Introduction
The treatment of rheumatoid arthritis (RA) has
evolved in recent years. Methotrexate (MTX), with or without
biologics, is currently the first-line therapy for RA, and is
widely administered as an anchor drug (1). The incidence of lymphoproliferative
disorder (LPD) is 2.0-5.5 times higher in patients with RA compared
with that in the general population, and MTX has been described as
a major cause of LPD (2). The World
Health Organization classification of lymphoid neoplasms describes
MTX-related LPD as an ‘iatrogenic immunodeficiency-associated LPD’,
which is similar to immunodeficiency-associated LPD, including
post-transplant LPD and human immunodeficiency-associated LPD
(3). Approximately 40-50% of
MTX-related LPD (MTX-LPD) cases occur in extranodal sites, with the
gastrointestinal tract, skin, liver, lung and kidney reported as
the most susceptible (4,5). The onset of MTX-LPD is shorter compared
with that of lymphomas that develop in general RA patients
(6). The causative role of MTX in
MTX-LPD pathophysiology is supported by the observation that
discontinuing treatment with MTX results in resolution of LPD
(7). The onset of MTX-LPD is
considered to be dose-independent, as is the case with MTX-related
interstitial pneumonia (8). Previous
studies to date have demonstrated that the mean duration and mean
total dose of MTX were 38-54 months and 940-984 mg, respectively
