved cytocidal Cyclin G1 construct. Int J Mol Med. 8:19–30.
2001.PubMed/NCBI
|
|
78
|
Waehler R, Russell SJ and Curiel DT:
Engineering targeted viral vectors for gene therapy. Nature Rev
Genet. 8:573–587. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hall FL, Gordon EM, Wu L, Zhu NL, Skotzko
MJ, Starnes VA and Anderson WF: Targeting retroviral vectors to
vascular lesions by genetic engineering of the MoMLV gp70 envelope
protein. Hum Gene Ther. 8:2183–2192. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Weimin Wu B, Cannon PM, Gordon EM, Hall FL
and Anderson WF: Characterization of the proline-rich region of
murine leukemia virus envelope protein. J Virol. 72:5383–5391.
1998.PubMed/NCBI
|
|
81
|
Hall FL, Liu L, Zhu NL, Stapfer M,
Anderson WF, Beart RW and Gordon EM: Molecular engineering of
matrix-targeted retroviral vectors incorporating a surveillance
function inherent in von Willebrand factor. Hum Gene Ther.
11:983–993. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhu NL, Gordon EM, Liu L, Terramani T,
Anderson WF and Hall FL: Collagen-targeted retroviral vectors
displaying domain D2 of von Willebrand factor (vWF-D2) enhance gene
transfer to human tissue explants. Int J Pediatr Hematol Oncol.
7:325–335. 2001.
|
|
83
|
Gordon EM, Zhu NL, Forney Prescott M, Chen
ZH, Anderson WF and Hall FL: Lesion-targeted injectable vectors for
vascular restenosis. Hum Gene Ther. 12:1277–1287. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Behrens A, Gordon EM, Li L, Liu PX, Chen
Z, Peng H, La Bree L, Anderson WF, Hall FL and McDonnell PJ:
Retroviral gene therapy vectors for prevention of excimer
laser-induced corneal haze. Invest Ophthalmol Vis Sci. 43:968–977.
2002.PubMed/NCBI
|
|
85
|
Song JC, McDonnell PJ, Gordon EM, Hall FL
and Anderson WF: Phase I/II evaluation of safety and efficacy and a
matrix-targeted retroviral vector bearing a dominant negative
cyclin G1 construct (Mx-dnG1) as adjunctive intervention for
superficial corneal opacity/corneal scarring. Hum Gene Ther.
14:306–309. 2003.PubMed/NCBI
|
|
86
|
Gordon EM, Liu PX, Chen ZH, Liu L, Whitley
MD, Gee C, Groshen S, Hinton DR, Beart RW and Hall FL: Inhibition
of metastatic tumor growth in nude mice by portal vein infusions of
matrix-targeted retroviral vectors bearing a cytocidal cyclin G1
construct. Cancer Res. 60:3343–3347. 2000.PubMed/NCBI
|
|
87
|
Gordon EM, Liu PX, Chen ZH, Liu L, Whitley
M, Liu L, Wei D, Groshen S, Hinton DR, Anderson WF, Beart RW Jr and
Hall FL: Systemic administration of a matrix-targeted retroviral
vector is efficacious for cancer gene therapy in mice. Hum Gene
Ther. 12:193–204. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lenz HJ, Anderson WF, Hall FL and Gordon
EM: Tumor site specific phase I evaluation of safety and efficacy
of hepatic arterial infusion of a matrix-targeted retroviral vector
bearing a dominant negative Cyclin G1 construct as treatment for
colorectal carcinoma metastatic to liver. Hum Gene Ther.
13:1515–1537. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Le Tourneau C, Lee JJ and Siu LL: Dose
escalation methods in phase I cancer clinical trials. J Natl Cancer
Inst. 101:708–720. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Gordon EM, Cornelio GH, Lorenzo CC III,
Levy JP, Reed RA, Liu L and Hall FL: First clinical experience
using a ‘pathotropic’ injectable retroviral vector (Rexin-G) as
intervention for stage IV pancreatic cancer. Int J Oncol.
24:177–185. 2004.PubMed/NCBI
|
|
91
|
Gordon EM, Lopez FF, Cornelio GH, Lorenzo
CC III, Levy JP, Reed RA, Liu L, Bruckner HW and Hall FL:
Pathotropic nanoparticles for cancer gene therapy Rexin-G IV:
Three-year clinical experience. Int J Oncol. 29:1053–1064.
