Introduction
The BRAF protein is a member of the RAF-MEK-ERK
signal transduction pathway (1).
Mutations of BRAF kinase are actively involved in oncogenic
proliferation through its constitutive activity (2). Approximately 3% of non-small-cell lung
cancer (NSCLC) cases harbor BRAF mutations (3). However, research on BRAF gene mutations
are rarely focused on NSCLC. Targeted therapies have significantly
modified the treatment of NSCLC (4),
with a large number of targeted therapies for NSCLC already
available or currently in clinical trials. However, tumor tissue
may be difficult to obtain for gene detection. It has been
demonstrated that next-generation sequencing (NGS) tests are
superior in terms of sensitivity and specificity compared with
non-NGS methods. Additionally, the coincidence rate of gene
mutations between the plasma and tumor tissue is 60–80% (5), suggesting that plasma NGS may be
recommended for selection of targeted drugs.
Case report
In April 2016, a 71-year-old man with a 46-year
history of smoking was diagnosed with lung adenocarcinoma of the
right middle lobe during a medical examination. A computed
tomography (CT) scan revealed a mass in the middle lobe of the
right lung with multiple metastatic nodules in both lungs.
Pathological assessment confirmed the diagnosis of pulmonary
adenocarcinoma. The patient was wild-type for epidermal growth
factor receptor (EGFR), Kirsten rat sarcoma viral oncogene homolog