(6,7). We herein describe a case of MTX-LPD
with the longest duration of MTX treatment identified in the
literature to date, and the highest total dose of MTX in a patient
with RA.
Case report
A 67-year-old woman presented to the Kindai
University Hospital (Osaka, Japan) in May 2012 with complaints of
fever and neck swelling. The patient had a history of RA, for which
she had been receiving oral methotrexate (MTX) 8 mg/week,
prednisolone 2.5 mg/day and bucillamine (BUC) 100 mg/day. The
patient had been taking MTX for 20 years, and her cumulative dose
at the time of presentation to our hospital was 7,636 mg.
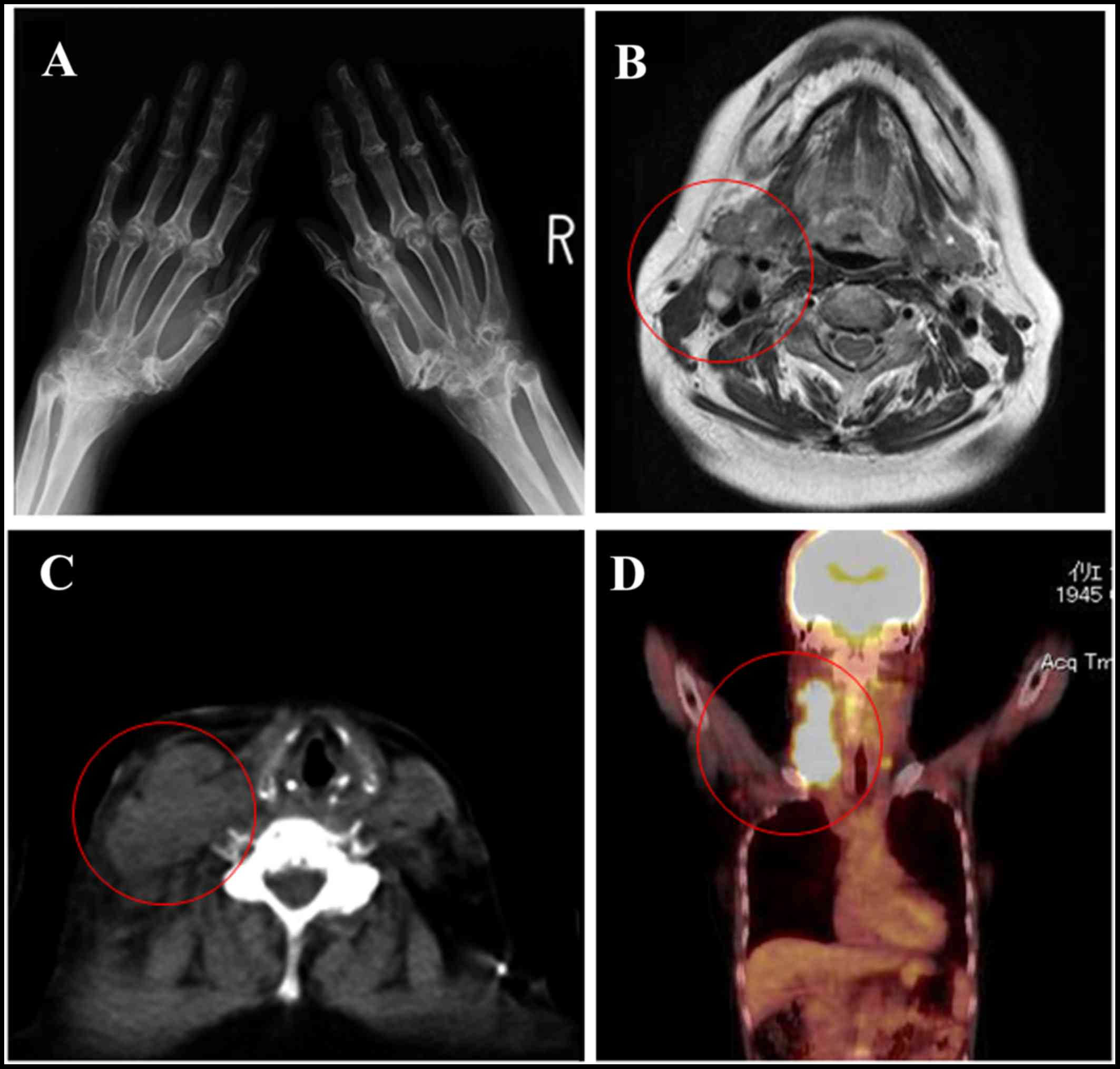
Radiography revealed destruction of cartilage and bone, and joint
hyperextension (Fig. 1A). The
patient was diagnosed with stage III disease according to the
Steinbrocker classification (9).
There was no evidence of lung involvement on radiographic
examination (data not shown). Clinical examination of the neck
revealed several non-tender, elastic, fluctuant, solid masses,
measuring ~1-3 cm in greatest diameter. The masses were
well-adhered to the overlying skin. Blood test results revealed an
inflammatory response, high soluble IL-2 receptor (sIL-2R) levels,
and Ebstein-Barr virus (EBV) antibody positivity (Table I). Computed tomography revealed an
isointense mass in the right side of the neck (Fig. 1B), which was also noted on
