Introduction
Endometrial carcinoma (EMC) is a common malignancy
in developed countries and its mortality is approaching that of
cervical cancer. The age of patients with EMC ranges widely, from
young adults to elderly women, but the overall population is
currently aging. The majority of data in the literature indicate
that advanced age is a predictor of poor outcome in patients with
EMC (1–5). Focusing on elderly women (aged ≥70
years), EMC specific to this subpopulation may be of particular
interest. The number of EMC patients aged ≥70 years has gradually
increased in Japan as well, recently reaching ~20% of all EMC cases
(6).
The prognosis of patients with EMC is associated
with variable pathological factors, including histological type,
tumor grade (7), depth of myometrial
invasion and lymphovascular invasion (8), as well as clinicopathological stage as
determined by the International Federation of Gynecology and
Obstetrics (FIGO) classification (9), which is a critical determinant of the
prognosis of EMC, irrespective of its histological type. Tumor
grade is essentially prognostic for endometrioid carcinoma, but not
for type II EMC, as this type has the potential to behave
aggressively (10). Type II EMC,
which comprises serous carcinoma and clear cell carcinoma, accounts
for the higher proportion of cases among post-menopausal rather
than pre-menopausal women. Advancing age is associated with an
increasing tendency toward high-grade endometrioid carcinoma and a
likelihood of type II EMC, but the histological characteristics do
not appear to be able to fully explain the unfavorable prognosis of
elderly EMC patients. An additional factor may be that a relative
lack of immune competence may be more prevalent among elderly
patients (11).
Estrogen receptor (ER) is highly expressed in
endometrioid carcinoma, particularly G1/2, showing a strong
dependency on estrogenic growth. However, ER expression in type II
EMC, i.e., high-grade carcinoma, such as endometrioid carcinoma G3,
serous carcinoma, clear cell carcinoma and carcinosarcoma, is
negative or mild, if present (12).
The expression of p53 tends to be enhanced in type II EMC,
represented by serous carcinoma (13,14). The
immunohistochemical ‘ER (+) and p53 (−)’ staining pattern is an
indicator of endometrioid carcinoma G1/2, whereas the reverse
pattern favors type II EMC. Ki-67 is an excellent marker for
defining the proliferation status of tumor cells (15), but the prognostic value of Ki-67
expression in EMC also remains controversial (16–20).
In the present study, attention was focused on age
specificity (≥70 years) in early-stage EMC (FIGO I or II), and an
attempt was made to divide elderly EMC patients into subpopulations
based on outcome.
Patients and methods
Patient selection
The cutoff age for elderly women with EMC was
defined as ≥70 years. These elderly patients were selected from the
archives of all EMC patients (n=1,171) who had been treated at
Kanagawa Cancer Center (Yokohama, Japan) between 1985 and 2011.
Among patients aged ≥70 years, 79 were preoperatively staged as
FIGO I or II, and the clinicopathological factors were
postoperatively confirmed after total abdominal hysterectomy and
salpingoophorectomy, with or without regional lymphadenectomy.
Neither preoperative chemotherapy nor irradiation had been
performed. The study protocol was approved by the Institutional
Review Board of the Kanagawa Cancer Center, and informed consent
was obtained from all patients.
Endometrioid carcinoma G1/2 and mucinous carcinoma
were categorized as type I, whereas other types of carcinomas,
including serous carcinoma, clear cell carcinoma, undifferentiated
carcinoma and carcinosarcoma, were categorized as type II EMC.
Immunohistochemistry
Representative formalin-fixed and paraffin-embedded
tissue blocks were cut into 4-μm sections for the
immunohistochemical staining of ER, Ki-67 and p53. The sections
were deparaffinized, and endogenous peroxidase activity was
quenched with 0.3% hydrogen peroxide. Heat-induced antigen
retrieval was applied using an autoclave in citrate buffer (10 mM,
pH 6.0) at 121°C for 15 min. The sections were incubated with the
following primary antibodies: ER (rabbit monoclonal, clone SP1,
1:1, Ventana, Tucson, AZ, USA); Ki-67 (mouse monoclonal, clone
MIB-1, 1:50, Dako, Glostrup, Denmark), and p53 (mouse monoclonal,
clone DO-7, 1:50, Dako). After rinsing in phosphate-buffered saline
(10 mM/l, pH 7.2), the sections were incubated with the Envision
Kit (Dako) for 30 min at room temperature. The reaction products
were visualized with diaminobenzidine tetrahydrochloride.
