Introduction
Oxidative stress is a chief cause of vascular
endothelial damage. It increases the levels of reactive oxygen
species, which subsequently induces vascular endothelial
dysfunction via the inhibition of nitric oxide production (1).
In the prostate, ischemia increases the levels of
reactive oxygen species, growth factors, and cytokines, and induces
the development of angiogenesis, resulting in cancer progression
(2). Recent studies, using a
prostate hyperplasia mouse model have demonstrated that ischemia in
the prostatic epithelium induces oxidative stress, resulting in
cell proliferation involved by inflammatory cytokines and several
growth factors (3,4).
These findings indicate that oxidative stress plays
an important role in the prostate. No report has described the
tissue 8-hydroxyguanosine (8-OHdG) expression in prostatic
specimens. In the present study, we compared the expression levels
of an oxidative stress marker 8-hydroxyguanosine between prostate
adenocarcinoma and non-neoplastic prostate tissues.
Materials and methods
Immunohistochemistry was performed, as described
previously (5) in sections from a
prostatic tissue microarray composed of 10 cases of prostatic
adenocarcinoma and 70 cases of non-neoplastic prostate (PR804; US
Biomax, Rockville, MD, USA). This company obtained informed consent
(https://www.biomax.us/FAQs,Q10) Briefly,
5-µm-thick slides were dewaxed with xylene, hydrated with gradient
ethanol, and microwaved at high level for 2 min., followed by 30
min. microwaved at middle-low levels for heat antigen retrieval
(Target Retrieval Solution pH9, Dako, Carpinteria, CA, USA). After
3% hydrogen peroxidase block, the samples were incubated overnight
at 4°C with a primary antibody to anti-8-OHdG (ab48508, diluted to
1:200; Abcam, Cambridge, MA, USA) and a broad-spectrum secondary
antibody (Histostain-SP kit; Invitrogen, Grand Island, NY, USA) for
2 h (Table I). The slides were then
examined by a single pathologist (HM) blinded to sample identify.
The score of immunoreactivity was determined by multiplying the
percentage of immunoreactive cells (0% = 0; 1–10% = 1; 11–50% = 2;
51–80% = 3; 81–100% = 4) by staining intensity (negative = 0; weak
= 1; moderate = 2; strong = 3). The immunohistochemical scores
(ranging from 0 to 12) were considered negative (0; 0–1), weakly
positive (1+; 2–4), moderately positive (2+; 6–8), and strongly
positive (3+; 9–12) for 8-OHdG expression (5).
 | Table I.First and second antibody for
immunohistochemistry analysis. |
Table I.
First and second antibody for
immunohistochemistry analysis.
| Name | Cat. no. | Company |
|---|
| First antibody |
|
|
|
8-Hydroxyguanosine polyclonal
antibody | ab48508 | Abcam |
| Second antibody and
staining kit |
|
|
|
Histostain-SP kit | 95–9943 | Invitrogen |
The 8-OHdG expression was analyzed by chi-square
test using the Graph Pad Prism software program (Graph Pad
Software, La Jolla, CA, USA).
Results
