Introduction
Undifferentiated pleomorphic sarcoma (UPS), which
was formerly known as malignant fibrous histiocytoma, is a
high-grade sarcoma, which mainly arises from the soft tissue of the
extremities and can appear at any age, but typically presents in
the 5th-7th decades of life and exhibits a slight male predominance
(1). Histopathologically, it is
characterized by pleomorphic and spindle-shaped tumor cells and a
storiform growth pattern; however, it lacks a definitive line of
differentiation (2). To date,
several cases of UPS that arose in the gastrointestinal tract have
been reported, but these reports did not include long-term
follow-up data. We report a case of UPS, in which the patient
survived for more than 7 years after the surgical resection of the
primary gastric tumor and metachronous metastases.
Case presentation
A 70-year-old male visited his primary care doctor,
complaining of abdominal fullness and a palpable large mass in his
lower abdomen. He was referred to our hospital for further
investigation. He had a history of hypertension and had undergone
resection of the right upper pulmonary lobe for tuberculosis 50
years ago. His blood examination findings only showed an elevated
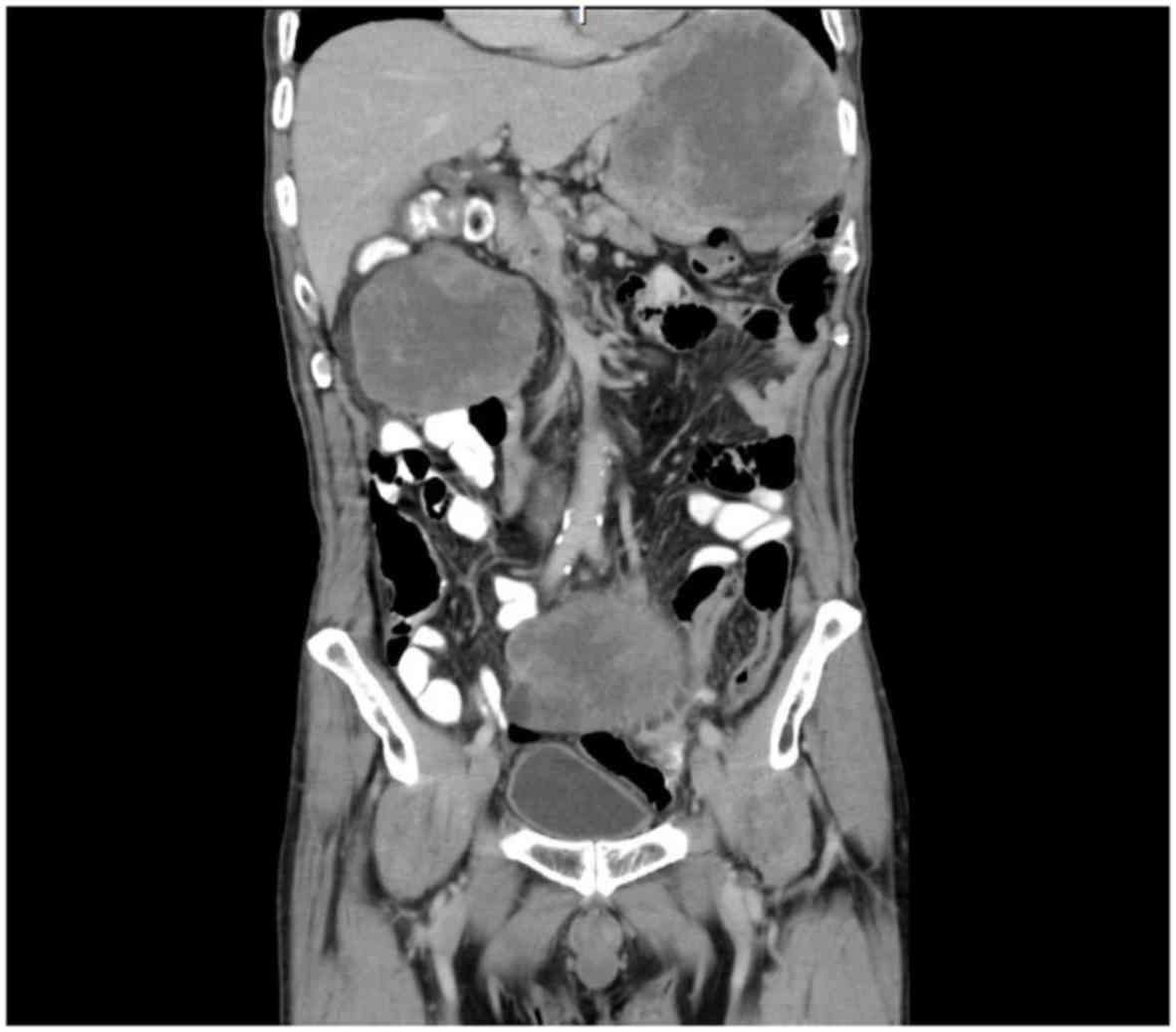
C-reactive protein level. An abdominal computed tomography (CT)
scan revealed a 14-cm oval tumor in the wall of the stomach and two
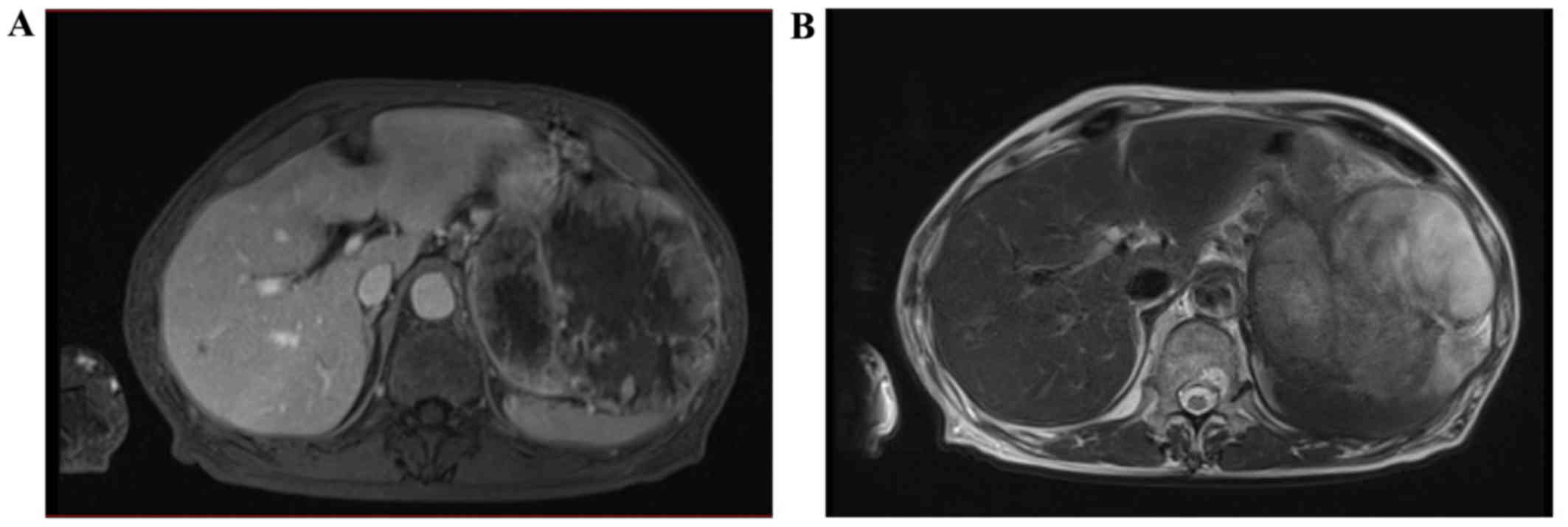
10-cm tumors in the mesentery of the small intestine (Fig. 1). During magnetic resonance imaging
(MRI), the tumor exhibited low signal intensity on T1-weighted
images and high and diffuse signal intensity on T2-weighted images
(Fig. 2). It also demonstrated a
diffusional constraint on diffusion-weighted images. Upper
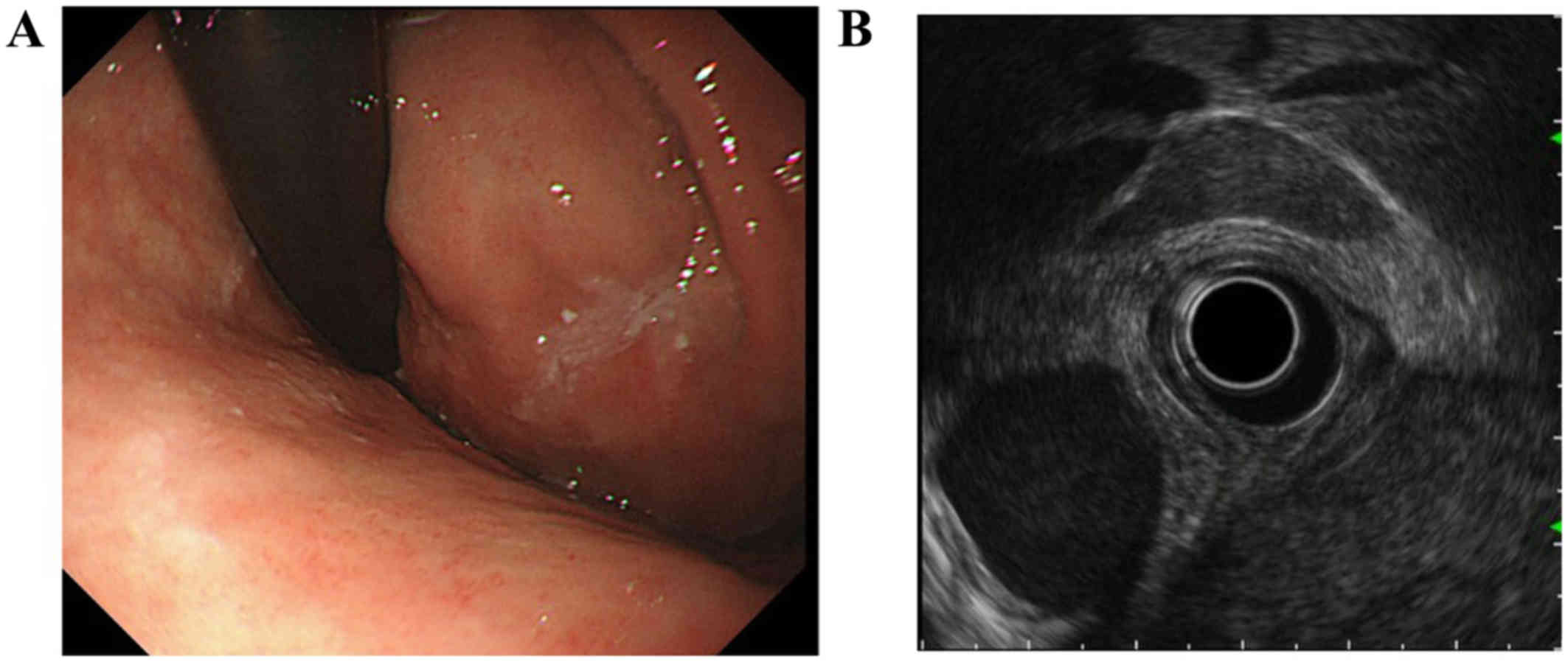
gastrointestinal endoscopy showed a submucosal tumor, and
endoscopic ultrasound (EUS) revealed a hypoechoic solid tumor,
which was contiguous with the proper muscular layer (Fig. 3). An endoscopic ultrasound-guided
fine-needle biopsy (EUS-FNB) was performed, and the specimen was
found to contain atypical cells with pleomorphic or bizarre nuclei
and a necrotic background, which was consistent with high-grade
sarcoma. Atypical cells showed no definite differentiation by
immunohistochemically, which were only positive for vimentin, and
negative for α-smooth muscle actin, cytokeratin (AE1/AE3),
epithelial membrane antigen, desmin, S-100 protein, c-Kit, CD34 and
Melan-A. Based on these findings, we decided to perform total
gastrectomy combined with resection of the accompanying tumors
because it was considered that systemic therapy would not be
effective. Twelve days after the EUS-FNB examination, the patient
underwent total gastrectomy and combined resection of the small
intestinal tumors. An intraoperative examination confirmed that the
largest tumor originated from the anterior wall of the upper
stomach and occupied the space between the stomach and the
transverse colon, whereas the other tumors located in the ileal
mesentery. There were no other masses in the abdominal cavity.