The immunoexpression of ER, Ki-67 and p53 was
semi-quantitatively evaluated as low (−) or high (+) as follows: ER
(−) <30% vs. (+) ≥30%; Ki-67 (−) <30% vs. (+) ≥30%; and p53
(−) <80% vs. (+) ≥80%.
Statistical analysis
The Fisher's exact test was used to analyse the
association between outcome and pathological factors, namely
histological type, myometrial invasion depth and vascular invasion.
The outcome was determined using disease-free survival (DFS) and
overall survival (OS) rates. DFS was defined as the time from the
date of surgery to the date of first recurrence, which was detected
by imaging examinations. OS was calculated from the date of surgery
to the date of death. OS and DFS were estimated using the
Kaplan-Meier method, and the differences between survival rates
were compared by the log-rank test. Data from survivors were
censored at the last follow-up. Multivariate analyses applying the
Cox proportional hazards regression model were used to assess an
independency as a prognostic factor. Differences with P<0.05
were considered as statistically significant. All statistical
analyses were performed using SPSS ver. 20 (IBM Corp., Armonk, NY,
USA).
Results
Pathological presentations
According to the histological diagnosis of EMC, the
79 patients were grouped into 54 cases with type I EMC (52
endometrioid carcinomas G1/2 and 2 mucinous carcinomas) and 25
cases with type II EMC (11 endometrioid carcinomas G3, 8 serous
carcinomas, 3 clear cell carcinomas, 2 carcinosarcomas and 1
undifferentiated carcinoma). If an EMC was mixed (type I and type
II), it was categorized as type II EMC (Table I). In the univariate log-rank
analysis, the histological type tended to adversely affect the
outcome, but a critical correlation between the two was not
statistically confirmed (Table II).
Neither myometrial invasion depth nor lymphovascular invasion were
apparently correlated with the outcome (Table II).
 | Table I.Histological and immunohistochemical
profile of patients with endometrial carcinoma aged ≥70 years. |
Table I.
Histological and immunohistochemical
profile of patients with endometrial carcinoma aged ≥70 years.
|
|
| ER | Ki-67 | p53 |
|---|
|
|
|
|
|
|
|---|
| Type of cancer | n=79 | (+) | (−) | (+) | (−) | (+) | (−) |
|---|
| Endometrioid Ca | 63 | 50 | 13 | 16 | 47 | 16 | 47 |
| G1 | 31 | 26 | 5 | 5 | 26 | 5 | 26 |
| G2 | 21 | 18 | 3 | 7 | 14 | 8 | 13 |
| G3 | 11 | 6 | 5 | 4 | 7 | 3 | 8 |
| Mucinous Ca | 2 | 2 | 0 | 2 | 0 | 1 | 1 |
| Serous Ca | 8 | 5 | 3 | 5 | 3 | 8 | 0 |
| Clear cell Ca | 3 | 0 | 3 | 1 | 2 | 2 | 1 |
| Undifferentiated
Ca | 1 | 0 | 1 | 0 | 1 | 1 | 0 |
| Carcinosarcoma | 2 | 0 | 2 | 0 | 2 | 1 | 1 |
 | Table II.Univariate and multivariate
analyses. |
Table II.
Univariate and multivariate
analyses.