(KRAS) and anaplastic lymphoma kinase (ALK).
In May 2016, the patient was treated with
carboplatin and pemetrexed (400 and 800 mg/day, respectively) for a
total of 6 cycles. A partial response (PR) was achieved. Therefore,
in November 2016, the patient was administered pemetrexed
maintenance monotherapy (800 mg/day) for 6 cycles. However, the CT
scan after 6 cycles of maintenance therapy revealed progressive
disease (PD) indicated by an increase in the size of the lung
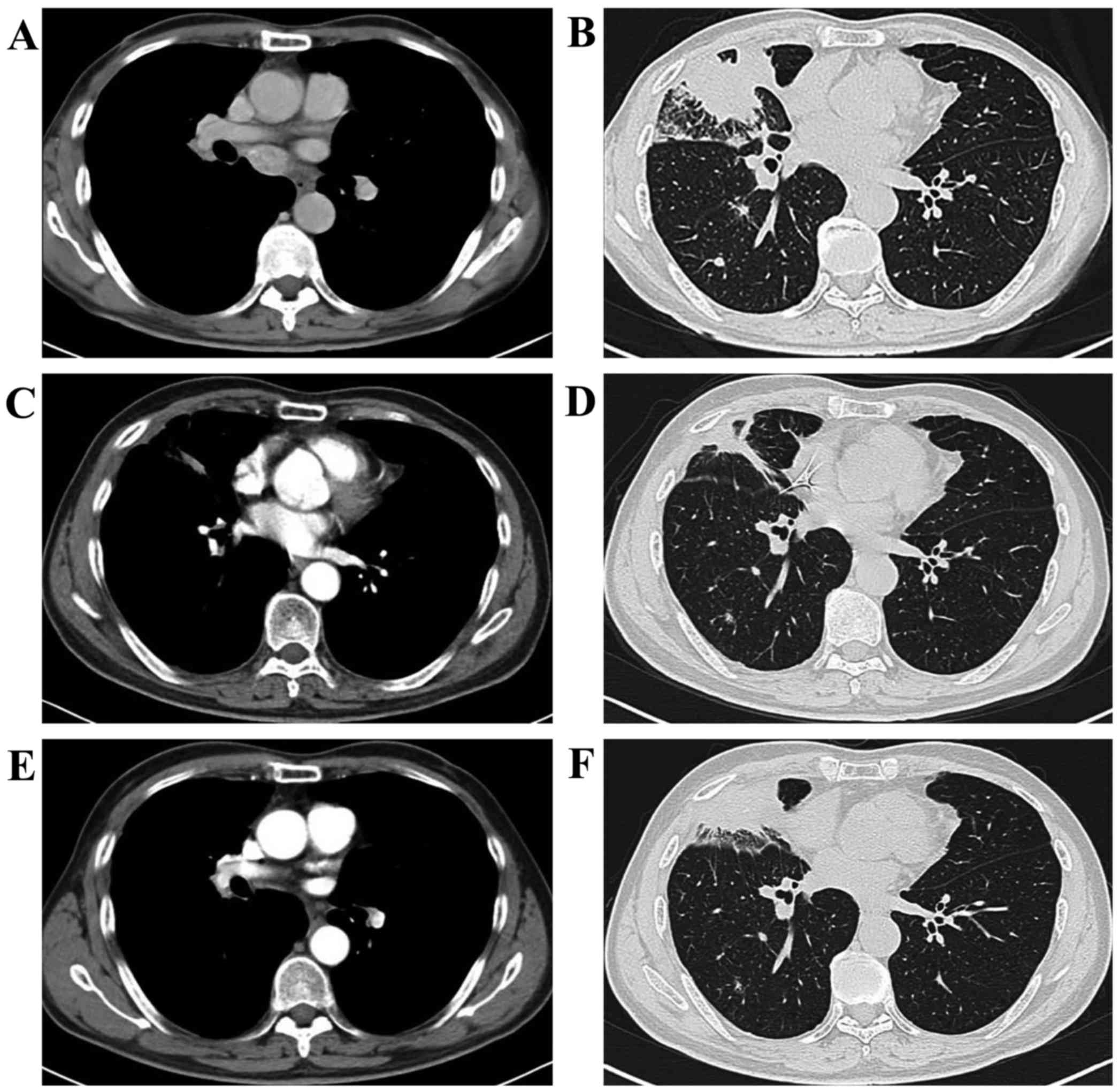
lesions (Fig. 1). The patient again
received chemotherapy with carboplatin and pemetrexed (450 mg twice
daily and 800 mg/day, respectively). After 2 cycles of
chemotherapy, the appearance of new liver lesions indicated PD. In
July 2017, the patient was administered docetaxel (100 mg/day).
After 2 cycles of this single-drug chemotherapy, PD was indicated
by an increase in the size of the lung lesions and the appearance
of new lesions in the pancreas and kidney. The performance status
(PS) of the patient quickly deteriorated to 3, with complaints of
abdominal distention and chest pain. In August 2017, plasma NGS
analysis revealed a V600E BRAF mutation in exon 15, with a mutation
abundance of 18.62%. Treatment with vemurafenib was initiated at a
dose of 720 mg (BID) on August 25, 2017 and the dose was increased
to 960 mg from September 1, 2017 to September 5, 2017 to improve
