Introduction
Invasive mucinous adenocarcinoma (IMA), formerly
referred to as mucinous bronchioloalveolar carcinoma (BAC),
accounts for ~3–4% of all lung cancers and exhibits an increasing
tendency annually (1). Due to the
lack of specific clinical manifestations, the majority of the early
cases were misdiagnosed as pneumonia, tuberculosis, and other
diffuse pulmonary diseases. Furthermore, the pathogenesis,
classification of subtypes and, particularly, the treatment
protocols of IMA, have not yet been fully elucidated. In recent
years, the platinum-based regimen with pemetrexed (PEM; a folic
acid metabolism antagonist) and bevacizumab (BEV) was reported as
an effective choice for patients with IMA (2,3). We
herein present the case of a patient with IMA who achieved a rapid
and stable response to an initial 6-cycle course and a subsequent
4-cycle course of combination chemotherapy with bevacizumab and
pemetrexed/cisplatin. In addition, a review of the relevant
literature on the treatment of IMA is presented.
Case report
A 42-year-old man, who was a current smoker
(Brinkman index: 500; his father had died of lung cancer),
presented with a sore throat and productive cough, night sweats,
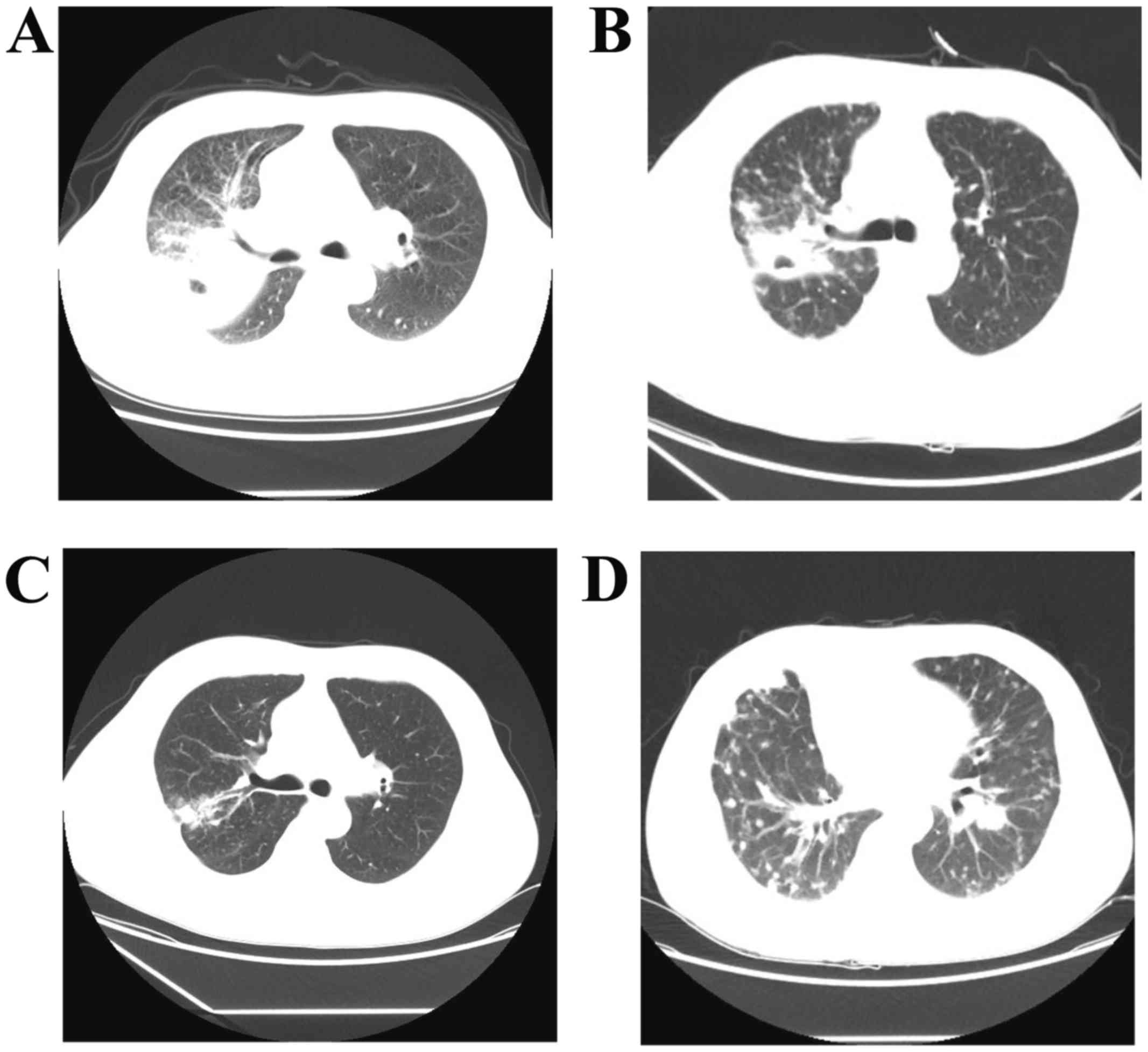
but no fever. A chest computed tomography (CT) scan revealed
exudation and a cavity in the upper lobe of the right lung (URL),
with enlarged mediastinal lymph nodes. No relief of the symptoms
was achieved by a 2-week treatment with antibiotics. With obvious
progression on imaging, the patient was diagnosed with pulmonary
tuberculosis (Fig. 1A) and received
diagnostic anti-tuberculosis therapy including pyrazinamide and
ethambutol. One week later, the patient developed chest pain and
dyspnea. Fibrotic bronchoscopy identified swelling and voluminous
secretions in the URL, without any readily evident neoplasms.