| Variables | HR (95% CI) | P-value |
|---|
| Univariate
analysis |
|
Histological type | 4.836
(0.885-26.43) | 0.069 |
| Depth
of myometrial invasion | 2.000
(0.365-10.95) | 0.424 |
|
Lymphovascular invasion | 0.794
(0.145-4.346) | 0.791 |
| ER | 0.162
(0.300-0.885) | 0.036 |
|
p53 | 10.21
(1.191-87.46) | 0.034 |
|
Ki-67 | 3.688
(0.940-19.61) | 0.126 |
| Multivariate
analysis |
|
|
| ER | 0.066
(0.006-0.774) | 0.030 |
|
p53 | 14.55
(1.280-165.3) | 0.031 |
Immunohistochemical analysis
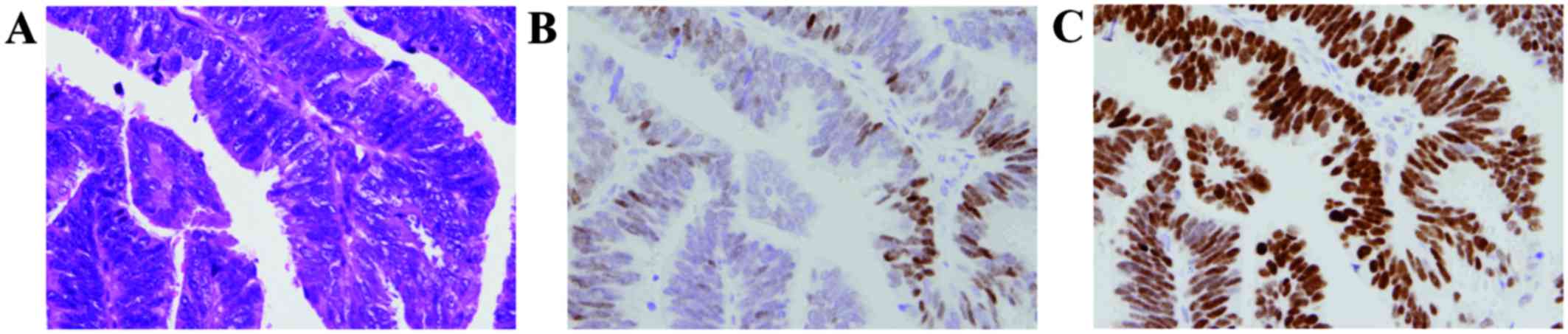
According to the semi-quantitative evaluation of
immunohistochemical staining (Fig. 1
and Table I), 57 cases had ER (+)
vs. 22 cases with ER (−); 24 cases had Ki-67 (+) vs. 55 cases with
Ki-67 (−); 29 cases had p53 (+) vs. 50 cases with p53 (−). The
percentage of cases with ER (+), Ki-67 (+) and p53 (+) for type I
EMC was 85.2, 25.9 and 25.9 %, respectively, and for type II, 44,
40 and 60%, respectively. The univariate log-rank analysis was
followed by multivariate Cox regression analysis, in which ER
(P=0.03) and p53 (P=0.03) expression were demonstrated to be
independent prognosticators for shorter survival.
Kaplan-Meier method
The follow-up period for survivors ranged from 0 to
120 months, with a median of 87 months. At the last follow-up,
92.4% of the patients were still alive, 10.1% experienced
recurrence, and 7.6% had succumbed to their disease. Kaplan-Meier
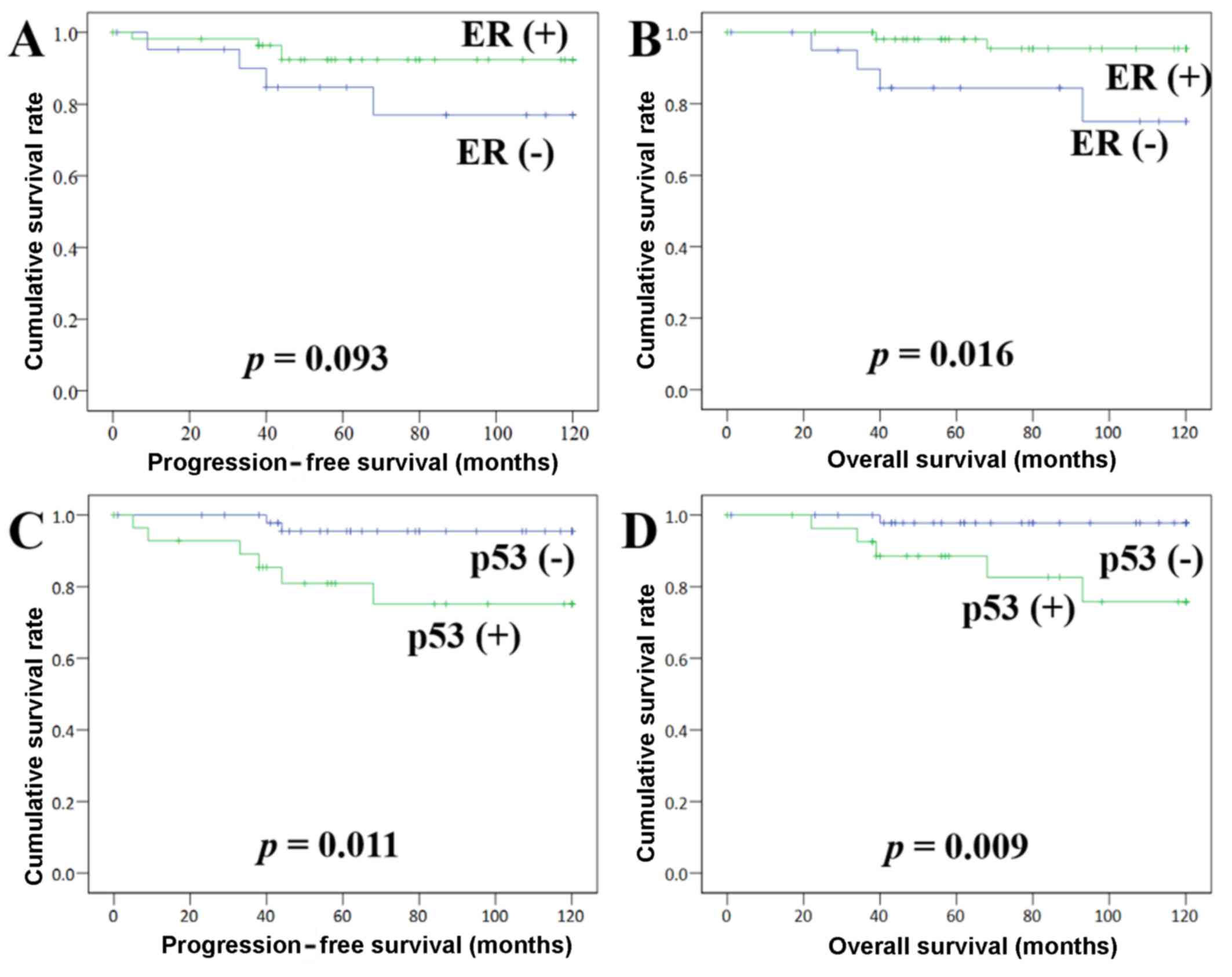
survival curves demonstrated that ER is associated with DFS: (+)
vs. (−) = 113.4 vs. 101.9 months, respectively (P=0.09), and that
ER (−) is significantly associated with shorter OS: (+) vs. (−) =
117.1 vs. 103.8 months, respectively (P=0.01) (Fig. 2). p53 (+) was found to be closely
associated with shorter DFS: (+) vs. (−) = 99.2 vs. 116.5 months,
respectively (P=0.01), as well as a shorter OS: (+) vs. (−) = 105.0
vs. 118.3 months, respectively (P=0.01) (Fig. 2). Ki-67 (+) was found to be
associated with shorter DFS: (+) vs. (−) = 102.8 vs. 113.6 months,
respectively (P=0.09), but not with OS: (+) vs. (−) = 102.8 vs.
115.8 months, respectively (P=0.10).