Intraoperative peritoneal washing cytology was negative for tumor
cells. As none of the tumors had invaded the adjacent organs, they
were wholly resected via total gastrectomy and partial resection of
the ileum. The gastric tumor was resected en bloc combined with D2
lymphadenectomy according to the Japanese Classification of Gastric
Carcinoma guidelines (3).
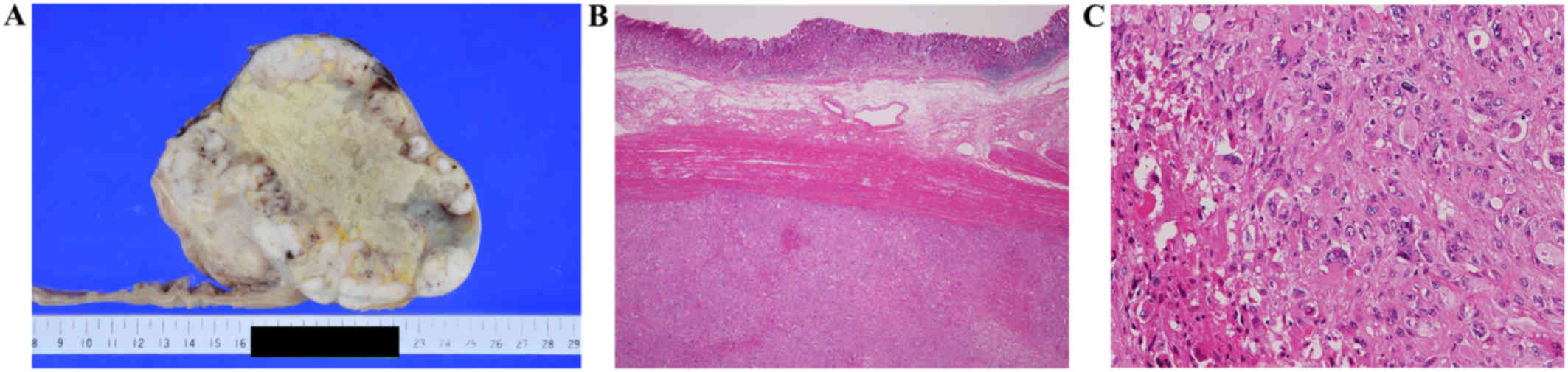
Macroscopically, the resected gastric tumor was a
well-demarcated solid grayish-white tumor, measuring 14×12×10 cm in
size, which displayed extensive central necrosis and peripheral
lobulation and protruded into the abdominal cavity (Fig. 4A and B). The other extra-gastric
tumors measured 12.5×11×8.5 and 11.5×9.5×9 cm in size,
respectively. Microscopically, the tumors were composed of atypical
spindle-shaped and pleomorphic cells with large pleomorphic or
bizarre nuclei (Fig. 4C). Mitotic
figures, including abnormal mitoses, were frequently encountered
(5–6/high-power field). The tumor displayed expansive growth
without infiltrating into the adjacent tissue. No vascular invasion
or regional lymph node metastasis was observed (0/44).
Immunohistochemically, the atypical cells were positive for
vimentin, but negative for cytokeratin (AE1/AE3 and CAM 5.2),
epithelial membrane antigen, desmin, S-100 protein, c-Kit, CD34,
mouse double minute 2 homolog (MDM2), cyclin-dependent kinase 4
(CDK4), and melan-A. The final pathological diagnosis was UPS of
the stomach with peritoneal dissemination. The patient's
postoperative course was uneventful; however, 6 months later,
follow-up CT showed a tumor located in the ligament of the sigmoid
colon, and so partial resection of the sigmoid colon was performed.
The histological and immunohistochemical features of the resected
tumor were almost the same as those of the primary tumor.
Peritoneal washing cytology was positive for atypical cells. No
adjuvant chemotherapy was administered because there is no standard
chemotherapy regimen for abdominal UPS, UPS exhibits a low
chemotherapy response rate, and chemotherapy can have side effects.
Seven years after the surgery, the patient remains tumor-free.