Adenocarcinoma cells were found in the bronchoalveolar lavage
fluid. A positron emission tomography/CT scan demonstrated that the
wall of the cavity was thick and the metabolic activity was
increased [standardized uptake value (SUV)=6.3]; the right hilar
lymph nodes were also enlarged (SUV=3.9). Following CT-guided
percutaneous transthoracic needle biopsy in April 29, 2014, and
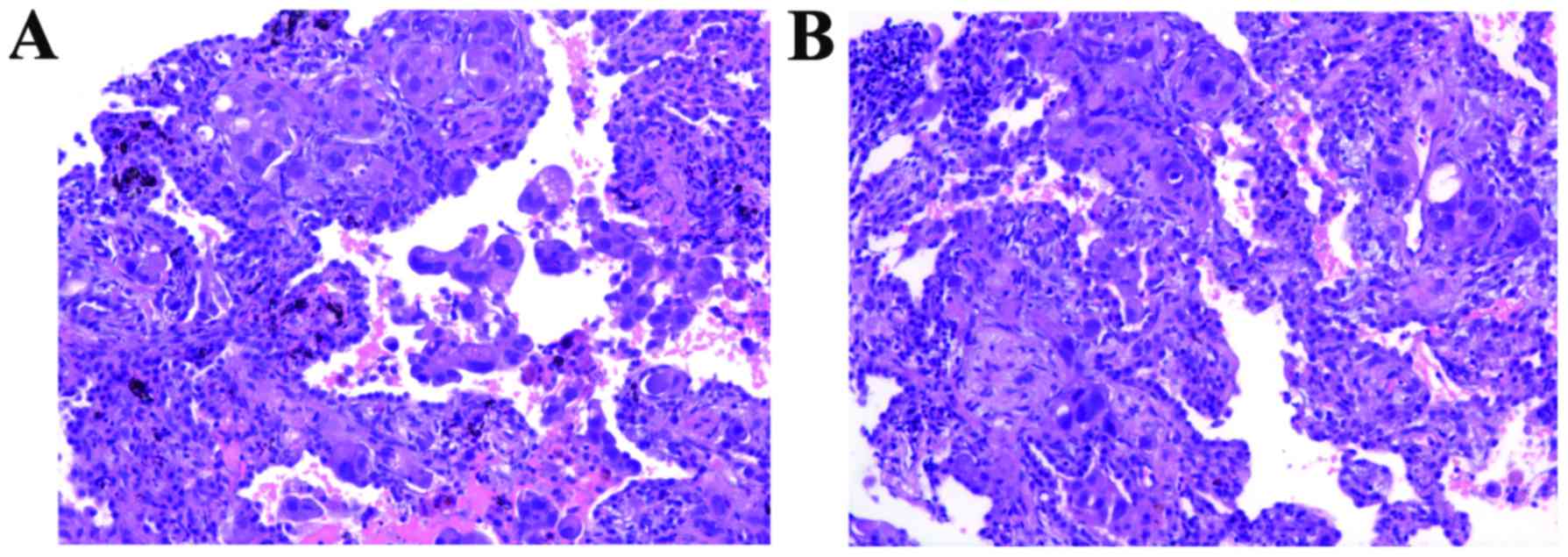
subsequent histological analysis, the patient was diagnosed with
IMA (Fig. 2A). An
immunohistochemical analysis was positive for carcinoembryonic
antigen, cytokeratin (CK)7, CK20 and epithelial membrane antigen,
and negative for thyroid transcription factor-1. Brain magnetic
resonance imaging and bone scintigraphy revealed no evidence of
extrathoracic metastasis. Thus, the clinical stage was IIIb
(cT4N2M0).