Discussion
It was reported that poor outcome is correlated with
lymphovascular invasion (8),
aggressive histology and cervical invasion in early (stage I or II)
EMC in the multivariate analysis (1). In addition, elderly EMC patients (≥70
years) appear to have worse outcomes compared with younger
patients, regardless of other poor prognostic factors, and EMC also
appears to be intrinsically more aggressive in elderly patients.
The cutoff age of 70 years is arbitrary, but it has been used
extensively in the medical literature when comparing elderly and
younger patients (1). Since the
greater majority of patients with EMC are in the seventh decade of
life or older and have acquired comorbidities, it is a reasonable
hypothesis that overall survival will be age-dependent (1,21–25).
However, the significance of age as a prognostic factor remains
controversial when risk-adjusted for the higher prevalence of
adverse prognostic factors in elderly EMC patients (21).
Among EMCs arising in elderly women, type II EMC has
a higher incidence compared with that in younger women (25). In our experience, type II EMC,
including mixed carcinoma, accounts for ~40% of the cases. Serous
carcinoma with an aggressive behavior (10,26),
representing type II EMC, is often found to have an extrauterine
spread (stage IV), irrespective of the absence of myometrial
invasion or lymphovascular invasion (7,27–29). In
the present study, the examined EMC patients aged ≥70 years were
confined to stage I or II, and advanced cases with extrauterine
spread were not included. Our preliminary study with stage I or II
EMC, arising in patients in their fifth (368 cases) and sixth (251
cases) decades of life, indicated that histological type and
lymphovascular invasion are associated with outcome (data not
shown). Subsequently, an association of myometrial invasion depth
with outcome was noted in patients in the fifth decade, but not in
those in the sixth decade of life. In patients aged ≥70 years, no
statistically significant correlations were demonstrated for these
factors (Table II).