T1-weighted magnetic resonance imaging (MRI) (Fig. 1C). T2-weighted MRI revealed a
high-intensity mass (data not shown). Positron emission tomography
with 2-deoxy-2-(fluorine-18)-fluoro-D-glucose integrated with
computed tomography (18F-FDG PET/CT) demonstrated FDG
accumulation in the right neck mass (Fig. 1D). Based on these findings, needle
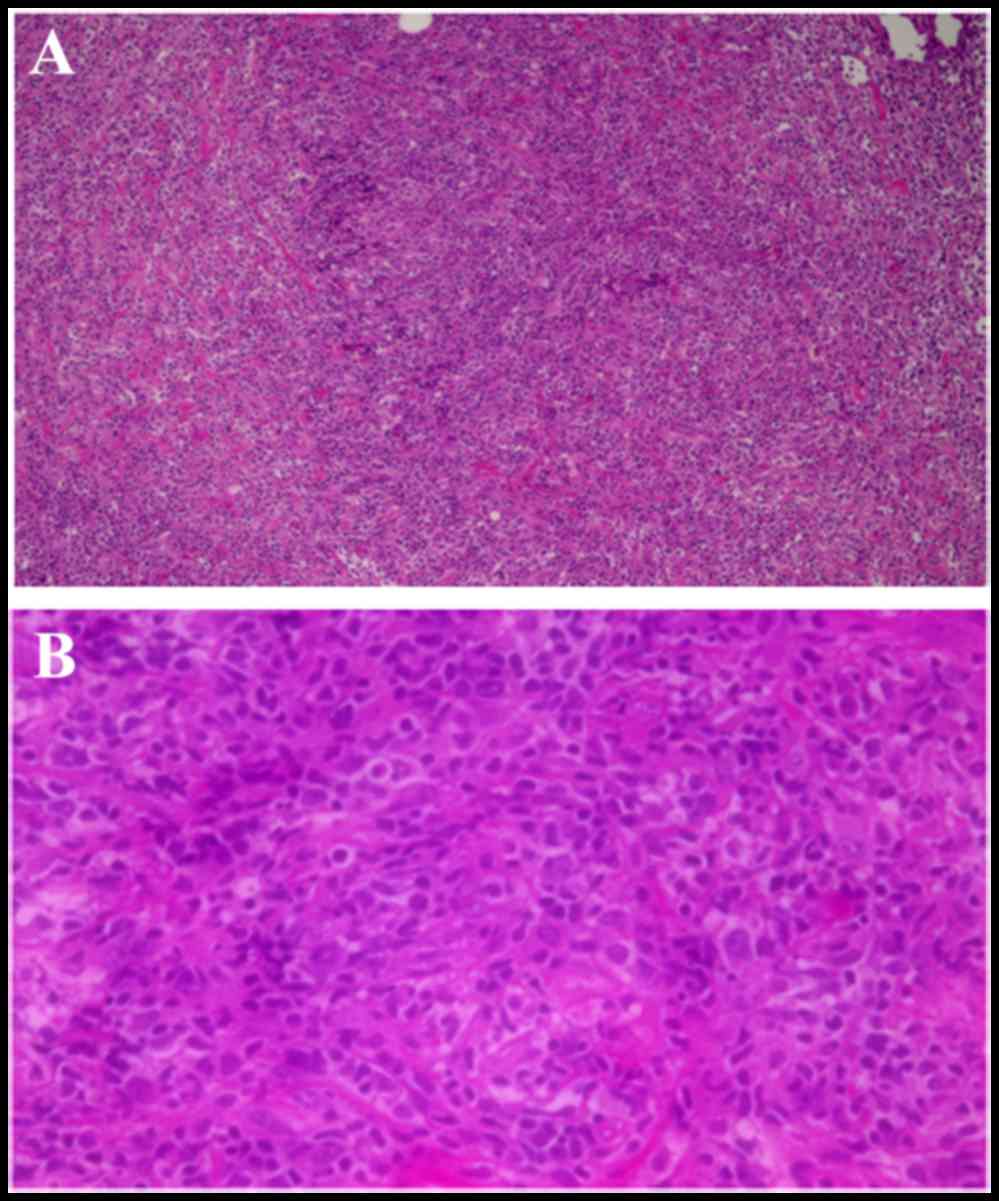
biopsy of the mass was performed. Histological examination of the
bioptic material revealed increased proliferation of atypical
lymphocytes with nuclear enlargement, and lymph node structure with
ambiguous histology on hematoxylin and eosin staining (Fig. 2A and B). Following discontinuation of
oral MTX and BUC treatment, the lymphadenopathy temporarily
disappeared. However, the right cervical lymphadenopathy became
more prominent ~1 month later. Repeat laboratory investigations at
that time revealed elevated levels of sIL-2R and lactate
dehydrogenase, which was consistent with disease relapse.
Therefore, the patient was initiated on high-dose steroid therapy.
Complete remission was observed at 4 years after treatment, and
there was no recurrence at 6 months after the end of treatment.
Treatment for RA was resumed, and good control was obtained with
prednisolone 2.5 mg/day and salazosulfapyridine 1,000 mg/day. The
patient has subsequently been followed up every 5 years.
 | Table I.Blood test results. |
Table I.