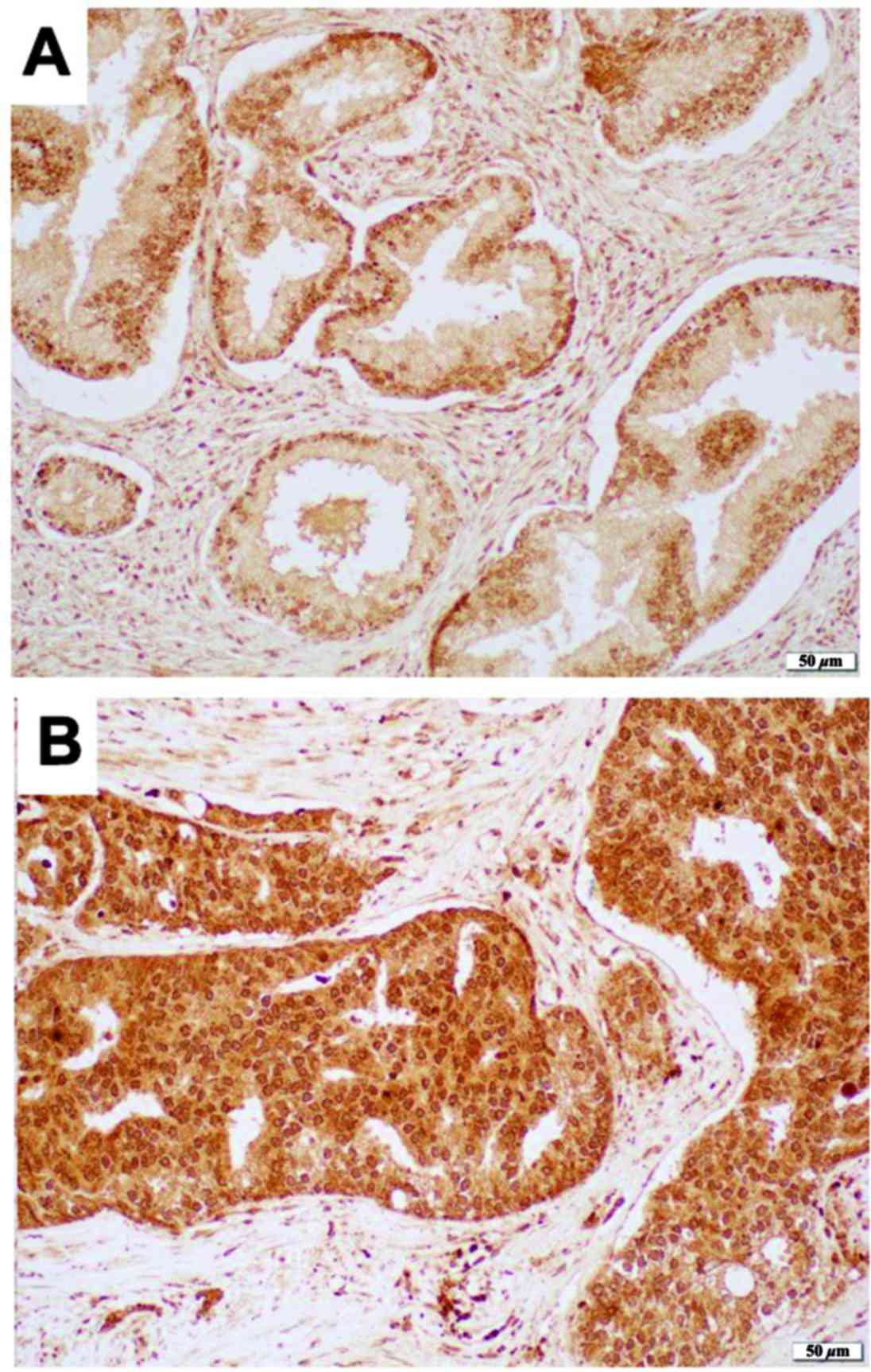
We immunohistochemically stained for 8-OHdG in a
prostate tissue microarray. Positive signals were detected
predominantly in cytoplasm of epithelial cells as well as in
nucleus of epithelial cells only in a few cases (Fig. 1). 8-OHdG was immunoreactive in all 10
prostatic adenocarcinomas, including 3 (30%) cases with moderate
(2+) expression and 7 (70%) cases with strong (3+) expression.
Similarly, 8-OHdG was moderately and strongly positive in 50
(71.4%) and 20 (28.6%) cases of benign prostatic hyperplasia
(Table II). Thus, the levels of
8-OHdG expression in prostatic adenocarcinoma tissue specimens were
significantly higher than those in non-neoplastic prostate tissue
specimens (P<0.01).
 | Table II.Immunohistochemical staining of
8-hydroxyguanosine (P<0.01). |
Table II.
Immunohistochemical staining of
8-hydroxyguanosine (P<0.01).
| Immunoreactive
score | 0 | 1+ | 2+ | 3+ |
|---|
| Prostate
adenocarcinoma | 0 (0.0%) | 0 (0.0%) | 3 (30.0%) | 7 (70.0%) |
| Non-neoplastic
tissue | 0 (0.0%) | 0 (0.0%) | 50 (71.4%) | 20 (28.6%) |
Discussion
Our results showed that prostate adenocarcinoma
showed higher 8-OHdG expression than non-neoplastic prostate
tissue. Miyake et al showed that prostate cancer patients
had significantly higher levels of urine 8-OHdG/Cre than those
without cancer (6). In patients with
prostate cancer, urine 8-OHdG/Cre levels were not markedly
different before and after radical prostatectomy. In contrast,
their levels were decreased significantly after androgen
deprivation therapy. These findings indicate that androgen
deprivation therapy reduces oxidative stress.
The detailed mechanism underlying the cancer
progression induced by 8-OHdG is still unknown. Guanine is easily
damaged by anti-oxidant stress, resulting in a change to 8-OHdG.
8-OHdG is structurally stable and widely used as an oxidant-stress
biomarker (6,7). H2O2 has also been
shown to increase 8-OHdG in a time-dependent manner in prostatic
epithelial cells, which is one potential mechanism underlying
cancer progression, presumably due to continuous anti-oxidant
damage (8,9). Based on these findings, oxidant stress
produced 8-OHdG in prostate tissue, resulting in the time-dependent
accumulation of 8-OHdG and subsequent carcinogenesis due to the
damage of DNA. Wu and Ni showed the correlation between 8-OHdg and
carcinogenesis and speculated that hypomethylation and regional
hypermethylation contribute to carcinogenesis (10). In gastric cancer, a correlation has
been reported between gastric cancer and cytotoxin-associated genes
that induces cancer progression. However, the detailed mechanisms
are still unknown (11). While
further studies are needed, the findings from the present study
suggest that 8-OHdG might be a candidate tumor marker for detecting
prostate cancer.
Tadalafil, a PDE-5 inhibitor, inhibits cGMP in
smooth muscle cells and induces reflexes smooth muscle (12,13).