the efficacy. However, the vemurafenib dosage was again reduced to
720 mg (BID) due to adverse events such as hand-foot syndrome,
liver dysfunction and hypodynamia. The side effects diminished
following dosage reduction. After treatment with vemurafenib, the
patient's symptoms of abdominal distention and chest pain were
ameliorated, and the PS improved to 1. A PR was achieved. However,
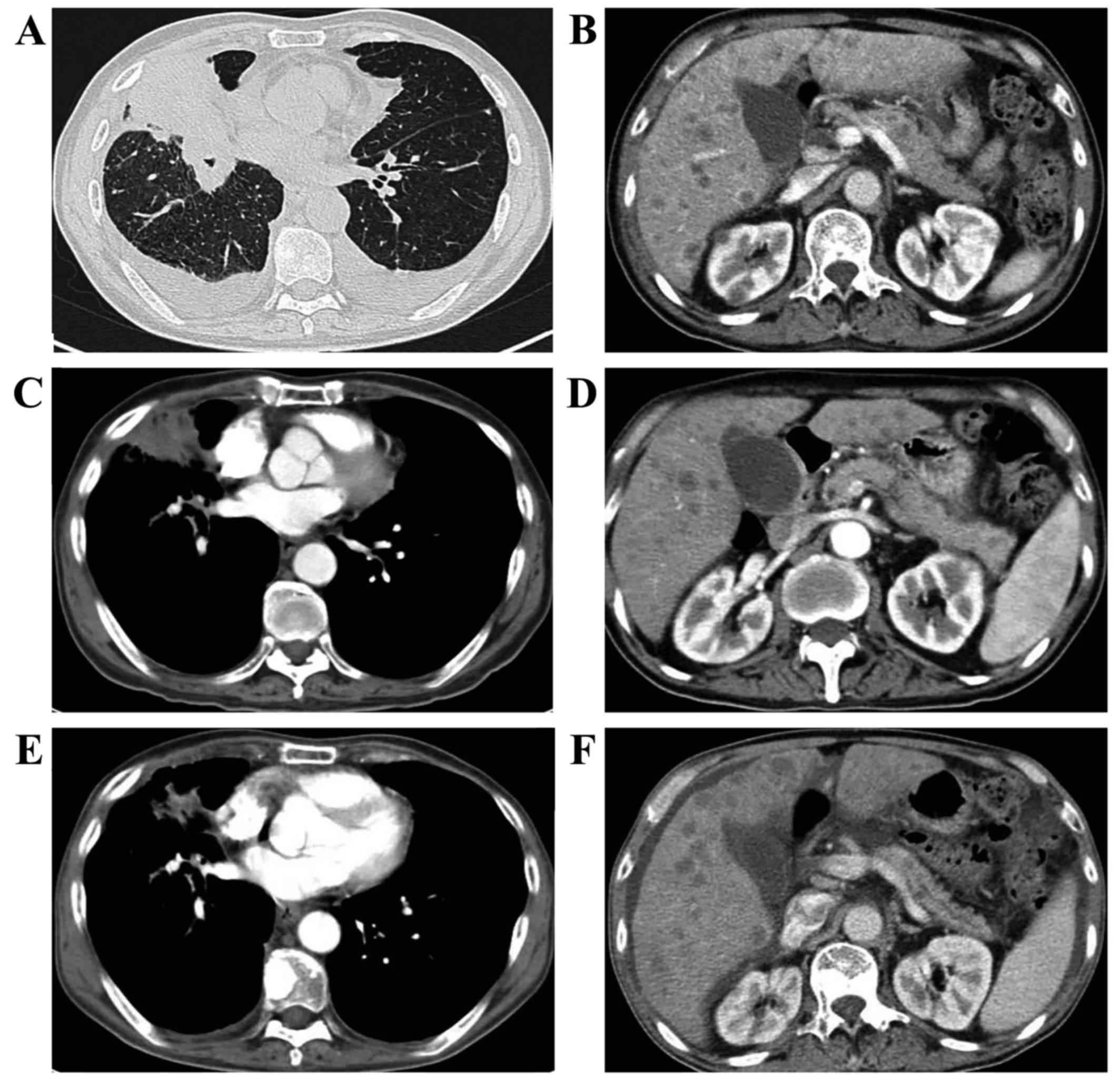
in December 2017, a CT scan revealed that, although the primary
lesion in the lung had shrunk, new liver lesions had appeared, and
the treatment efficacy evaluation was again PD (Fig. 2). Furthermore, the PS quickly
deteriorated to 3, and the patient again exhibited symptoms of
abdominal distension. The patient finally succumbed to the disease
on the day of discharge (December 24, 2017), and the cause of death
was multiple organ failure. The overall duration of vemurafenib
treatment was 3.2 months, and the patient's survival following lung
cancer diagnosis was 19.2 months.
Discussion
Currently, treatments for NSCLC include surgery,
chemotherapy, radiotherapy, targeted therapy and immunotherapy.
Targeted therapies have significantly changed the treatment of
NSCLC (4). However, with regards to
the tumor heterogeneity and differences among tissues and organs,
the effects of the same targeted agent on tumors located in
different areas, even in the same region, may vary greatly
(6,7).