Blood test results.
| Tests (units) | Values |
|---|
| C-reactive protein
(mg/dl) | 1.41 |
| Aspartate
transaminase (U/l) | 36 |
| Alanine transaminase
(U/l) | 25 |
| White blood cells
(×103 µl) | 7.3 |
| Hemoglobin
(g/dl) | 13.7 |
| Platelets
(×104 µl) | 18.8 |
| Blood urea nitrogen
(mg/dl) | 16 |
| Creatinine
(mg/dl) | 0.64 |
| IgG | 1,003 |
| sIL-2R (U/ml) | 2,067 |
| β2-MG (mg/dl) | 3.2 |
| EBV VCA IgG | 7.9 (+) |
| Squamous cell
carcinoma tumor marker | (−) |
Discussion
MTX-LPD is a serious complication in patients
treated with MTX. The incidence of MTX-LPD has increased, due in
part to the increased use of MTX as an anchor drug in the
management of RA. Given the important implications, there is a need
to further elucidate the incidence, demographic characteristics and
risk factors for this condition. To this end, we herein describe a
case of MTX-LPD in an elderly RA patient on long-term oral
treatment with MTX.
Although the mechanism of onset is unknown, the
combination of immunodeficiency as a result of RA and the
immunosuppressive effect of MTX has been implicated in the
pathogenesis of MTX-LPD (5,6). In RA, self-reactive T cells of a
specific clone stimulate B cells that produce autoantibodies, such
as rheumatoid factor (10). When MTX
is administered, its immunosuppressive effect reactivates viral
infections, including subclinical infections, and it is
hypothesized that this triggers clonal proliferation of cells
(11). In particular, EBV is
considered to be an important viral infection associated with LPD
that develops in the context of rheumatological diseases (8). Activation of EBV is identified in the
majority of MTX-LPD cases (8),
including the present case, which was characterized by the presence
of positive EBV antibody serology.
The clinical characteristics of MTX-LPD include
superficial and deep lymphadenopathy, as well as systemic symptoms,
such as fever and weight loss (5,6). The
frequency of extralymphatic involvement is relatively high, with
lesions most commonly affecting the skin, soft tissues and lung
(5,6,11). In
the present case, no lung lesions were observed.
Although the histological characteristics of MTX-LPD
are diverse, the most common finding is diffuse large B-cell
lymphoma (DLBCL), which accounts for ~50% of all cases, followed by
Hodgkin's lymphoma (HL) in ~20% of the cases (5,12). The
patient in the present case was diagnosed with DLBCL. A
characteristic feature of MTX-LPD pathology is the presence of B, T
and natural killer cells, as well as other cell subtypes derived
from lymphocytes (12). Complicated
phenotypes, including pathological findings of DLCBCL mixed with
lymphoma-like granulomatosis and peripheral T-cell lymphoma,
non-specific type, have also been reported (12).
A previous report suggested that other
immunosuppressants also contribute to the onset of MTX-LPD
(13). However, no study to date has
implicated BUC. The patient described herein was receiving both MTX
and BUC; therefore, the possible involvement of BUC in the
development of MTX-LPD cannot be excluded.
The duration and dose of MTX treatment has varied
across studies. A previous report of an RA patient receiving MTX
therapy who developed MTX-LPD described a median duration of 38
months and total dose of 984 mg (7).
Another previous study reported a median duration and total dose of
54 months and 940 mg, respectively (6). To the best of our knowledge, the
patient described in the present case had the most delayed onset of
MTX-LPD following initiation of MTX treatment, and the highest
cumulative dose of MTX prior to disease onset.
Spontaneous resolution of LPD after withdrawal of
MTX treatment is observed in ~50% of all affected patients
(12,14,15).