Thereafter, the patient underwent first-line
treatment with PEM (500 mg/m2), cisplatin (DDP; 75
mg/m2) and BEV (15 mg/kg) starting on day 1 every 21-day
cycle, along with folic acid and vitamin B12 supplementation. The
patient was evaluated for partial response (PR) after 6 cycles,
with favorable radiological improvement (Fig. 1B). During follow-up, he exhibited
radiological progression after 2 months (Fig. 1C) and he received 2 cycles of
gemcitabine plus carboplatin, and 2 cycles of paclitaxel plus DDP
(d1) combined with Conmana [icotinib; an epidermal growth factor
receptor tyrosine kinase (EGFR-TKI) (d8-d21)]. However,
multiple metastatic lung nodules were identified on CT examination,
indicating disease progression (Fig.
1D).
The second biopsy was also diagnosed as
adenocarcinoma (Fig. 2B) with
KRAS gene mutation. The patient was then administered
another 4 cycles of PEM + DDP + BEV and again achieved stable
disease. After the disease progressed again, vinorelbine and
oxaliplatin combined with BEV were selected as the chemotherapy
regimen. However, the patient did not respond to treatment, and
succumbed to the disease in October 2016.
Discussion
We herein report that IMA may present as pneumonia
mimicking pulmonary tuberculosis. Based on the unique radiological,
morphological and genetic characteristics, BAC was renamed as IMA
and classified as a new distinct category of lung cancer (4). The imaging findings are as follows
(5): The solitary nodule type
displays the characteristics of peripheral adenocarcinoma, which is
lobulated or has scalloped margins, with heterogeneous density on
CT scans, located subpleurally. The segmental type comprises
multisegmental or multilobular lesions on bronchiolography, located
in the lower lung. Finally, the diffuse type includes bilateral
diffuse nodules of various sizes and distributions. In the present
case, diffuse patchy shadows and a cavity in the URL were
identified on chest CT, which displayed all the radiological
characteristics mentioned above.
As regards the treatment of IMA, surgery remains the
first choice for patients diagnosed as stage I or II (6,7). A wide
variety of chemotherapeutic options are available for advanced BAC
or IMA (8,9), but with a poor sensitivity rate.
According to the results of 30 relevant studies, including 19 case
reports and 11 clinical trials on IMA treatment (Table I), IMA exhibits a poor response to
traditional chemotherapy, such as paclitaxel (10,11),
navebine (12) and platinum-based
chemotherapy (13,14), with a median progression-free
survival (PFS) ranging from 2.2 to 5 months, and an overall
survival (OS) ranging from 13 to 23 months.
 | Table I.Response to systemic chemotherapy in
patients with IMA. |
Table I.
Response to systemic chemotherapy in
patients with IMA.