2006.PubMed/NCBI
|
|
92
|
Galanis E, Carlso SK, Foster NR, Lowe V,
Quevedo F, McWilliams RR, Grothey A, Jatoi A, Alberts SR and Rubin
J: Phase I trial of a pathotropic retroviral vector expressing a
cytocidal cyclin G1 construct (Rexin-G) in patients with advanced
pancreatic cancer. Mol Ther. 16:979–984. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chawla SP, Chua VS, Mohan V, Alzwahereh K,
Kalra A, Quon D, Gordon EM and Hall FL: Phase I/II study of
targeted gene delivery in vivo-intravenous infusions of Rexin-G
demonstrate significant biologic activity by FDG PET-CT without
toxicity in patients with progressive chemo-resistant sarcoma,
breast cancer and pancreatic cancer. J Clin Oncol. 26
15-Suppl:S14509. 2008. View Article : Google Scholar
|
|
94
|
Chawla SP, Chua VS, Fernandez L, Quon D,
Saralou A, Blackwelder WC, Hall FL and Gordon EM: Evaluation of the
safety and efficacy of ‘pathotropic’ nanoparticles bearing a
dominant-negative Cyclin G1 construct (Rexin-G) as monotherapy for
chemo-resistant osteosarcoma and other sarcomas-phase I/II and
phase II studies. J Clin Oncol. 27:10513. 2009.
|
|
95
|
Chawla SP, Chua VS, Fernandez L, Quon D,
Saralou A, Blackwelder WC, Hall FL and Gordon EM: Phase I/II and
phase II studies of targeted gene delivery in vivo: intravenous
Rexin-G for chemotherapy-resistant sarcoma and osteosarcoma. Mol
Ther. 17:1651–1657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chawla SP, Chawla NS, Quon D, Chua-Alcala
V, Blackwelder WC, Hall FL and Gordon EM: An advanced phase 1/2
study using an XC-targeted gene therapy vector for chemotherapy
resistant sarcoma. Sarcoma Res Int. 3:1024–1031. 2016.
|
|
97
|
Chawla SP, Chua VS, Fernandez L, Quon D,
Blackwelder WC, Gordon EM and Hall FL: Advanced phase I/II studies
of targeted gene delivery in vivo: Intravenous Rexin-G for
gemcitabine-resistant metastatic pancreatic cancer. Mol Ther.
18:435–441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Gordon EM and Hall FL: Rexin-G, a targeted
genetic medicine for cancer. Expert Opin Biol Ther. 10:819–832.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gordon EM, Chan MT, Geraldino N, Lopez FF,
Cornelio GH, Lorenzo CC III, Levy JP, Reed RA, Liu L and Hall FL:
Le morte du tumour: Histological features of tumor destruction in
chemo-resistant cancers following intravenous infusions of
pathotropic nanoparticles bearing therapeutic genes. Int J Oncol.
30:1297–1307. 2007.PubMed/NCBI
|
|
100
|
Gordon EM and Hall FL: A primer on
pathotropic medicine. In ‘one hundred years of the FDA and the
future of global health. Brooklands New Media Ltd; Shopshire UK:
pp. 842007
|
|
101
|
Kim S, Federman N, Gordon EM, Hall FL and
Chawla SP: Rexin-G®, a tumor-targeted retrovector for
malignant peripheral nerve sheath tumor: A case report. Mol Clin
Oncol. 6:861–865. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Feng Z, Zhang C, Wu R and Hu W: Tumor
suppressor p53 meets microRNAs. J Mol Cell Biol. 3:44–50. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Huang S and He X: The role of microRNAs in
liver cancer progression. Br J Cancer. 104:235–240. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Gramantieri L, Ferracin M, Fornari F,
Veronese A, Sabbioni S, Liu CG, Calin GA, Giovannini C, Ferrazzi E,
Grazi LG, et al: Cyclin G1 is a target of miR-122a, a microRNA
frequently down-regulated in human hepatocellular carcinoma. Cancer
Res. 67:6092–6099. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Coulouarn C, Factor VM, Andersen JB,
Durkin ME and Thorgeirsson SS: Loss of miR-122 expression in liver
cancer correlates with suppression of the hepatic phenotype and
gain of metastatic properties. Oncogene. 28:3526–3536. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Fornari F, Gramantieri L, Giovannini C,
Veronese A, Ferracin M, Sabbioni S, Calin GA, Grazi GL, Croce CM,
Tavolari S, et al: MiR-122/cyclin G1 interaction modulates p53
activity and affects doxorubicin sensitivity of human
hepatocarcinoma cells. Cancer Res. 69:5761–5767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wu X, Wu S, Tong L, Luan T, Lin L, Lu S,
Zhao W, Ma Q, Liu H and Zhong Z: miR-122 affects the viability and
apoptosis of hepatocellular carcinoma cells. Scand J
