Introduction
Oligosaccharides are frequently found as components
of glycoproteins or glycolipids; therefore, these molecules are
often used as chemical markers, particularly for cell recognition.
When oligosaccharides are consumed, any undigested remnants act as
fuel for the intestinal microflora. The effects exerted on the
bacterial groups may be either stimulatory or suppressive,
depending on the type of oligosaccharide (1,2).
Clinical studies have demonstrated that the administration of
fructo-oligosaccharides, galacto-oligosaccharides or inulin can
enhance the levels of useful intestinal bacteria and simultaneously
reduce the number of harmful bacteria (3,4).
The sialyl-Lewis(x) oligosaccharide, identified in a
study in 2011 (5), is believed to
be the most abundant carbohydrate receptor in the outer coating, or
zona pellucida (ZP), of human female ova. This oligosaccharide has
been suggested to play a pivotal role in the binding of sperm to
the ZP, leading to fertilization. Sialyl-Lewis(x) branches are more
abundant on the ova than on any other type of body cell, forming a
complete coating. The identification this prominently featuring
receptor on the human ova may enhance research into the field of
infertility (5).
In numerous organisms, the production of energy for
the fueling of biological processes is dependent on oxidation.
However, the uncontrolled production of superoxide anion free
radicals is involved in the onset of a number of diseases,
including cancer, atherosclerosis and degenerative processes
associated with aging (6). Thus,
it is essential to develop effective and natural antioxidants to
protect the human body from free radicals and certain chronic
diseases (7).
Hericium erinaceus is a type of fungi
belonging to the Hericium family, which grows in Xiaojin
county of Sichuan in China at altitudes of ~3,700 m (8). In the present study, water-soluble
oligosaccharides were extracted and purified from the fruiting
bodies of Hericium erinaceus using diethylaminoethyl
(DEAE)-cellulose and Sephadex G-200 column chromatography. To the
best of our knowledge, the present study is the first to
characterize the chemical structure of the extracted Hericium
erinaceus oligosaccharide (HEO-A). The antioxidant
activity of HEO-A was evaluated using three biochemical
methods.
Materials and methods
Chemicals
The fruiting bodies of Hericium erinaceus
were collected in Xiaojin county of Sichuan, China, and were
authenticated by Professor Zhirong Yang (College of Life Sciences,
Sichuan University, Chengdu, China). At the same time, a voucher
specimen was preserved in the Key Laboratory of Southwest China
Wildlife Resources Conservation (Nanchong, China). DEAE-cellulose
52 and Sephadex G-200 were purchased from Sigma-Aldrich (Shanghai,
China). Monosaccharide standards and Dextran T-500, T-110, T-70,
T-40, and T-10, were purchased from Beijing Biodee Biotechnology
Co., Ltd. (Beijing, China). All other reagents used were of
analytical grade.
Extraction, purity and fractionation of
oligosaccharides from Hericium erinaceus
Once the fruiting bodies (200 g) of Hericium
erinaceus were soaked with 95% ethanol (EtOH), the residue was
dried and then extracted with boiling water three times (3 h each).
Following the concentration, dialysis and centrifugation of the
filtrate, three equivalents of 95% EtOH were added to the
supernatant to precipitate the crude oligosaccharides (HEO, 21.7 g;
recovery, 10.85%). Following deproteination using the Sevag method
(9), LEO (5 g) was purified using
a DEAE-cellulose column (Tris-HCl, pH 7.0, 4.5×50 cm,
Cl−) and eluted stepwise with 0, 0.1, 0.2, 0.3, 0.4, 0.5
and 1.0 M NaCl. The eluate was monitored by the phenol-sulfuric
acid method (10). The 0 M NaCl
eluate was concentrated, lyophilized and purified on a Sephadex
G-200 column (2.6×60 cm). The resulting HEO-A was obtained by the
above processes at a yield rate of 0.12% (0.24 g) for the starting
material.
Assessment of molecular weight
High-performance gel permeation chromatography was
performed to measure the molecular weight of HEO-A (11). The column was calibrated with
standard T-series Dextran (T-500, T-110, T-70, T-40 and T-10). Data
were processed using the Waters Millennium32 Gel Permeation
Chromatography software (Waters Corp. Milford, MA, USA).