| Year | Authors | n | Pathological type
(cases) | Treatment (no. of
cases) | Response | (Refs.) |
|---|
| 2005 | West et
al | 58 | BAC (58) | PTX (27) | ORR 14%; PFS 5
months; OS 12 months | (11) |
| 2005 | Scagliotti et
al | 19 | BAC (19) | PTX (19) | ORR 11.1%; DCR 54%;
PFS 2.2 months; OS 8.6 months | (10) |
| 2007 | Dziadziuszko et
al | 1 | BAC (1) | WN (1) | Sx. improved; CR 6
months | (12) |
| 2013 | Dirican et
al | 44 | BAC (44) | Platinum-based
(21) | ORR 33.3% (4 PR, 3
CR); SD 42.8%; PD 23.8% | (13) |
| 2016 | Luo et al | 3,681 | Pure IMA (97) Mixed
IMA (48) Ade. (3,536) | Platinum-based | DFS (P=0.003); OS
(P=0.514) (78/36/2,753) | (14) |
| 2013 | Lau et
al | 27 | Pure BAC (6) | PEM (24) | ORR 23%; PFS 6
months; | (17) |
|
|
|
| Mixed BAC (18) |
| OS 25 months |
|
| 2011 | Okuda et
al | 1 | BAC (1) | PEM (1) | Sx. improved; CR-SD
12 months | (16) |
| 2012 | Duruisseaux et
al | 88 | BAC (88) | TAX-based (29) | ORR 21%; DCR 56%;
PFS 3 months | (19) |
|
|
|
|
| GEM-based (12) |
|
|
|
|
|
|
| PEM (2) |
|
|
|
|
|
|
| Bortezomib (3) |
|
|
|
|
|
|
| Erlotinib (1) |
|
|
| 2010 | Manson et
al | 1 | BAC (1) | PEM (1) | Sx. improved; CR-SD
12.6 months | (18) |
| 2013 | Koma et
al | 2 | IMA (2) | DDP/PEM/BEV
(2) | Sx. improved; CR-SD
6.1 months | (2) |
| 2013 | Yamakawa et
al | 2 | IMA (2) | DDP/PEM/BEV
(2) | Sx. improved; CR-SD
4.7 months; | (3) |
|
|
|
|
|
| CR-SD 7.2
months |
|
PEM is a new member of the antifolate class that
acts by inhibiting thymidylate synthesis, dihydrofolate reductase
and glycinamide ribonucleotide formyltransferase, promoting S phase
arrest of tumor cells (15). The
response of IMA to PEM has been reported to be good, with fewer
side effects (16,17), even in patients insensitive to
gefitinib and/or erlotinib (18,19).
BEV, a recombinant humanized monoclonal antibody developed against
vascular endothelial growth factor that may prevent receptor
binding and inhibit endothelial cell proliferation and vessel
formation, has been used as a molecular-targeted treatment for
malignant tumors in recent years (20,21). As
a cell stabilizer, BEV exerts a synergistic effect with PEM. To a
certain extent, PEM + BEV as second-line therapy for non-small-cell
lung cancer (NSCLC) appears promising, with a PFS of 4 months and
an OS of 8.6 months (22). The
present case demonstrated the clinical efficacy and survival
benefit of PEM/DDP and BEV in the treatment of IMA. The initial 6
cycles of treatment were effective and well-tolerated. The benefit
of this combination therapy was consistent with that of an
additional 4 cases reported in Japan (2,3).
Over the last decades, selective EGFR-TKIs achieved
excellent results in the treatment of NSCLC (Table II). Gefitinib (23–32) and
erlotinib (33–35), as first-generation EGFR-TKIs,
significantly prolonged the OS to 13.2–23 months, although the PFS
remained at 2.9–13 months. However, IMA derived from metaplasia of
bronchiolar epithelia, is strongly associated with KRAS
mutations and absence of EGFR mutations (36,37),
indicating that EGFR-TKIs would not be beneficial for this
patient.
 | Table II.Response to EGFR-TKI in patients with
IMA. |
Table II.
Response to EGFR-TKI in patients with
IMA.
| Year | Authors | n | Pathological type
(no. of cases) | Treatment (no. of
cases) | Evaluation | Response | (Refs.) |
|---|
| 2003 | Yano et
al | 2 | BAC (2) | ZD1839 (2) | Sx., S | Sx. improved;
Sputum cytology (−); CR 8–13 months | (24) |
| 2003 | Chang et
al | 2 | BAC (2) | ZD1839 (2) | Sx., S | Sx. improved; CR 2
weeks - 2 months | (23) |
| 2004 | Bayle et
al | 1 | BAC (1) | Gefitinib (1) | Sx., S | PR 12 months | (25) |
| 2005 | Milton et
al | 2 | BAC (2) | Gefitinib (2) | Sx., S | Sx. improved; CR
4–7 weeks | (27) |
| 2005 | Kitazaki et
al | 2 | BAC (2) | Gefitinib (1) | Sx., S | Sx. improved; CR 2
−2.7 weeks | (26) |
| 2005 | Taja-Chayeb et
al | 1 | BAC (1) | Gefitinib (1) | Sx., S | Sx. improved; CR 4
weeks | (28) |
| 2006 | West et
al | 136 | BAC (136) | Gefitinib
(136) | ORR, CRs, OS | ORR 17%; CRs 6%
(untreated), 9% (pretreated); OS 13 months | (29) |
| 2007 | Kijima et
al | 1 | BAC (1) | Gefitinib (1) | Sx., S, OS | Sx. improved; CR-SD
8.5 months; OS 26 months | (30) |
| 2009 | Cadranel et
al | 88 | BAC (88) | Gefitinib (88) | DCR, PFS, OS | DCR 29.4%; PR
12.9%; SD 16.4%; PFS 2.9 months; OS 13.2 months | (31) |
| 2012 | Popat et
al | 1 | BAC (1) | Gefitinib (1) | Sx. | Sx. improved | (32) |
| 2008 | Miller et
al | 101 | BAC (12) Ade.