BRAF mutations are a contributor to the
heterogeneity of lung cancer. The BRAF protein is a member of the
RAF-MEK-ERK signal transduction pathway that controls a variety of
biological processes (1). Mutations
of BRAF kinase are actively involved in oncogenic proliferation
through its constitutive activity (2). BRAF mutations include V600E and
non-V600E mutations. Approximately 3% of NSCLC cases harbor BRAF
mutations, which are closely associated with adenocarcinoma
(3). The BRAF V600E mutation is more
frequent among women and is significantly associated with
non-smokers (3). Approximately 55%
of NSCLC patients with a BRAF mutation have been found to harbor
the V600E mutation (8). Overall
survival (OS) with chemotherapy has improved by the use of
vemurafenib (a BRAF inhibitor), which was approved by the Food and
Drug Administration and European Medicines Agency for the treatment
of metastatic melanoma with a BRAF mutation (9). There are studies and clinical trials
showing that BRAF inhibitors are effective against BRAF-mutated
NSCLC (10,11). One phase 2 trial included 57 NSCLC
cases with the BRAF V600E mutation, and the overall response rate
(ORR) was 63.2%, indicating that dabrafenib plus trametinib were
promising as novel targeted therapy in BRAF V600E-mutant NSCLC
(11). In a histology-independent
phase 2 ‘basket’ study of vemurafenib, the response rate of the
NSCLC cohort was 42%, and the median progression-free survival
(PFS) was 7.3 months (12). In the
case described herein, BRAF-mutated NSCLC responded to vemurafenib
treatment.
In the present case, the dose of vemurafenib was
reduced due to adverse events, including increased levels of total
bilirubin and PS 3. The most common adverse reactions associated
with vemurafenib include diarrhea, fever, rash, photosensitivity,
hand-foot syndrome, joint pain, abnormal liver function and QT
interval prolongation. In Chinese studies, the adverse reactions of
vemurafenib were mainly grade 1–2, whereas events of grade ≥3 were
rarely reported. The incidence of melanotic nevus was high, but
there are no reports of melanotic nevus progression, and the use of
vemurafenib in cutaneous squamous cell carcinoma has not yet been
reported. In other studies, the adverse reactions of vemurafenib
were mainly grade 1–2 with grade ≥3 reactions rarely reported; the
majority of the grade 3 reactions were observed in cutaneous
squamous cell carcinoma.
There is currently a great number of targeted
therapies for NSCLC that are already available or undergoing
evaluation in clinical trials. Therefore, molecular
characterization of tumors using NGS technology has become a
valuable tool that aids treatment decision-making and the clinical
management of NSCLC patients (13).
Non-NGS testing includes a number of tests for alterations of 11
genes known to be involved in lung cancer (14,15). All
four types of DNA alterations in cancer may be diagnosed though
hybrid capture-based NGS platforms with better sensitivity and
specificity compared with non-NGS tests (13). One study indicated that hybrid
capture-based NGS identified actionable genomic alterations in 65%
of tumors with genomic alterations that were not detected by more
dated extensive non-NGS testing (14). Furthermore, dysregulated miRNA
profiles may be specifically identified using NGS technology in
resectable NSCLC, which may be a potential predictor of
recurrence-free survival and OS (16).
At present, plasma NGS technology is applied in
clinical practice. Sensitive polymerase chain reaction analysis
techniques and high-throughput NGS technologies have been developed
to perform genetic analyses of circulating free DNA. The
coincidence rate of gene mutations between the plasma and tumor
tissue is 60–80% (5). Therefore, in
cases where tumor tissue is difficult to obtain, plasma NGS may be
used to detect gene mutations. Limitations of gene detection in
tumor tissues are overcome by plasma NGS technology. The high
specificity of NGS may be directly recommended for the selection of
targeted drugs based on the results of the plasma DNA analysis
(17).