Monosaccharide composition analysis
The HEO-A (5.0 mg) was hydrolyzed with 2 M
trifluoroacetic acid at 110°C for 6 h by acid-catalyzed hydrolysis
(12). Excess acid was removed by
co-distillation with methyl alcohol following the completion of
hydrolysis. The hydrolysate was used for thin layer chromatography
(TLC) analysis as previously described (13) using
acetoacetate-pyridine-EtOH-water (8:5:1.5:1) as the developing
solvent and a diphenylamine-aniline system [85% phosphoric acid
solution (140 ml) containing 8 ml diphenylamine and 8 g aniline] as
the developer system.
Polarimetry (ORD), ultraviolet (UV) and
infrared (IR) spectroscopic analysis
HEO-A was assessed using UV spectroscopy at
wavelengths of 200–600 nm. IR analysis of the HEO sample was
obtained by grinding a mixture of oligosaccharide with dry KBr and
then pressing into a mold. Spectra were run in the 4,000–400
cm−1 range (14). ORD
analysis of the HEO sample was obtained using using an HK7_SGW_1
automatic optical polarimeter (Shanghai Jingke Scientific
Instrument Co., Ltd., Shanghai, China) at room temperature.
Nuclear magnetic resonance (NMR)
experiment
1H-NMR and 13C-NMR spectra
were recorded on a Varian Unity INOVA 400/45 (Varian Medical
Systems, Palo Alto, CA, USA) in D2O with
tetramethylsilane as the internal standard (15).
Assessment of the
1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging
activity of HEO-A
The DPPH− radical scavenging activity of
HEO-A was measured according to the method described by Braca et
al (16). The percentage
scavenging activity was calculated using the following formula:
Scavenging effect (%) = (1−A sample/A control) ×100,
where A control is the absorbance of the control (DPPH
solution without sample) and A sample is the absorbance of
the test sample (DPPH solution plus test sample or positive
control). Vitamin c (Vc) and butylated hydroxytoluene (BHT) were
used as positive controls.
Assessment of the
2,2′-azino-bis(3-ethylbenzthiazoline-6-sufonic acid) diammonium
(ABTS) radical scavenging activity
To determine the ABTS+ scavenging
activity, the assay was performed as described by Auddy et
al (14). ABTS+
radicals were produced by reacting ABTS and ammonium persulfate and
incubating the mixture at room temperature in the dark for 16 h. A
total of 2 ml HEO-A solution at different concentrations and 2 ml
ABTS+ radical solution (0.7 mM) were then added. The
absorbance was measured immediately at 734 nm. A control reaction
was performed without the extract. The percentage scavenging of
ABTS+ radicals was calculated as follows: Scavenging
effect (%) = [1-(A sample − A sample blank)/A
control] ×100, where A control was the absorbance of the
control group in the ABTS+ radical generation system,
A sample was the absorbance of the test group and A
sample blank was the absorbance of the sample only. Vc was used as
a positive control.
Assessment of the hydroxyl radical
scavenging activity
The ability of HEO-A to scavenge hydrogen peroxide
was determined according to the method of Smirnoff and Cumbes
(17). The percentage scavenging
of hydroxyl radicals was calculated as follows: Scavenging effect
(%) = [1-(A sample − A sample blank)/A
control] ×100, where A control was the absorbance of the
control group in the hydroxyl radical generation system, A
sample was the absorbance of the test group and A sample
blank was the absorbance of the samples only. Vc was used as a
positive control.
Statistical analysis
All values are expressed as the mean ± standard
deviation of three replications. Statistical analyses were
performed using the Student’s t-test and one-way analysis of
variance. Values of P<0.05 were considered to indicate a
statistically significant difference.
Results and Discussion
Extraction, purity and composition of
oligosaccharides
The crude oligosaccharide was obtained from the
fruiting bodies of Hericium erinaceus with a yield of 8.7%.