Discussion
Gastric mesenchymal tumors are much rarer than
gastric epithelial tumors. However, gastric mesenchymal tumors
include both benign and malignant tumors. Among them,
gastrointestinal stromal tumors (GIST) are the most common type of
mesenchymal tumor, and most GIST exhibit benign behavior so there
is little difficulty with their pathological diagnosis. Conversely,
gastric sarcomas are reported to account for <1–3% of all
gastric tumors, and they are difficult to diagnose based on their
clinical and pathological findings (4). UPS is the commonest type of high-grade
malignant sarcoma found in elderly people and most are asymptomatic
but some case was reported fever as chief complaint because UPS
producing activity cytokines as G-CSF (5). It is characterized as a pleomorphic
sarcoma involving spindle-shaped cells, which is not very
disease-specific, and does not exhibit marked cellular
differentiation. Therefore, it is essentially diagnosed by
exclusion, so it is necessary to carefully exclude other sarcomas
whose histology overlap with that of UPS using electron microscopy
and immunohistochemical techniques.
Regarding primary sarcoma of gastric origin, the
main differential diagnoses for such cases include leiomyosarcoma
and malignant peripheral nerve sheath tumors. However, in the
present case these tumors were excluded because of the lack of
neurogenic marker and smooth muscle marker expression (6,7). In
addition, a rare type of malignant GIST, dedifferentiated GIST, in
which a high-grade malignant sarcoma component is found in an
ordinary GIST, was also considered; however, it was excluded due to
the lack of a typical GIST component and the fact that the tumor
was completely negative for c-kit (8). In this case, multiple tumors were
detected in the abdominal cavity, including both simultaneous and
metachronous tumors. Therefore, we also had to consider
dedifferentiated liposarcoma (DLPS), which is a more common type of
sarcoma of intra-abdominal/retroperitoneal origin that can involve
the stomach and produce multiple tumor nodules. Although the
histology of the dedifferentiated component of DLPS is
indistinguishable from that of UPS, it typically contains a
well-differentiated component, which exhibits marked adipocytic
differentiation. Cytogenetically, DLPS is characterized by gene
amplification of the 12q13-15 region, resulting in the
overexpression of MDM2 and CDK4 (9).
In the current case, DLPS was excluded because the tumor lacked
adipocytic differentiation and did not overexpress MDM2 or
CDK4.
Although there are no CT findings specific to UPS as
far as we know, previous review of peritoneal sarcomatosis reported
that peritoneal implants and mesenteric involvement were
well-defined, and neither diffuse thickening nor calcifications
(10). Also in our case, tumors were
found to have well-defined shapes and central hypo-density areas as
common characteristics of peritoneal sarcomas. The other report
indicated the apparent diffusion coefficient (ADC) values
caluculated from diffusion-weighted MRI was independent prognostic
factor in gastric cancer (11). We
might be able to apply ADC value of gastric UPS for prognostic
indication.
With regard to the evaluation of the tumor's
histological grade, the UPS was a large undifferentiated tumor
containing frequent mitoses and extensive necrosis. According to
the French Federation of Comprehensive Cancer Centers grading
system for soft tissue sarcomas, it exhibited a tumor
differentiation score of 3, a mitotic count score of 3, and a
necrosis score of 2 and had an overall classification of grade 3,
which is the highest grade (12). It
is worth specifically mentioning that the patient in this case
demonstrated an unexpectedly good prognosis in spite of the tumor's
high-grade nature and the presence of intra-abdominal
dissemination.
A review of the literature revealed 8 reported cases
of primary gastric UPS (13–18). Based on an analysis of the 9 reported
cases of primary gastric UPS, including ours, the mean age of the
patients was 68 years (range: 42–79 years), and the male-to-female
ratio was 8:1 (Table I). The tumors
measured between 3.5 and 12.0 cm in maximum diameter. As for the
location of the sarcoma, it was located on the gastric body in 5
cases. There were no cases in which neoadjuvant or adjuvant
chemotherapy was administered.
 | Table I.Cases of primary gastric
undifferentiated pleomorphic sarcoma. |
Table I.