However, cases of patients with LPD progression after the
discontinuation of MTX have also been reported (12,15). The
progression or resolution of LPD is not only affected by MTX
treatment per se, but also by the immune status of the host, which,
as previously described, is affected by RA pathogenesis. However,
the majority of reports recommend interrupting MTX treatment upon
development of MTX-LPD (12,14–16). In
a previous study, 23 patients (76.7%) achieved regression of LPD by
MTX withdrawal (16). In another
study, the majority of DLBCL-type MTX-LPD patients (81%) achieved
remission with MTX discontinuation alone (17). By contrast, the majority of patients
with classical HL-type MTX-LPD (76%) required additional
chemotherapy (17). Corticosteroids
are used in combination with cyclophosphamide or chemotherapy for
the treatment of malignant lymphoma, whereas a previous study
reported the use of rituximab (18).
In keeping with our results, another study also demonstrated that
corticosteroid pulse therapy was effective (12). EBV has been described as an important
prognostic factor. A previous study reported that a significantly
higher positive rate of peripheral blood EBV DNA was observed in
MTX-LPD patients belonging to the chemotherapy-free group compared
with patients requiring chemotherapy (9/9 vs. 0/3, respectively;
P=0.0002), suggesting that EBV DNA positivity in the peripheral
blood is a marker of better outcome in these patients (16). This finding is also consistent with
our experience, as the patient in the present case was EBV-positive
and developed no recurrence.
In conclusion, we herein reported a case of LPD in
an elderly female RA patient with a history of long-term oral MTX
administration. In order to facilitate early diagnosis and
intervention, clinicians must be aware of the risk of MTX-LPD in
such patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
Data was acquired and analyzed by KH and MA. KH and
MA prepared the manuscript.
Ethics approval and consent to
participate
The patient gave their consent to participate in
this study.
Patient consent for publication
Consent for publication of the case details and
associated images was obtained from the patient.
Competing interests
All the authors declare that they have no competing
interests to disclose.
References
|
1
|
Smolen JS, Landewé R, Bijlsma J, Burmester
G, Chatzidionysiou K, Dougados M, Nam J, Ramiro S, Voshaar M, van
Vollenhoven R, et al: EULAR recommendations for the management of
rheumatoid arthritis with synthetic and biological
disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis.
76:960–977. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baecklund E, Iliadou A, Askling J, Ekbom
A, Backlin C, Granath F, Catrina AI, Rosenquist R, Feltelius N,
Sundström C, et al: Association of chronic inflammation, not its
treatment, with increased lymphoma risk in rheumatoid arthritis.
Arthritis Rheum. 54:692–701. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ellman MH, Hurwitz H, Thomas C and Kozloff
M: Lymphoma developing in a patient with rheumatoid arthritis
taking low dose weekly methotrexate. J Rheumatol. 18:1741–1743.
1991.PubMed/NCBI
|
|
4
|
Ishiguro K, Hayashi T, Aoki Y, Murakami R,
Ikeda H and Ishida T: Other iatrogenic immunodeficiency-associated
lymphoproliferative disorder presenting as primary bone lymphoma in
a patient with rheumatoid arthritis. Intern Med. 55:2259–2264.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kameda T, Dobashi H, Miyatake N, Inoo M,
Onishi I, Kurata N, Mitsunaka H, Kawakami K, Fukumoto T, Susaki K,
et al: Association of higher methotrexate dose with
lymphoproliferative disease onset in rheumatoid arthritis patients.