Following fractionation using DEAE-cellulose 52 and Sephadex G-200
column chromatography, 180 mg HEO-A was obtained from the 0 M NaCl
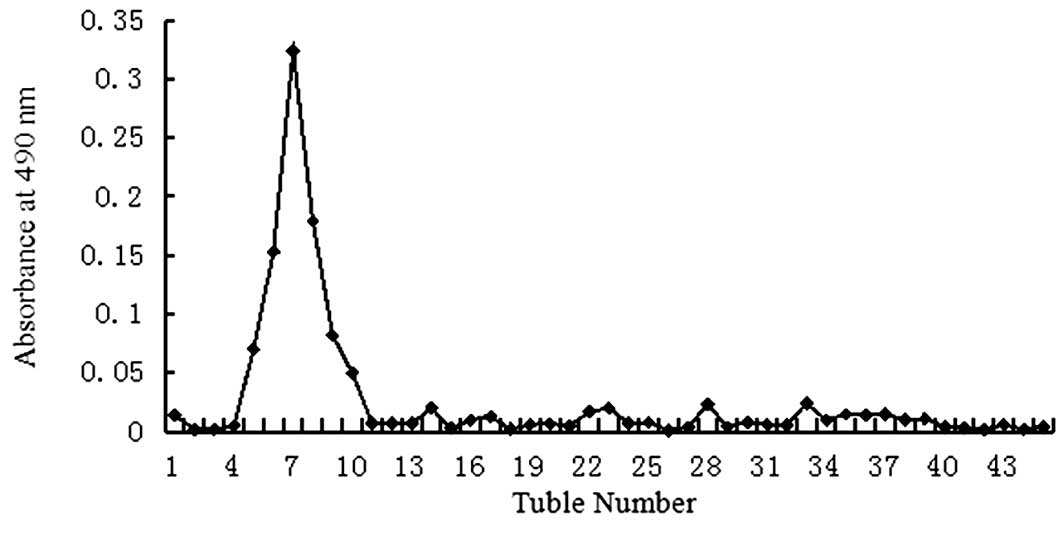
eluate and detected using the phenol-sulfuric acid assay as a
single peak (Figs. 1 and 2). The homogeneity of the oligosaccharide
was then elucidated. An absence of absorption at 280 and 260 nm in
the UV absorption spectra of HEO-A demonstrated the absence of
protein and nucleic acid in this oligosaccharide. In addition,
HEO-A exhibited the same optical rotation,
[α]20D -11.2° (concentration, 0.5 g/100 ml
water), in different low concentrations (5, 10 and 15%) of EtOH, as
shown using an HK7-SGW-1 automatic optical polarimeter at room
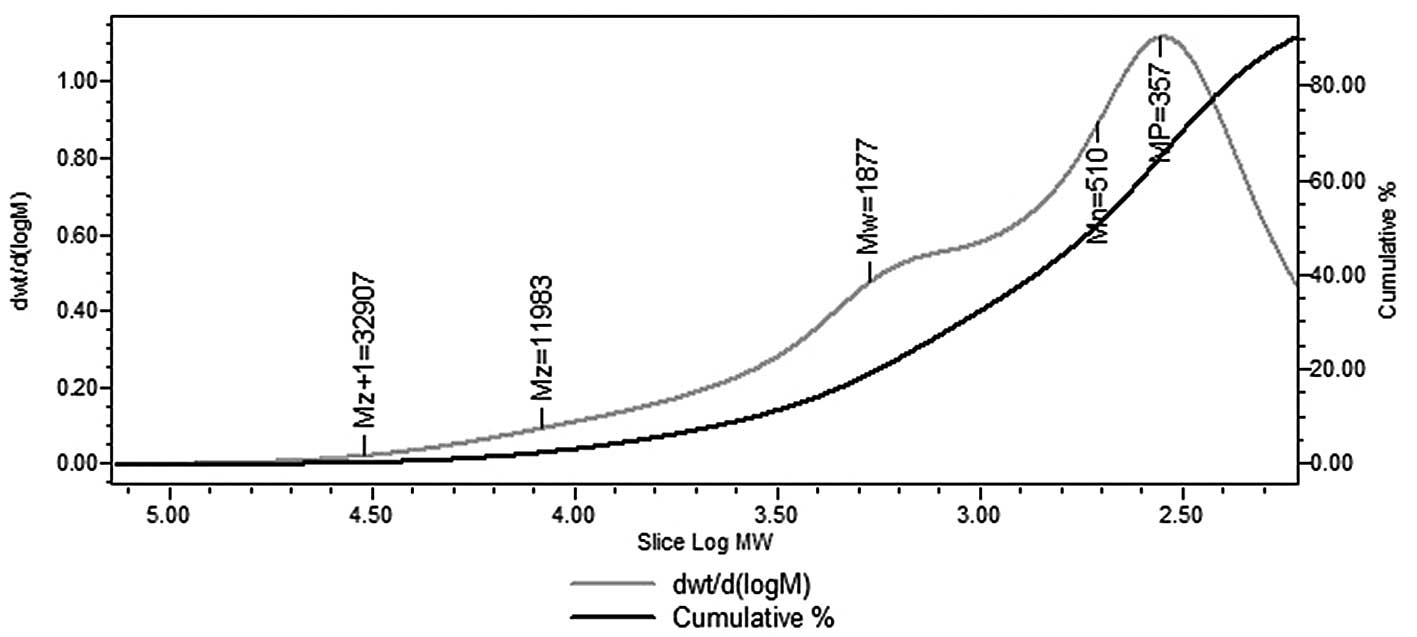
temperature. The weight-average molecular weight of HEO-A was
~1,877 Da (Fig. 3). The two