Due to the tumor heterogeneity, different patients
may harbor different gene mutations, such as the multiple mutations
of EGFR. The mutation abundance is associated with the therapeutic
effect of EGFR-tyrosine kinase inhibitors. Patients with EGFR
mutations had a significantly longer PFS compared with those
without EGFR mutations (18).
Patients with a high level of EGFR mutation abundance exhibited
better response to treatment compared with patients in the
low-level group (19). However,
there are no relevant studies on the abundance of BRAF mutations.
It remains unknown whether BRAF mutation abundance affects the
response of patients to targeted drug therapy to the same extent as
EGRF mutation abundance, and further experiments are required to
elucidate this hypothesis.
In conclusion, we herein described the case of a
heavy smoker with BRAF-mutated NSCLC. The patient was wild-type for
EGFR and ALK. Following failure of the second-line treatment, the
patient was administered vemurafenib when the NGS analysis revealed
a V600E BRAF mutation in exon 15 and a mutation abundance of
18.62%, after which time the CT scan revealed a PR. However, in
December 2017, the CT scan revealed PD (PFS of 3.2 months). This
case provides an example of vemurafenib administration based on
NGS, and highlights the potential value of vemurafenib in the
treatment of advanced lung cancer harboring a BRAF mutation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81500012), the
Zhejiang Provincial Natural Science Foundation of China (grant no.
LQ16H010001) and the Medical and Health Technology Program of
Zhejiang Province (grant nos. 2015111464 and 2017204226).
Availability of data and materials
Not applicable.
Authors' contributions
YY contributed to the patient's treatment plan. XL
and XF collected and interpreted the data. HH, XF and YC
contributed to the management of patient. All the authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient's family consent to the publication of
the case details and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peyssonnaux C and Eychène A: The
Raf/MEK/ERK pathway: New concepts of activation. Biol Cell.
93:53–62. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huebner C, Weber R and Lloydd R: A HRM
assay for identification of low level BRAF V600E and V600K
mutations using the CADMA principle in FFPE specimens. Pathology.
49:776–783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen D, Zhang LQ, Huang JF, Liu K, Chuai
ZR, Yang Z, Wang YX, Shi DC, Liu Q, Huang Q, et al: BRAF mutations
in patients with non-small cell lung cancer: A systematic review
and meta-analysis. PLoS One. 9:e1013542014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamiya M, Tamiya A, Shiroyama T, Takeoka
S, Naito Y, Omachi N, Kimura Y, Morishita N, Suzuki H, Okamoto N,
et al: Phase1 study of cisplatin plus pemetrexed with erlotinib and
bevacizumab for chemotherapy-naïve advanced non-squamous non-small
cell lung cancer with EGFR mutations. Invest New Drugs. Nov
4–2017.(Epub ahead of print). PubMed/NCBI
|
|
5
|
Oxnard GR, Paweletz CP and Sholl LM:
Genomic analysis of plasma cell-free DNA in patients With Cancer.
JAMA Oncol. 3:740–741. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grzywa TM, Paskal W and Włodarski PK:
Intratumor and intertumor heterogeneity in melanoma. Transl Oncol.
10:956–975. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martelotto LG, Ng CK, Piscuoglio S,
Weigelt B and Reis-Filho JS: Breast cancer intra-tumor
heterogeneity. Breast Cancer Res. 16:2102014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Villaruz LC, Socinski MA, Abberbock S,
Berry LD, Johnson BE, Kwiatkowski DJ, Iafrate AJ, Varella-Garcia M,
Franklin WA, Camidge DR, et al: Clinicopathologic features and
outcomes of patients with lung adenocarcinomas harboring BRAF
mutations in the Lung Cancer Mutation Consortium. Cancer.
121:448–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Czirbesz K, Gorka E, Balatoni T, Pánczél
G, Melegh K, Kovács P, Gézsi A and Liszkay G: Efficacy of
vemurafenib treatment in 43 metastatic melanoma patients with BRAF
mutation. Single-institute retrospective analysis, early real-life
survival data. Pathol Oncol Res. Sep 29–2017.(Epub ahead of print).