Gastroenternol. 44:1332–1339. 2009. View Article : Google Scholar
|
|
108
|
Ma L, Liu J, Shen J, Liu L, Wu J, Li W,
Luo J, Chen Q and Qian C: Expression of miR-122 mediated by
adenoviral vector induces apoptosis and cell cycle arrest of cancer
cells. Cancer Biol Ther. 9:554–561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hsu SH, Wang B, Kota J, Yu J, Costinean S,
Kutay H, Yu L, Bai S, La Perle K, Chivukula RR, et al: Essential
metabolic, anti-inflammatory, and anti-tumorigenic functions of
miR-122 in liver. J Clin Invest. 122:2871–2883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Bandopadhyay M, Sarkar N, Datta S, Das D,
Pal A, Panigrahi1 R, Banerjee A, Panda CK, Das C, Chakrabarti S and
Chakravarty R: Hepatitis B virus X protein mediated suppression of
miRNA-122 expression enhances hepatoblastoma cell proliferation
through cyclin G1-p53 axis. Infect Agent Cancer. 11:402016.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Reimer CL, Borras AM, Kurdistani SK,
Garreau JR, Chung M, Aaronson SA and Lee SW: Altered regulation of
Cyclin G in human breast cancer and its specific localization at
replication foci in response to DNA damage in p53+/+ cells. J Biol
Chem. 274:11022–11029. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Perez R, Wu N, Klipfel AA and Beart RW Jr:
A better cell cycle target for gene therapy of colorectal cancer:
Cyclin G. J Gastrointest Surg. 7:884–889. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wen W, Ding J, Sun W, Fu J, Chen Y, Wu K,
Ning B, Han T, Huang L, Chen C, et al: Cyclin G1-mediated
epithelial-mesenchymal transition via phosphoinositide 3-kinase/Akt
signaling facilitates liver cancer progression. Hepatology.
55:1787–1798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Weinstein B and Joe A: Oncogene addiction.
Cancer Res. 68:3077–3080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Li H, Okamoto K, Peart MJ and Prives C:
Lysine-independent turnover of Cyclin G1 can be stabilized by
B'alpha subunits of protein phosphatase 2A. Mol Cell Biol.
29:919–928. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Piscopo DM and Hinds PW: A role for the
cyclin box in the ubiquitin-mediated degradation of cyclin G1.
Cancer Res. 68:5581–5590. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Seo HR, Kim J, Bae S, Soh JW and Lee YS:
Cdk5-mediated phosphorylation of c-Myc on Ser-62 is essential in
transcriptional activation of cyclin B1 by Cyclin G1. J Biol Chem.
283:15601–15610. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Menssen A and Hermeking H:
Characterization of the c-MYC-regulated transcriptome by SAGE:
Identification and analysis of c-MYC target genes. Proc Natl Acad
Sci USA. 99:6274–6279. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Morita N, Kiryu S and Kiyama H:
p53-independent Cyclin G expression in a group of mature neurons
and its enhanced expression during nerve regeneration. J Neurosci.
16:5961–5966. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Sultana R and Butterfield DA: Regional
expression of key cell cycle proteins in brain from subjects with
amnestic mild cognitive impairment. Neurochem Res. 32:655–662.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yang Y, Mufson EJ and Herrup K: Neuronal
cell death is preceded by cell cycle events at all stages of
Alzheimer's disease. J Neurosci. 23:2557–2563. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lee MS, Kwon YT, Li M, Peng J, Friedlander
RM and Tsai LH: Neurotoxicity induces cleavage of p35 to p25 by
calpain. Nature. 405:360–364. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ko J, Humbert S, Bronson RT, Takahashi S,
Kulkarni AB, Li E and Tsai L: p35 and p39 are essential for
cyclin-dependent kinase 5 function during neurodevelopment. J
Neurosci. 21:6758–6771. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Cheung ZH, Gong K and Ip NY:
Cyclin-dependent kinase 5 supports neuronal survival through
phosphorylation of Bcl-2. J Neurosci. 28:4872–4877. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Cruz JC, Tseng HC, Goldman JA, Shih H and
Tsai LH: Aberrant Cdk5 activation by p25 triggers pathological
events leading to neurodegeneration and neurofibrillary tangles.