monosaccharides, D-glucose (D-Glu) and D-xylose (D-Xyl), were
identified by analyzing the hydrolysate of HEO-A using TLC.
Structure elucidation of HEO-A
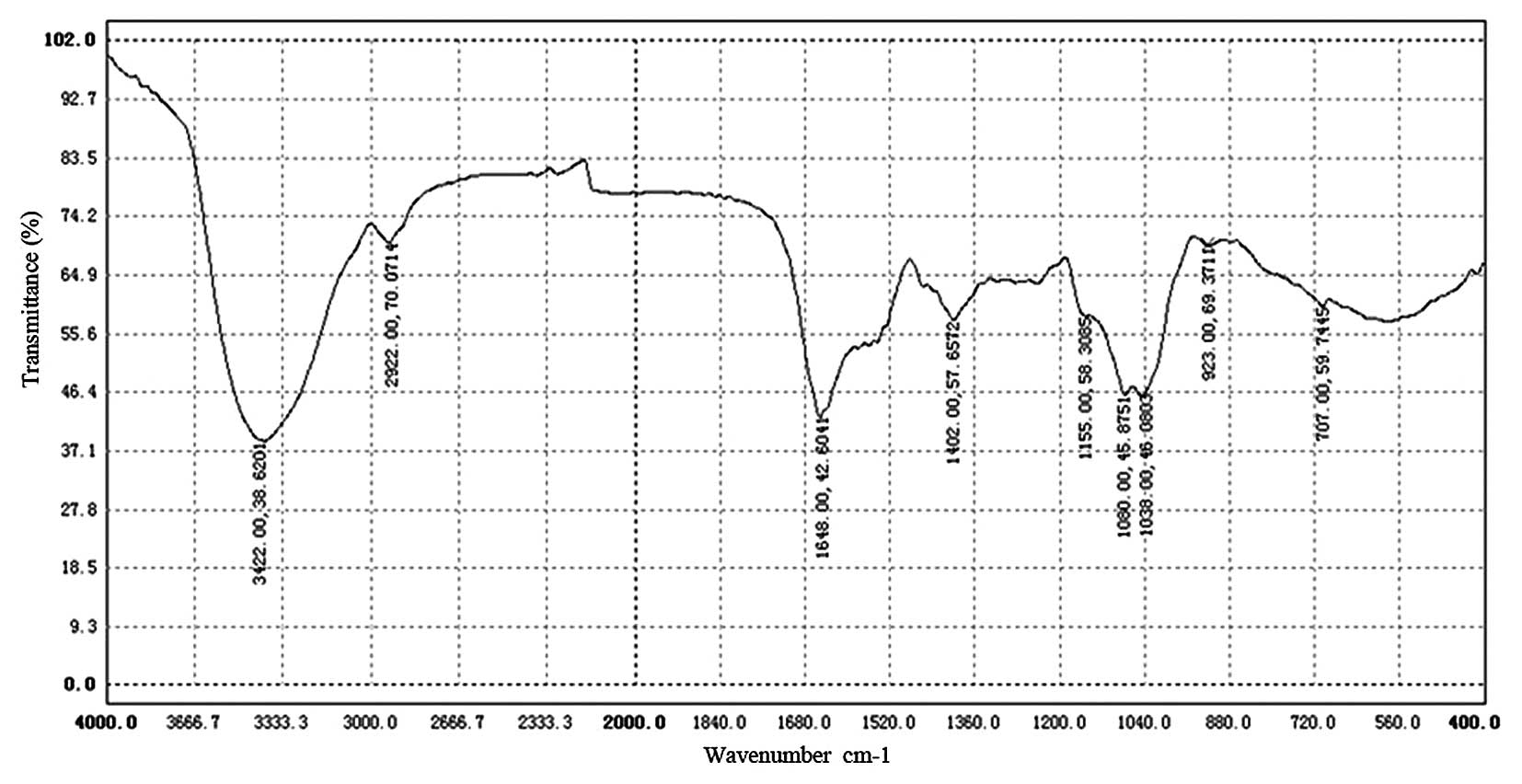
The intensity of bands around 3,422.38
cm−1 in the IR spectrum (Fig. 4) was due to the hydroxyl stretching
vibration of the oligosaccharide and, as expected, they were broad.
The bands in the region of 2,922.70 cm−1 were due to a
C-H stretching vibration and the bands in the region of 1,648.42
cm−1 were due to associated water (18). The strong absorption bands at
1,155.58 and 1,060.45 cm−1 in the range of 1,200–1,000
cm−1 in the IR spectrum suggested that the
monosaccharide in HEO-A had a pyranose ring. The strong absorption
bands at 1,402.57 cm−1 were due to the C-H bending
vibration, and the bands in the region of 923.69–707.59
cm−1 were due to the C-H rocking vibration. In addition,