(89) | Erlotinib
(101) | ORR, OS, PFS | ORR 22%; OS 4
months (BAC), 19 months (Ade); PFS 4 months. | (33) |
| 2012 | Yuyama et
al | 1 | BAC (1) | Erlotinib (1) | Sx. | Sx. improved | (34) |
| 2014 | Sanz Rubiales et
al | 1 | BAC (1) | Erlotinib (1) | S | PR 8 months | (35) |
Finally, this patient with IMA had a better
prognosis, with a 10-month PFS and 30-month OS, compared with 4
cases reporting a 12.6-month OS (2,3).
Therefore, early treatment with BEV combined with PEM and DDP may
be beneficial in terms of prolonged IMA survival.
Acknowledgements
Not applicable.
Funding
This study was supported by the Shanghai Key
Discipline for Respiratory Diseases (grant no. 2017ZZ02014).
Availability of data and materials
Not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and the
accompanying images that have been submitted together with this
manuscript.
Authors' contributions
XS, YD and YZ carried out the design and
coordination of the study, PC, YY and JS performed the data and
statistical analysis. XS and QL drafted the manuscript. All the
authors have read and approved the final version of this
manuscript.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BEV
|
bevacizumab
|
|
DDP
|
cisplatin
|
|
IMA
|
invasive mucinous adenocarcinoma
|
|
PEM
|
pemetrexed
|
References
|
1
|
Read WL, Page NC, Tierney RM, Piccirillo
JF and Govindan R: The epidemiology of bronchioloalveolar carcinoma
over the past two decades: Analysis of the SEER database. Lung
Cancer. 45:137–142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koma Y, Nakashima N, Koyama M, Goto K,
Yokota N, Kimura K, Matsumoto Y, Yoshida C, Matsuoka H, Masuya D,
et al: Two cases of recurrent invasive mucinous adenocarcinoma of
the lung showing marked responses to platinum-based
chemotherapyregimens with pemetrexed and bevacizumab. Gan To Kagaku
Ryoho. 40:1525–1528. 2013.(In Japanese). PubMed/NCBI
|
|
3
|
Yamakawa H, Takayanagi N, Ishiguro T,
Kagiyama N, Shimizu Y and Sugita Y: A favorable response to
cisplatin, pemetrexed and bevacizumab in two cases of invasive
mucinous adenocarcinoma formerly known as pneumonic-type mucinous
bronchioloalveolar carcinoma. Intern Med. 52:2781–2784. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger K, Yatabe Y, Powell CA, Beer D, Riely G,
Garg K, et al: International Association for the Study of Lung
Cancer/American Thoracic Society/European Respiratory Society:
International multidisciplinary classification of lung
adenocarcinoma: Executive summary. Proc Am Thorac Soc. 8:381–385.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Masuzawa K, Minematsu N, Sasaki M, Ohsawa
K, Yamamoto T, Iwamaru A, Ogata K, Betsuyaku T and Murakami M:
Invasive mucinous adenocarcinoma of the lung presenting as a large,
thin-walled cyst: A case report and literature review. Mol Clin
Oncol. 6:433–437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levy BP, Drilon A, Makarian I, Patel AA
and Grossbard ML: Systemic approaches for multifocal
bronchioloalveolar carcinoma: Is there an appropriate target?