The FIGO grading system of endometrioid carcinoma
relies first and foremost on the glandular architecture. The
architecture usually corresponds well to nuclear grade, but nuclear
grade is often a more reliable indicator of prognosis (30,31). The
majority of endometrioid carcinomas G1/2 react with ER on
immunohistochemical staining, and staining for ER and progesterone
receptor (PgR) may prove to be of some value in determining which
patients may be suitable for hormonal therapy (32). p53 mutations are found in 5-10% of
all endometrioid carcinomas, but occur in 50% of endometrioid
carcinomas G3, and even more frequently (90%) in serous carcinoma
(12–14,33–35). On
the contrary, ER and PgR expression is absent or only weak in
serous carcinomas (28).
As shown in the Kaplan Meier curves, the ‘ER (−) and
p53 (+)’ pattern is considered a promising prognostic indicator for
predicting an unfavorable clinical outcome in elderly EMC patients
(Fig. 2). However, this pattern is
usually an indicator of serous carcinoma with the risk of an
aggressive clinical course. Some of endometrioid carcinomas G1
exhibit high-grade nuclear atypia without solid growth (identical
to grade 1 architecture), which is the basis for raising the grade
from 1 to 2 (Fig. 1), particularly
in elderly patients. Therefore, it is considered that these cases
should be treated as high-grade/type II EMC rather than
endometrioid carcinoma G2 (low-grade/type I EMC).
With the aid of immunohistochemical staining
(36–38), prognostication of EMC may be achieved
to a greater extent. In order to predict EMC with aggressive
potential, which appears to increase in association with patient
age, the ‘ER (−) and p53 (+)’ pattern may become an easily
applicable indicator. Elderly patients with EMC must be closely
followed up, as these tumors occasionally exhibit an intrinsic
aggressiveness, regardless of low-grade/type I appearance of
endometrioid carcinoma.
Acknowledgements
This article is dedicated to the late Dr Hiroki
Nakayama (Head of the Department of Gynecology, Kanagawa Cancer
Center, Yokohama, Japan) for his authentic and conscientious
instructions throughout the course of this study.
Funding
No funding was received.
Availability of data and materials
All datasets generated in this study are available
from the corresponding author upon reasonable request.
Authors' contributions
All the authors have read and approved the final
version of this manuscript. NO contributed to the conception,
design, acquisition, analysis and interpretation of data, and
drafting of the manuscript. MY and TK took part in acquisition and
analysis of data. SK critically revised the manuscript for
important intellectual content. HK, YK, SH, and MY contributed to
the conception and design of the manuscript for important
intellectual content.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board of the Kanagawa Cancer Center, and informed consent
was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests to disclose.
References
|
1
|
Alektiar KM, Venkatraman E, Abu-Rustum N
and Barakat RR: Is endometrial carcinoma intrinsically more
aggressive in elderly patients? Cancer. 98:2368–2377. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kosary CL: FIGO stage, histology,
histologic grade, age and race as prognostic factors in determining
survival for cancers of the female gynecological system: An
analysis of 1973-87 SEER cases of cancers of the endometrium,
cervix, ovary, vulva, and vagina. Semin Surg Oncol. 10:31–46. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zaino RJ, Kurman RJ, Diana KL and Morrow
CP: Pathologic models to predict outcome for women with endometrial
adenocarcinoma: The importance of the distinction between surgical
stage and clinical stage-a Gynecologic Oncology Group study.
Cancer. 77:1115–1121. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abeler VM and Kjørstad KE: Endometrial
adenocarcinoma in Norway. A study of a total population. Cancer.