the characteristic absorptions at 707.59 cm−1 indicated
α-configurations existing in the oligosaccharide, which was
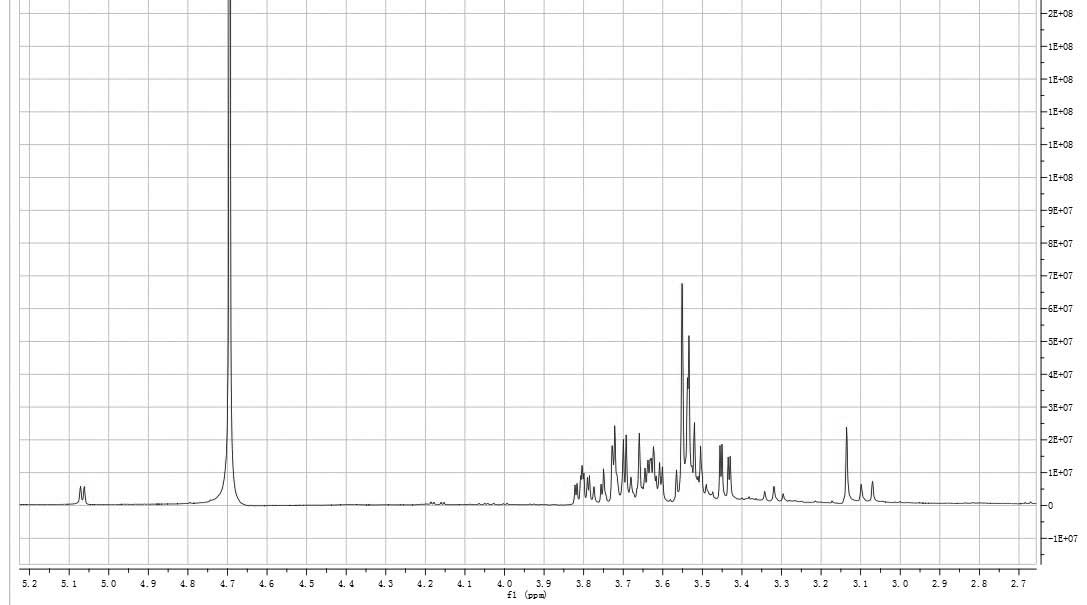
consistent with the anomeric proton signals at δ 5.06 and 5.07 in
the 1H-NMR (400 MHz) spectrum (Fig. 5). The signals at δ 3.0–3.9 were the
signal peaks of remaining protons, which were mostly formed by a
number of overlapping signal peaks. Among them, the signals at δ
3.79, 3.78 and 3.77 were ascribed to the α-H of the methyl group in
xylose and the signals at δ 3.55 corresponded with the β-H of the
methyl group in xylose. Signals at δ 3.53, 3.52 and 3.45 were the
signal peaks of the other hydrogen atoms in xylose. The signals at
δ 3.65 and 3.63 were the signal peaks of the hydroxy-methyl group
in glucose and the signals at δ 3.80 indicated the hydrogen atom of