Oncology (Williston Park). 24(888–898): 9002010.PubMed/NCBI
|
|
7
|
Whitson BA, Groth SS, Andrade RS, Mitiek
MO, Maddaus MA and D'Cunha J: Invasive adenocarcinoma with
bronchoalveolar features: A population-based evaluation of the
extent of resection in bronchoalveolar cell carcinoma. J Thorac
Cardiovasc Surg. 143(591–600): e12012.PubMed/NCBI
|
|
8
|
Miller VA, Hirsch FR and Johnson DH:
Systemic therapy of advanced bronchioloaIveolar cell carcinoma:
Challenges and opportunities. J Clin Oncol. 23:3288–3293. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kris MG, Giaccone G, Davies A, Fukuoka M,
Garfield DH, Jassem J, Quoix EA, Sandler AB, Scagliotti GV, Van
Meerbeeck JP and West H: Systemic therapy of bronchioloalveolar
carcinoma: Results of the first IASLC/ASCO consensus conference on
bronchioloalveolar carcinoma. J Thorac Oncol. 1 9 Suppl:S32–S36.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scagliotti GV, Smit E, Bosquee L, O'Brien
M, Ardizzoni A, Zatloukai P, Eberhardt W, Smid-Geirnaerdt M, de
Bruin HG, Dussenne S, et al: A phase II study of paclitaxel in
advanced bronchioloalveolar carcinoma (EORTC trial 08956). Lung
Cancer. 50:91–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
West HL, Crowley JJ, Vance RB, Franklin
WA, Livingston RB, Dakhil SR, Giguere JK, Rivkin SE, Kraut M,
Chansky K, et al: Advanced bronchioloalveolar carcinoma: A phase II
trial of paclitaxel by 96-hour infusion (SWOG 9714): A Southwest
Oncology Group study. Ann Oncol. 16:1076–1080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dziadziuszko R, Siemiatkowska A, Limon J,
Rzyman W, Jassem J, Bunn PA Jr, Varella-Garcia M and Hirsch FR:
Unusual chemosensitivity of advanced bronchioalveolar carcinoma
after gefitinib response and progression: A case report. J Thorac
Oncol. 2:91–92. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dirican N, Baysak A, Cok G, Goksel T and
Aysan T: Clinical characteristics of patients with
bronchioloalveolar carcinoma: A retrospective study of 44 cases.
Asian Pac J Cancer Prev. 14:4365–4368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo J, Wang R, Han B, Zhang J, Zhao H,
Fang W, Luo Q, Yang J, Yang Y, Zhu L, et al: Analysis of the
clinicopathologic characteristics and prognostic of stage I
invasive mucinous adenocarcinoma. J Cancer Res Clin Oncol.
142:1837–1845. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Joerger M, Omlin A, Cerny T and Früh M:
The role of pemetrexed in advanced non-small cell lung cancer:
Special focus on pharmacology and mechanism of action. Curr Drug
Targets. 11:37–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okuda C, Kim YH, Takeuchi K, Togashi Y,
Masago K, Sakamori Y, Mio T and Mishima M: Successful treatment
with pemetrexed in a patient with mucinous bronchioloalveolar
carcinoma: Long-term response duration with mild toxicity. J Thorac
Oncol. 6:641–642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lau DH, Moon J, Davies AM, Sanborn RE,
Hirsch FR, Franklin WA, Ruzich JC, Redman MW and Gandara DR:
Southwestern oncology group phase II trial (S0526) of pemetrexed in
bronchioloalveolar carcinoma subtypes of advanced adenocarcinoma.
Clin Lung Cancer. 14:351–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Manson GV and Ma PC: Response to
pemetrexed chemotherapy in lung adenocarcinoma-bronchioloalveolar
carcinoma insensitive to erlotinib. Clin Lung Cancer. 11:57–60.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duruisseaux M, Baudrin L, Quoix E, Wislez
M, Moro-Sibilot D, Coëtmeur D, Monnet I, Mourlanette P, Morère JF,
Soria JC, et al: Chemotherapy effectiveness after first-line
gefitinib treatment for advanced lepidic predominant adenocarcinoma
(formerly advanced bronchioloalveolar carcinoma): Exploratory
analysis of the IFCT-0401 trial. J Thorac Oncol. 7:1423–1431. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roviello G, Francini E, Perrella A, Laera
L, Mazzei MA, Guerrini S and Petrioli R: An exceptional overall
survival using bevacizumab beyond progression in a patient with
non-small cell lung cancer. Cancer Biol Ther. 16:1720–1725. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arjaans M, Schröder CP, Oosting SF, Dafni
U, Kleibeuker JE and de Vries EG: VEGF pathway targeting agents,
vessel normalization and tumor drug uptake: From bench to bedside.