Gotoh et al demonstrated that tadalafil improved the bladder
blood flow and increased the level of 8-OHdG in tissue (14). Tadalafil has also been reported to
show anti-inflammatory activity in the prostate and might be a
prevention effect from prostate cancer (15,16).
The limitations of this study include no staining in
completely normal prostatic tissue. Further studies, using normal
prostate tissues and/or cell lines, are also required to reveal the
underlying mechanism for the involvement of 8-OHdG in prostate
carcinogenesis and cancer progression.
We found that 8-OHdG expression was elevated in
prostate cancer tissues compared with non-neoplastic prostate
tissues. 8-OHdG may therefore play an important role in prostate
cancer development.
Acknowledgements
Not applicable.
Funding
Funding was received from Kakenhi grants (grant no.
16K20152) for the Ministry of Education, Culture, Sports, Science
and Technology of Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon request
Authors' contributions
SO, TK and HU conceived and designed the
experiments. SO, TK, YI, TT, SK, KI, HM and HU performed the
experiments. SO, TK and HM wrote the manuscript. All authors have
read and approved the manuscript
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cai H and Harrison DG: Endothelial
dysfunction in cardiovascular diseases: The role of oxidant stress.
Circ Res. 87:840–844. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Nunzio C, Aronson W, Freedland SJ,
Giovannucci E and Parsons JK: The correlation between metabolic
syndrome and prostatic diseases. Eur Urol. 61:560–570. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sychlowy A, van der Gaag H and
Hannen-Hofheinz I: Hyperkalemia-life-threatening early complication
of asphyxia in premature infants. Monatsschr Kinderheilkd.
138:62–65. 1990.(In German). PubMed/NCBI
|
|
4
|
Saito M, Tsounapi P, Oikawa R, Shimizu S,
Honda M, Sejima T, Kinoshita Y and Tomita S: Prostatic ischemia
induces ventral prostatic hyperplasia in the SHR; possible
mechanism of development of BPH. Sci Rep. 4:38222014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shim V, Gauthier ML, Sudilovsky D, Mantei
K, Chew KL, Moore DH, Cha I, Tlsty TD and Esserman LJ:
Cyclooxygenase-2 expression is related to nuclear grade in ductal
carcinoma in situ and is increased in its normal adjacent
epithelium. Cancer Res. 63:2347–2350. 2003.PubMed/NCBI
|
|
6
|
Miyake H, Hara I, Kamidono S and Eto H:
Oxidative DNA damage in patients with prostate cancer and its
response to treatment. J Urol. 171:1533–1536. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nelson WG, DeWeese TL and DeMarzo AM: The
diet, prostate inflammation, and the development of prostate
cancer. Cancer Metastasis Rev. 21:3–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanwal R, Pandey M, Bhaskaran N, Maclennan
GT, Fu P, Ponsky LE and Gupta S: Protection against oxidative DNA
damage and stress in human prostate by glutathione S-transferase
P1. Mol Carcinog. 53:8–18. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koul HK, Kumar B, Koul S, Deb AA, Hwa JS,
Maroni P, van Bokhoven A, Lucia MS, Kim FJ and Meacham RB: The role
of inflammation and infection in prostate cancer: Importance in
prevention, diagnosis and treatment. Drugs Today (Barc).
46:929–943. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu Q and Ni X: ROS-mediated DNA
methylation pattern alterations in carcinogenesis. Curr Drug
Targets. 16:13–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raza Y, Khan A, Farooqui A, Mubarak M,
Facista A, Akhtar SS, Khan S, Kazi JI, Bernstein C and Kazmi SU:
Oxidative DNA damage as a potential early biomarker of Helicobacter
pylori associated carcinogenesis. Pathol Oncol Res. 20:839–846.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukumoto K, Nagai A, Hara R, Fujii T and
Miyaji Y: Tadalafil for male lower urinary tract symptoms improves
endothelial function. Int J Urol. 24:206–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yokoyama O, Ozeki A, Suzuki N and Murakami
M: Early improvement of storage or voiding symptoms by tadalafil
predicts treatment outcomes in patients with lower urinary tract
symptoms from benign prostatic hyperplasia. Int J Urol. 25:240–245.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gotoh D, Torimoto K, Tatsumi Y, Hori S,
Yamada A, Miyake M, Morizawa Y, Aoki K, Tanaka N, Hirayama A, et
al: Tadalafil, a phosphodiesterase type 5 inhibitor, improves
bladder blood supply and restores the initial phase of lower
urinary tract dysfunction in diabetic rats. Neurourol Urodyn.
35:444–449. 2017.
|
|
15
|
Vignozzi L, Gacci M, Cellai I, Morelli A,
Maneschi E, Comeglio P, Santi R, Filippi S, Sebastianelli A, Nesi
G, et al: PDE5 inhibitors blunt inflammation in human BPH: A
potential mechanism of action for PDE5 inhibitors in LUTS.
Prostate. 73:1391–1402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zarifpour M, Nomiya M, Sawada N and
Andersson KE: Protective effect of tadalafil on the functional and
structural changes of the rat ventral prostate caused by chronic
pelvic ischemia. Prostate. 75:233–241. 2015. View Article : Google Scholar : PubMed/NCBI
|