C-3 of glucose. The hydrogen signal of water was observed at δ
4.67. The 1H-NMR data were consistent with
monosaccharide analysis data. According to previous studies, the
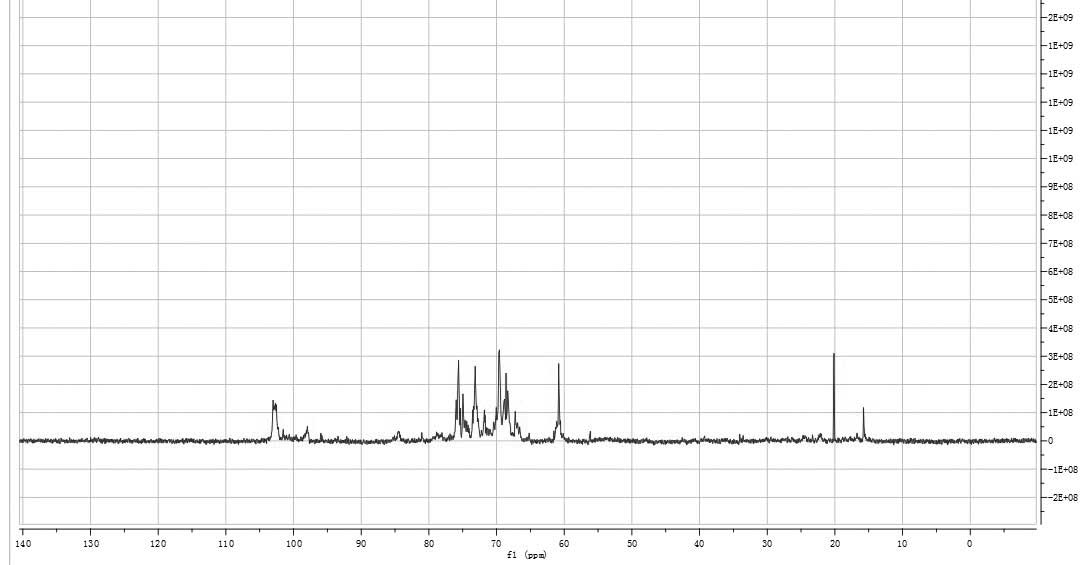
resonances in the region of 95–100 ppm in the 13C NMR
(400 MHz) spectrum of HEO-A were attributed to the anomeric carbon
atoms of D-Glu and D-Xyl (Fig. 6)
(19).
Determination of the DPPH−
radical scavenging activity of HEO-A
Previous studies have shown that oligosaccharides
composed of different monosaccharides and with different molecular
weights may exhibit different antioxidant activities, particularly
in terms of scavenging free radicals (20). As excess free radicals are harmful
to human health, the free radical scavenging activity of the
extracted oligosaccharide against DPPH−, hydroxyl
radicals and superoxide anions was evaluated.