View Article : Google Scholar
|
|
10
|
Noeparast A, Teugels E, Giron P,
Verschelden G, De Brakeleer S, Decoster L and De Grève J: Non-V600
BRAF mutations recurrently found in lung cancer predict sensitivity
to the combination of Trametinib and Dabrafenib. Oncotarget.
8:60094–60108. 2016.PubMed/NCBI
|
|
11
|
Planchard D, Besse B, Groen HJM, Souquet
PJ, Quoix E, Baik CS, Barlesi F, Kim TM, Mazieres J, Novello S, et
al: Dabrafenib plus trametinib in patients with previously treated
BRAF(V600E)-mutant metastatic non-small cell lung cancer: An
open-label, multicentre phase 2 trial. Lancet Oncol. 17:984–993.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hyman DM, Puzanov I, Subbiah V, Faris JE,
Chau I, Blay JY, Wolf J, Raje NS, Diamond EL, Hollebecque A, et al:
Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600
Mutations. N Engl J Med. 373:726–736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsoulos N, Papadopoulou E, Metaxa-Mariatou
V, Tsaousis G, Efstathiadou C, Tounta G, Scapeti A, Bourkoula E,
Zarogoulidis P, Pentheroudakis G, et al: Tumor molecular profiling
of NSCLC patients using next generation sequencing. Oncol Rep.
38:3419–3429. 2017.PubMed/NCBI
|
|
14
|
Drilon A, Wang L, Arcila ME,
Balasubramanian S, Greenbowe JR, Ross JS, Stephens P, Lipson D,
Miller VA, Kris MG, et al: Broad, Hybrid Capture-Based
Next-Generation Sequencing Identifies Actionable Genomic
Alterations in Lung Adenocarcinomas Otherwise Negative for Such
Alterations by Other Genomic Testing Approaches. Clin Cancer Res.
21:3631–3639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chaft JE, Arcila ME, Paik PK, Lau C, Riely
GJ, Pietanza MC, Zakowski MF, Rusch V, Sima CS, Ladanyi M, et al:
Coexistence of PIK3CA and other oncogene mutations in lung
adenocarcinoma-rationale for comprehensive mutation profiling. Mol
Cancer Ther. 11:485–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suh JH, Johnson A, Albacker L, Wang K,
Chmielecki J, Frampton G, Gay L, Elvin JA, Vergilio JA, Ali S, et
al: Comprehensive Genomic Profiling Facilitates Implementation of
the National Comprehensive Cancer Network Guidelines for Lung
Cancer Biomarker Testing and Identifies Patients Who May Benefit
From Enrollment in Mechanism-Driven Clinical Trials: Comprehensive
genomic profiling facilitates implementation of the national
comprehensive cancer network guidelines for lung cancer biomarker
testing and identifies patients who may benefit from enrollment in
mechanism-driven clinical trials. Oncologist. 21:684–691. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiong L, Cui S, Ding J, Sun Y, Zhang L,
Zhao Y, Gu A, Chu T, Wang H, Zhong H, et al: Dynamics of EGFR
mutations in plasma recapitulates the clinical response to
EGFR-TKIs in NSCLC patients. Oncotarget. 8:63846–63856. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shan L, Wang Z, Guo L, Sun H, Qiu T, Ling
Y, Li W, Li L, Liu X, Zheng B, et al: Concurrence of EGFR
amplification and sensitizing mutations indicate a better survival
benefit from EGFR-TKI therapy in lung adenocarcinoma patients. Lung
Cancer. 89:337–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Wang Y, Su F, Li J and Gong P:
Monitoring of cyclooxygenase-2 levels can predict EGFR mutations
and the efficacy of EGFR-TKI in patients with lung adenocarcinoma.
Int J Clin Exp Pathol. 8:5577–5583. 2015.PubMed/NCBI
|