Cases of primary gastric
undifferentiated pleomorphic sarcoma.
| Authors | Age (years)/sex | Tumor size | Location | Type of
operation | Gross features | Chemotherapy | Prognosis | (Refs.) |
|---|
| Agaimy et
al | 79/M | 8×7.5 cm | U | Total
gastrectomy | Polypoid ulcerated
and intramural, whitish | None | Died 2 weeks after op
for pulmonary embolism | (13) |
| Agaimy et
al | 68/M | 12×9 cm | M, Gre | Distal
gastrectomy | Extramural, gastric
wall infiltrated, cystic areas, fleshy whitish-yellow | None | No MTS, alive 6
months after op | (13) |
| Wada et
al | 78/M | 5.5×5 cm | M, post | Partial
gastrectomy | Ulcerated,
whitish | None | No MTS, alive 2 years
after op | (14) |
| Wada et
al | 77/M | 4×4 cm | M, Ant | Partial
gastrectomy | Ulcerated,
yellowish-white | None | No MTS, died 4 years
after op for pneumonia | (14) |
| Rathakrishnan et
al | 51/M | ND | ND | Feeding jejunostomy
(inoperable) | ND | None | Died a few weeks
after op | (15) |
| Wright et
al | 42/M | 5 cm | ND | Partial
gastrectomy | Polypoid ulcerated,
whitish | None | No MTS, died 17
months after op | (16) |
| Shibuya et
al | 60/M | 4.5×4 cm | M, Ant | Total
gastrectomy | Polypoid, solid,
whitish | None | No MTS, died 3 months
after op | (17) |
| Morita et
al | 60/F | 3.5×2.2 cm | M, Ant | Gatrectomy | Solid, yellowish | None | Recurrence 5 months
after op, died 7 months after op | (18) |
| Present case | 70/M | 14.0× 12.0 cm | U, Ant | Total
gastrectomy | Central necrosis,
solid, grayish-white | None | Recurrence 6 months
after op, alive 7 years after op |
|
This was a very significant case, in that we were
able to control a high-grade primary gastric sarcoma using surgical
resection alone. The administration of adjuvant or neoadjuvant
chemotherapy has been widely adopted as a treatment strategy for
soft tissue sarcomas; however, there is no standard chemotherapy
regimen for abdominal UPS, and we decided not to administer
chemotherapy in the present case based on the low response rate of
such tumors and the potential complications of chemotherapy
(19,20). According to the National
Comprehensive Cancer Network soft tissue sarcoma guidelines,
complete surgical resection with appropriate negative margins is
the standard primary treatment for UPS (21). In spite of smaller surgical margin of
gastric sarcoma than soft tissue sarcoma located at upper limbs or
lower limbs, we were able to control this tumor via resection
alone.
To the best of our knowledge, this is the first
report about a case of primary gastric UPS involving positive
peritoneal washing cytology results. Although the patient's good
clinical course is difficult to explain based on the fact that the
tumor was a high-grade sarcoma and peritoneal washing cytology
produced positive results, we speculate that abdominal sarcomas
like UPS do not have an invasive nature despite their high
histological grade, which is unusual for this type of tumor. In the
current case, continuous clinical observation for recurrence or
metastasis will be necessary in case the tumor becomes more
malignant.
In conclusion, this case deserves special mention,
as it involved a high-grade sarcoma that was not treated with
chemotherapy, and yet the patient exhibited a good prognosis.
Clinicians should be aware that some sarcomas might follow a
favorable course.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets obtained and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YO, HC and TM analyzed patient data and wrote the
manuscript. RO, HO and RT collected the data and critically revised
the manuscript.
Ethics approval and consent to
participate
This case report was approved by the institutional
review board, and written informed consent was obtained from the
patient.
Patient consent for publication
Written consent for publication was obtained from
the patient.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
UPS
|
undifferentiated pleomorphic
sarcoma
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
EUS
|
endoscopic ultrasound
|
|
FNB
|
fine-needle biopsy
|
|
MDM2
|
mouse double minute 2 homolog
|
|
CDK4
|
cyclin-dependent kinase 4
|
|
GIST
|
gastrointestinal stromal tumors
|
|
DLPS
|
dedifferentiated liposarcoma
|
References
|
1
|
Fletcher CDM, Bridge JA, Hogendoorn P and
Mertens F: WHO classification of tumours of soft tissue and bone.
(4th). IARC Press. (Lyon). pp120–122. 2013.
|
|
2
|
O'Brien JE and Stout AP: Malignant fibrous
xanthomas. Cancer. 17:1445–1455. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sano T and Aiko T: New Japanese
classifications and treatment guidelines for gastric cancer:
Revision concepts and major revised points. Gastric Cancer.