The stable DPPH− radical is commonly used
to measure the antioxidant activity of a sample (21). Furthermore, the DPPH−
assay is a more time-efficient method than other strategies. In the
presence of hydrogen-donating antioxidants, the characteristic
purple of an alcoholic solution containing DPPH−
radicals and unpaired electrons changes to yellow. This
discoloration occurs due to the single paired electrons. Thus, the
DPPH− radical scavenging activity of HEO-A was
determined by measuring the discoloration and absorbance values at
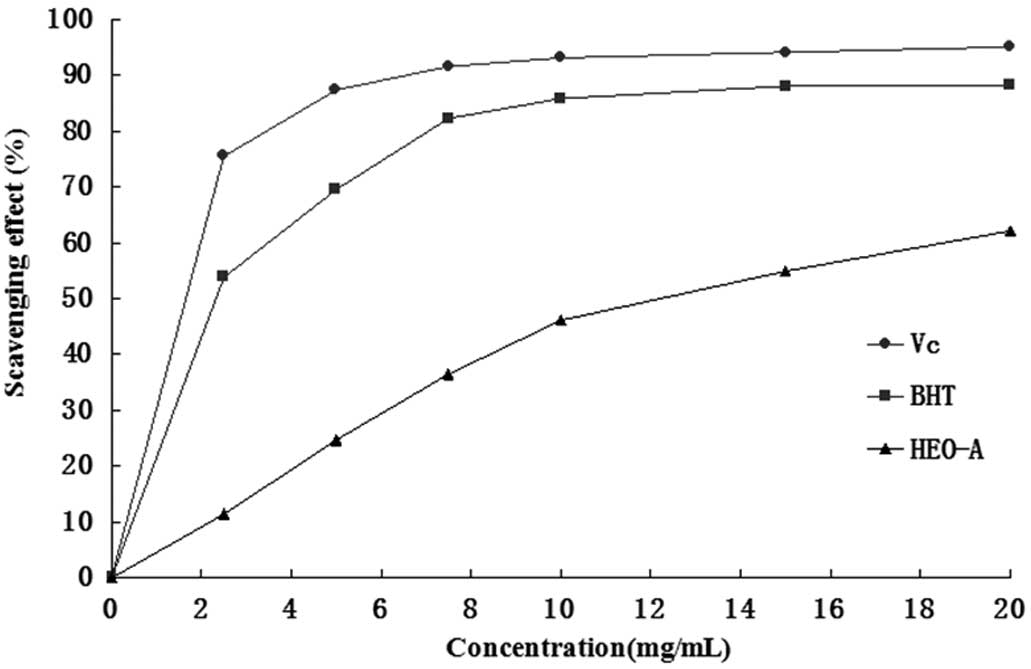
517 nm, and the results were compared with those of BHT. Fig. 7 shows the scavenging activity of
the purified oligosaccharide samples on the DPPH−
radical. These results showed that the IC50 value of
HEO-A for eliminating DPPH− radicals was ~12.5 mg/ml,
which indicated that HEO-A had a notable effect on scavenging
DPPH− radicals, particularly when added at high
quantities. However, the inhibition ability was lower than that of
BHT and Vc.