67:3093–3103. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Irwin C, Levin W, Fyles A, Pintilie M,
Manchul L and Kirkbride P: The role of adjuvant radiotherapy in
carcinoma of the endometrium-results in 550 patients with
pathologic stage I disease. Gynecol Oncol. 70:247–254. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saito T, Takahashi F and Katabuchi H: 2016
Committee on Gynecologic Oncology of the Japan Society of
Obstetrics and Gynecology: Annual Report of the Committee on
Gynecologic Oncology, Japan Society of Obstetrics and Gynecology:
Patient Annual Report for 2014 and Treatment Annual Report for
2009. J Obstet Gynaecol Res. 43:1667–1677. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carcangiu ML and Chambers JT: Early
pathologic stage clear cell carcinoma and uterine papillary serous
carcinoma of the endometrium: comparison of clinicopathologic
features and survival. Int J Gynecol Pathol. 14:30–38. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakaki M, Yasuda M, Yano M, Goto Y,
Nakamura M, Kayano H and Hasegawa K: The prognostic significance of
tumor lymphangiogenesis and lymphatic vessel density in
endometrioid carcinoma of the uterine corpus. Oncol Lett.
14:5313–5318. 2017.PubMed/NCBI
|
|
9
|
Ogane N, Yasuda M, Kato H, Kato T, Yano M,
Kameda Y and Kamoshida S: Cleaved caspase-3 expression is a
potential prognostic factor for endometrial cancer with positive
peritoneal cytology. Cytopathology. 29:254–261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carcangiu ML and Chambers JT: Uterine
papillary serous carcinoma: A study on 108 cases with emphasis on
the prognostic significance of associated endometrioid carcinoma,
absence of invasion, and concomitant ovarian carcinoma. Gynecol
Oncol. 47:298–305. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mutter GL and Prat J: Endometrial
adenocarcinoma. Pathology of the Female Reproductive Tract. 3rd
edition. Churchill Livingstone Elsevier; Boston, MA: pp.
3972014
|
|
12
|
Kounelis S, Kapranos N, Kouri E, Coppola
D, Papadaki H and Jones MW: Immunohistochemical profile of
endometrial adenocarcinoma: a study of 61 cases and review of the
literature. Mod Pathol. 13:379–388. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sherman ME, Bur ME and Kurman RJ: p53 in
endometrial cancer and its putative precursors: Evidence for
diverse pathways of tumorigenesis. Hum Pathol. 26:1268–1274. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lax SF, Kendall B, Tashiro H, Slebos RJ
and Hedrick L: The frequency of p53, K-ras mutations, and
microsatellite instability differs in uterine endometrioid and
serous carcinoma: Evidence of distinct molecular genetic pathways.
Cancer. 88:814–824. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kallakury BV, Ambros RA, Hayner-Buchan AM,
Sheehan CE, Malfetano JH and Ross JS: Cell proliferation-associated
proteins in endometrial carcinomas, including papillary serous and
endometrioid subtypes. Int J Gynecol Pathol. 17:320–326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salvesen HB, Iversen OE and Akslen LA:
Identification of high-risk patients by assessment of nuclear Ki-67
expression in a prospective study of endometrial carcinomas. Clin
Cancer Res. 4:2779–2785. 1998.PubMed/NCBI
|
|
18
|
Geisler JP, Geisler HE, Miller GA, Wiemann
MC, Zhou Z and Crabtree W: MIB-1 in endometrial carcinoma:
Prognostic significance with 5-year follow-up. Gynecol Oncol.
75:432–436. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Semczuk A, Skomra D, Cybulski M and
Jakowicki JA: Immunohistochemical analysis of MIB-1 proliferative
activity in human endometrial cancer. Correlation with
clinicopathological parameters, patient outcome, retinoblastoma
immunoreactivity and K-ras codon 12 point mutations. Histochem J.
33:193–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lundgren C, Auer G, Frankendal B, Moberger
B, Nilsson B and Nordstrom B: Nuclear DNA content, proliferative
activity, and p53 expression related to clinical and
histopathologic features in endometrial carcinoma. Int J Gynecol
Pathol. 12:110–118. 2002. View Article : Google Scholar
|
|
21
|
AlHilli MM, Bakkum-Gamez JN, Mariani A,
Weaver AL, McGree ME, Keeney GL, Jatoi A, Dowdy SC and Podratz KC:
Risk-adjusted outcomes in elderly endometrial cancer patients:
Implications of the contrasting impact of age on progression-free
and cause-specific survival. Gynecol Oncol. 138:133–140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fleming ND, Lentz SE, Cass I, Li AJ,
Karlan BY and Walsh CS: Is older age a poor prognostic factor in
stage I and II endometrioid endometrial adenocarcinoma? Gynecol
Oncol. 120:189–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Farley JH, Nycum LR, Birrer MJ, Park RC
and Taylor RR: Age-specific survival of women with endometrioid
adenocarcinoma of the uterus. Gynecol Oncol. 79:86–89. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jolly S, Vargas CE, Kumar T, Weiner SA,
Brabbins DS, Chen PY, Floyd W and Martinez AA: The impact of age on
long-term outcome in patients with endometrial cancer treated with
postoperative radiation. Gynecol Oncol. 103:87–93. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vance S, Yechieli R, Cogan C, Hanna R,
Munkarah A and Elshaikh MA: The prognostic significance of age in
surgically staged patients with Type II endometrial carcinoma.