14:97–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soufi M, Errougani A and Chekkof RM:
Primary gastric leiomyosarcoma in young revealed by a massive
hematemesis. J Gastrointest Cancer. 40:69–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kabashima A, Kimura K, Sanefuji K,
Masunari S, Haraoka S and Maekawa S: A case of primary gastric
undifferentiated high-grade pleomorphic sarcoma diagnosed with
chief complaint of fever: A case report and literature review. Surg
Case Rep. 3:412017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Welch JP: Smooth muscle tumors of the
stomach. Am J Surg. 130:279–285. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farid M, Demicco EG, Garcia R, Ahn L,
Merola PR, Cioffi A and Maki RG: Malignant peripheral nerve sheath
tumors. Oncologist. 19:193–201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Virgilio E, Mercantini P, Tarantino G,
Socciarelli F, Pilozzi E, Uccini S, Caterino S and Ziparo V:
Gastrointestinal stromal tumors with de novo anaplastic
dedifferentiation: Considerations on a little-known neoplastic
metamorphosis. Int J Surg Pathol. 22:3852014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McGovern Y, Zhou CD and Jones RL: Systemic
therapy in metastatic or unresectable
well-differentiated/dedifferentiated liposarcoma. Front Oncol.
7:2922017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Villanueva A, Pérez C, Sabaté JM, Llauger
J and Monill JM: CT manifestations of peritoneal
leiomyosarcomatosis. Eur J Radiol. 17:166–169. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giganti F, Orsenigo E, Esposito A, Chiari
D, Salerno A, Ambrosi A, Albarello L, Mazza E, Staudacher C, Del
Maschio A, et al: Prognostic role of diffusion weighted MR imaging
for resectable. Gastric cancer. Radiology. 276:444–452. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Italiano A, Delva F, Mathoulin-Pelissier
S, Le Cesne A, Bonvalot S, Terrier P, Trassard M, Michels JJ, Blay
JY, Coindre JM and Bui B: Effect of adjuvant chemotherapy on
survival in FNCLCC grade 3 soft tissue sarcomas: A multivariate
analysis of the French Sarcoma Group database. Ann Oncol.
21:2436–2441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agaimy A, Gaumann A, Schroeder J,
Dietmaier W, Hartmann A, Hofstaedter F, Wünsch PH and Mentzel T:
Primary and metastatic high-grade pleomorphic sarcoma/malignant
fibrous histiocytoma of the gastrointestinal tract: An approach to
the differential diagnosis in a series of five cases with emphasis
on myofibroblastic differentiation. Virchows Arch. 451:949–957.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wada Y, Matsushita T, Sarumaru S, Ryo J,
Isobe H, Satoh B, Kanaya S, Katayama T and Ohtoshi M: Malignant
fibrous histiocytoma of the stomach: Report of two cases. Surg
Today. 28:296–300. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rathakrishnan V, Arianayagam S and Kumar
G: Primary malignant fibrous histiocytoma of the stomach: (A case
report). Australas Radiol. 33:302–304. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wright JR Jr, Kyriakos M and
DeSchryver-Kecsemeti K: Malignant fibrous histiocytoma of the
stomach. A report and review of malignant fibrohistiocytic tumors
of the alimentary tract. Arch Pathol Lab Med. 112:251–258.
1988.PubMed/NCBI
|
|
17
|
Shibuya H, Azumi N, Onda Y and Abe F:
Multiple primary malignant fibrous histiocytoma of the stomach and
small intestine. Acta Pathol Jpn. 35:157–164. 1985.PubMed/NCBI
|
|
18
|
Morita T, Kato T, Furukawa K and Itoh Y: A
case report of malignant fibrous histiocytoma in the regions of
stomach and gall bladder. J Jpn Soc Clin Cytol. 23:425–429. 1984.
View Article : Google Scholar
|
|
19
|
Pasquali S and Gronchi A: Neoadjuvant
chemotherapy in soft tissue sarcomas: Latest evidence and clinical
implications. Ther Adv Med Oncol. 9:415–429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo J, Cui Q, Liu C, Sui J, Jiang N, Zhou
J, Li D and Zeng Y: Clinical report on transarterial neoadjuvant
chemotherapy of malignant fibrous histiocytoma in soft tissue. Clin
Transl Oncol. 15:370–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
NCCN Guidelines for Patients. Soft Tissue
Sarcoma. Version 1. 2014, https://www.nccn.org/patients/guidelines/sarcomaApril
12–2018
|