Neuron. 40:471–483. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Tansey WP: Mammalian MYC proteins and
cancer. New J Sci. 2014:Article ID 757534. 2014. View Article : Google Scholar
|
|
127
|
Dang CV, Reddy EP, Shokat KM and Soucek L:
Drugging the ‘undruggable’ cancer targets. Nat Rev Cancer.
17:502–508. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhang W, Xu J, Ji D, Li Z, He W, Yang F,
Lan H, Wang Y, Wu Z, Liu X, et al: Cyclin G1 amplification enhances
aurora kinase inhibitor-induced polyploid resistance and inhibition
of Bcl-2 pathway reverses the resistance. Cell Physiol Biochem.
43:94–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Russell P, Hennessy BT, Li J, Carey MS,
Bast RC, Freeman T and Venkitaraman AR: Cyclin G1 regulates the
outcome of taxane-induced mitotic checkpoint arrest. Oncogene.
31:2450–2460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Shang Y, Feng B, Zhou L, Ren G, Zhang Z,
Fan X, Sun Y, Luo G, Liang J, Wu K, et al: The
miR27b-CCNG1-P53-miR-508-5p axis regulates multidrug resistance of
gastric cancer. Oncotarget. 7:538–549. 2015.
|
|
131
|
Zhang H, Hao Y, Yang J, Zhou Y, Li J, Yin
S, Sun C, Ma M, Huang Y and Xi JJ: Genome-wide functional screening
of miR-23b as a pleiotropic modulator suppressing cancer
metastasis. Nat Commun. 2:5542011. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yan J, Jiang JY, Meng XN, Xiu YL and Zong
ZH: MiR-23b targets cyclin G1 and suppresses ovarian cancer
tumorigenesis and progression. J Exp Clin Cancer Res. 35:312016.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Uchihashi T, Ota K, Yabuno Y, Ohno S,
Fukushima K, Naito Y, Kogo M, Yabuta N and Nojima H: ELAS1 induces
apoptotic death in adenocarcinoma DU145 and squamous-cell carcinoma
SAS cancer cells, but not in normal KD cells. Oncotarget.
8:85868–85882. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Brown NR, Noble ME, Endicott JA, Garman
EF, Wakatsuki S, Mitchell E, Rasmussen B, Hunt T and Johnson LN:
The crystal structure of Cyclin A. Structure. 3:1235–1247. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Ferro ES, Hyslop S and Camargo AC:
Intracellullar peptides as putative natural regulators of protein
interactions. J Neurochem. 91:769–777. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
de Araujo CB, Russo LC, Castro LM, Forti
FL, do Monte ER, Rioli V, Gozzo FC, Colquhoun A and Ferro ES: A
Novel intracellular peptide derived from g1/s cyclin d2 induces
cell death. J Biol Chem. 289:16711–16726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Russo LC, Araujo CB, Iwai LK, Ferro ES and
Forti FL: A cyclin D2-derived peptide acts on specific cell cycle
phases by activating ERK1/2 to cause the death of breast cancer
cells. J Proteomics. 151:24–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Gondeau C, Gerbal-Chaloin S, Bello P,
Aldrian-Herrada G, Morris MC and Divita G: Design of a novel class
of peptide inhibitors of cyclin-dependent kinase/cyclin activation.
J Biol Chem. 280:13793–13800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Ahuja D, Sáenz-Robles MT and Pipas JM:
SV40 large T antigen targets multiple cellular pathways to elicit
cellular transformation. Oncogene. 24:7729–7745. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ohno S, Naito Y, Mukai S, Yabuta N and
Nojima H: ELAS1-mediated inhibition of the cyclin G1-B’γ
interaction promotes cancer cell apoptosis via stabilization and
activation of p53. Oncogene. 34:5983–5996. 2015. View Article : Google Scholar : PubMed/NCBI
|