Gynecol Oncol. 126:16–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Connelly PJ, Alberhasky RC and
Christopherson WM: Carcinoma of the endometrium. III. Analysis of
865 cases of adenocarcinoma and adenoacanthoma. Obstet Gynecol.
59:569–575. 1982.PubMed/NCBI
|
|
27
|
Goff BA, Kato D, Schmidt RA, Ek M, Ferry
JA, Muntz HG, Cain JM, Tamimi HK, Figge DC and Greer BE: Uterine
papillary serous carcinoma: Patterns of metastatic spread. Gynecol
Oncol. 54:264–268. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lax SF, Pizer ES, Ronnett BM and Kurman
RJ: Clear cell carcinoma of the endometrium is characterized by a
distinctive profile of p53, Ki-67, estrogen, and progesterone
receptor expression. Hum Pathol. 29:551–558. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jia L, Yuan Z, Wang Y, Cragun JM, Kong B
and Zheng W: Primary sources of pelvic serous cancer in patients
with endometrial intraepithelial carcinoma. Modern pathology : An
official journal of the United States and Canadian Academy of
Pathology. Inc. 28:118–127. 2015.
|
|
30
|
Creasman W: Revised FIGO staging for
carcinoma of the endometrium. Int J Gynaecol Obstet. 105:1092009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zaino RJ, Kurman RJ, Diana KL and Morrow
CP: The utility of the revised International Federation of
Gynecology and Obstetrics histologic grading of endometrial
adenocarcinoma using a defined nuclear grading system. A
Gynecologic Oncology Group study. Cancer. 75:81–86. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fukuda K, Mori M, Uchiyama M, Iwai K,
Iwasaka T and Sugimori H: Prognostic significance of progesterone
receptor immunohistochemistry in endometrial carcinoma. Gynecol
Oncol. 69:220–225. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matias-Guiu X, Catasus L, Bussaglia E,
Lagarda H, Garcia A, Pons C, Muñoz J, Argüelles R, Machin P and
Prat J: Molecular pathology of endometrial hyperplasia and
carcinoma. Hum Pathol. 32:569–577. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Berchuck A and Boyd J: Molecular basis of
endometrial cancer. Cancer. 76 Suppl:2034–2040. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
An HJ, Logani S, Isacson C and Ellenson
LH: Molecular characterization of uterine clear cell carcinoma.
Modern pathology : An official journal of the United States and
Canadian Academy of Pathology. Inc. 17:530–537. 2004.
|
|
36
|
Huvila J, Laajala TD, Edqvist PH,
Mardinoglu A, Talve L, Pontén F, Grénman S, Carpén O, Aittokallio T
and Auranen A: Combined ASRGL1 and p53 immunohistochemistry as an
independent predictor of survival in endometrioid endometrial
carcinoma. Gynecol Oncol. 149:173–180. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Obata T, Nakamura M, Mizumoto Y, Iizuka T,
Ono M, Terakawa J, Daikoku T and Fujiwara H: Dual expression of
immunoreactive estrogen receptor β and p53 is a potential predictor
of regional lymph node metastasis and postoperative recurrence in
endometrial endometrioid carcinoma. PLoS One. 12:e01886412017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Geels YP, van der Putten LJ, van Tilborg
AA, Lurkin I, Zwarthoff EC, Pijnenborg JM, van den Berg-van Erp SH,
Snijders MP, Bulten J, Visscher DW, et al: Immunohistochemical and
genetic profiles of endometrioid endometrial carcinoma arising from
atrophic endometrium. Gynecol Oncol. 137:245–251. 2015. View Article : Google Scholar : PubMed/NCBI
|