Arthritis Care Res (Hoboken). 66:1302–1309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoshida Y, Xu JX, Fujita S, Nakamichi I,
Ikeda J, Tomita Y, Nakatsuka S, Tamaru J, Iizuka A, Takeuchi T, et
al: Lymphoproliferative disorders in rheumatoid arthritis:
Clinicopathological analysis of 76 cases in relation to
methotrexate medication. J Rheumatol. 34:322–331. 2007.PubMed/NCBI
|
|
7
|
Miyazaki T, Fujimaki K, Shirasugi Y,
Yoshiba F, Ohsaka M, Miyazaki K, Yamazaki E, Sakai R, Tamaru J,
Kishi K, et al: Remission of lymphoma after withdrawal of
methotrexate in rheumatoid arthritis: Relationship with type of
latent Epstein-Barr virus infection. Am J Hematol. 82:1106–1109.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mariette X, Cazals-Hatem D, Warszawki J,
Liote F, Balandraud N and Sibilia J; Investigators of the Club
Rhumatismes et Inflammation, : Lymphomas in rheumatoid arthritis
patients treated with methotrexate: A 3-year prospective study in
France. Blood. 99:3909–3915. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuda K, Gotoh M, Mitsui Y, Yoshikawa E,
Kume S, Yano M, Honda S, Okawa T, Fukuda T, Higuchi F, et al:
Steinbrocker classification class IV to class II after multi-joint
surgery. Kurume Med J. 59(7): 9–82. 2012.10.
|
|
10
|
Lu DR, McDavid AN, Kongpachith S,
Lingampalli N, Glanville J, Ju CH, Gottardo R and Robinson WH: T
cell-dependent affinity maturation and innate immune pathways
differentially drive autoreactive B cell responses in rheumatoid
arthritis. Arthritis Rheumatol. May 31–2018.(Epub ahead of print).
View Article : Google Scholar
|
|
11
|
Mokuda S, Miyazaki T, Saeki Y, Masumoto J,
Kanno M and Takasugi K: Epstein-Barr virus-related MTX-LPD in
rheumatoid arthritis patients exhibits a viral pattern of the CD64
and CD35 expression on neutrophils: Three case reports. Mod
Rheumatol. 25:166–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tokuhira M, Watanabe R, Nemoto T, Sagawa
M, Tomikawa T, Tamaru J, Itoyama S, Nagasawa H, Amano K, Kameda H,
et al: Clinicopathological analyses in patients with other
iatrogenic immunodeficiency-associated lymphoproliferative diseases
and rheumatoid arthritis. Leuk Lymphoma. 53:616–623. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hashimoto A, Chiba N, Tsuno H, Komiya A,
Furukawa H, Matsui T, Nishino J and Tohma S: Incidence of
malignancy and the risk of lymphoma in Japanese patients with
rheumatoid arthritis compared to the general population. J
Rheumatol. 42:564–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ureshino H, Kadota C, Kurogi K, Miyahara M
and Kimura S: Spontaneous Regression of Methotrexate-related
Lymphoproliferative Disorder with T-cell Large Granular
Lymphocytosis. Intern Med. 54:2235–2239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ichikawa A, Arakawa F, Kiyasu J, Sato K,
Miyoshi H, Niino D, Kimura Y, Takeuchi M, Yoshida M, Ishibashi Y,
et al: Methotrexate/iatrogenic lymphoproliferative disorders in
rheumatoid arthritis: Histology, Epstein-Barr virus, and clonality
are important predictors of disease progression and regression. Eur
J Haematol. 91:20–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katsuyama T, Sada KE, Yan M, Zeggar S,
Hiramatsu S, Miyawaki Y, Ohashi K, Morishita M, Watanabe H,
Katsuyama E, et al: Prognostic factors of methotrexate-associated
lymphoproliferative disorders associated with rheumatoid arthritis
and plausible application of biological agents. Mod Rheumatol.
27:773–777. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zaidi A, Kampalath B, Peltier WL and
Vesole DH: Successful treatment of systemic and central nervous
system lymphomatoid granulomatosis with rituximab. Leuk Lymphoma.
45:777–780. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gion Y, Iwaki N, Takata K, Takeuchi M,
Nishida K, Orita Y, Tachibana T, Yoshino T and Sato Y:
Clinicopathological analysis of methotrexate-associated
lymphoproliferative disorders: Comparison of diffuse large B-cell
lymphoma and classical Hodgkin lymphoma types. Cancer Sci.
108:1271–1280. 2017. View Article : Google Scholar : PubMed/NCBI
|