Oncotarget. 7:21247–21258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adjei AA, Mandrekar SJ, Dy GK, Molina JR,
Adjei AA, Gandara DR, Ziegler KL, Stella PJ, Rowland KM Jr, Schild
SE and Zinner RG: Phase II trial of pemetrexed plus bevacizumab for
second-line therapy of patients with advanced non-small cell lung
cancer: NCCTG and SWOG study N0426. J Clin Oncol. 28:614–619. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang GC, Yang TY, Wang NS, Huang CM and
Chiang CD: Successful treatment of multifocal bronchioloalveolar
cell carcinoma with ZD1839 (Iressa) in two patients. J Formos Med
Assoc. 102:407–411. 2003.PubMed/NCBI
|
|
24
|
Yano S, Kanematsu T, Miki T, Aono Y, Azuma
M, Yamamoto A, Uehara H and Sone S: A report of two
bronchioloalveolar carcinoma cases which were rapidly improved by
treatment with the epidermal growth factor receptor tyrosine kinase
inhibitor ZD1839 (‘Iressa’). Cancer Sci. 94:453–458. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bayle S, Descourt R, Gouva S, Daniel C and
Robinet G: Efficacy of gefitinib (Iressa) in the treatment of an
inoperable bronchioloalveolar cell carcinoma. Rev Mal Respir.
21:153–157. 2004.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kitazaki T, Fukuda M, Soda H and Kohno S:
Novel effects of gefitinib on mucin production in
bronchioloalveolar carcinoma; two case reports. Lung Cancer.
49:125–128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Milton DT, Kris MG, Gomez JE and Feinstein
MB: Prompt control of bronchorrhea in patients with
bronchioloalveolar caricinoma treated with gefitinib (Iressa).
Support Care Cancer. 13:70–72. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taja-Chayeb L, Candelaria M, Brom R,
Trejo-Becerril C, Meza F and Duenas-Gonzalez A: Response to
gefitinib in bronchioloalveolar carcinoma in the absence of EGFR
mutation. Lung Cancer. 50:259–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
West HL, Franklin WA, McCoy J, Gumerlock
PH, Vance R, Lau DH, Chansky K, Crowley JJ and Gandara DR:
Gefitinib therapy in advanced bronchioloalveolar carcinoma:
Southwest Oncology Group Study S0126. J Clin Oncol. 24:1807–1813.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kijima T, Suzuki M, Ueda K, Minami S,
Takeda Y, Goya S, Matsuoka H, Kumagai T, Yoshida M, Osaki T, et al:
Short-term gefitinib treatment brought about a long-term regression
of bronchioloalveolar carcinoma without EGFR gene alterations: A
case report. Oncol Res. 16:489–495. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cadranel J, Quoix E, Baudrin L,
Mourlanette P, Moro-Sibilot D, Morere JF, Souquet PJ, Soria JC,
Morin F and Milleron B: IFCT-0401 Trial Group: IFCT-0401 trail: A
phase II study of gefitinib administered as first-line treatment in
advanced adenocarcinoma with bronchioloalveolar carcinoma subtype.
J Thorac Oncol. 4:1126–1135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Popat N, Raghavan N and Mclvor RA: Severe
bronchorrhea in a patient with bronchioloalveolar carcinoma. Chest.
141:513–514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miller VA, Riely GJ, Zakowski MF, Li AR,
Patel JD, Heelan RT, Kris MG, Sandler AB, Carbone DP, Tsao A, et
al: Molecular characteristics of bronchioloalveolar carcinoma and
adenocarcinoma, bronchioloalveolar carcinoma subtype, predict
response to erlotinib. J Clin Oncol. 26:1472–1478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yuyama K, Matsushita H, Egawa K, Osawa T,
Tsubouchi Y, Takahashi T and Miura H: A case of bronchioloalveolar
carcinoma successfully treated with low-dose, alternate-day
administration of erlotinib. Gan To Kagaku Ryoho. 39:433–435.
2012.(In Japanese). PubMed/NCBI
|
|
35
|
Rubiales Sanz A, de la Cruz V, Berezo JÁ
and Torres MÁ: Erlotinib or gefitinib as first-choice therapy for
bronchorrhea in bronchioloalveolar carcinoma. J Pain Symptom
Manage. 47:e7–e9. 2014. View Article : Google Scholar
|
|
36
|
Garfield DH, Cadranel J and West HL:
Bronchioloalveolar carcinoma: The case for two diseases. Clin Lung
Cancer. 9:24–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cha YJ, Kim HR, Lee HJ, Cho BC and Shim
HS: Clinical course of stage IV invasive mucinous adenocarcinoma of
the lung. Lung Cancer. 102:82–88. 2016. View Article : Google Scholar : PubMed/NCBI
|