Assessment of the ABTS+
radical cation scavenging activity of HEO-A
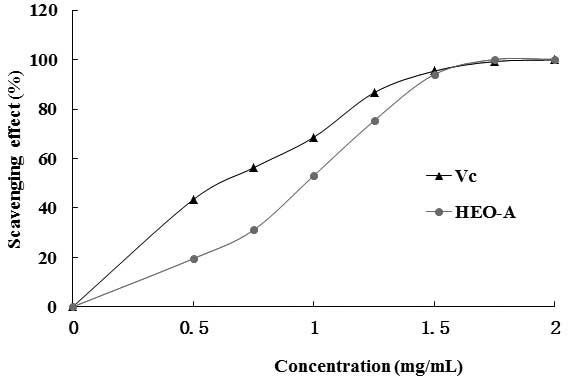
The ABTS+ radical scavenging activity of
HEO-A was measured spectrophotometrically at 734 nm. Upon
interaction with the extract at various concentrations, the
absorbance of the ABTS+ radical cations was decreased
dose dependently, and the IC50 value of HEO-A was 0.93
mg/ml (Fig. 8). However, the
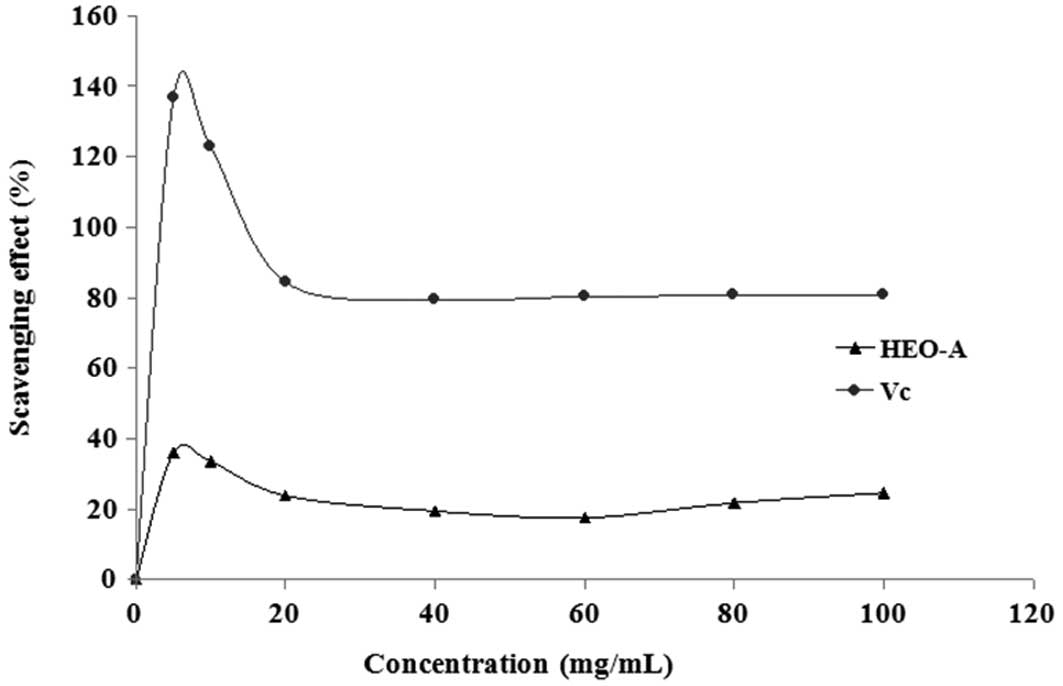
scavenging activity of HEO-A was lower than that of Vc.
Assessment of the hydroxyl radical
scavenging activity of HEO-A
The hydroxyl radical is one of the most reactive and
dangerous reactive oxygen species to human health. The hydroxyl
radicals generated through the Fenton reaction in this system were
scavenged by HEO-A. Fig. 9 shows
the percentage hydroxyl radical scavenging effects of HEO-A at
different doses. At the test concentrations, HEO-A exhibited a
concentration-dependent scavenging effect on the hydroxyl radicals,
which showed that the purified oligosaccharide exhibited weaker
hydroxyl radical-scavenging effects than Vc at the same dose.
Conclusion
According to the results above, Hericium
erinaceus may be introduced as a possible valuable source of
oligosaccharides, which exhibits unique antioxidant properties.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 31200012 and 31400016), the
Application Foundation Project of Sichuan Province (grant no.
2013JY0094), the Science and Technology Support Project of Sichuan
Province (grant nos. 2014SZ0020 and 2014FZ0024), The Cultivate
Major Projects of Sichuan Province (grant no. 14CZ0016), and the
Doctor Startup Foundation Project of China West Normal University
(grant nos. 11B019 and 11B020).
References
|
1
|
Bode L: Human milk oligosaccharides:
prebiotics and beyond. Nutr Rev. 67(Suppl 2): S183–S191. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Filippo C, Cavalieri D, Di Paola M,
Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G and
Lionetti P: Impact of diet in shaping gut microbiota revealed by a
comparative study in children from Europe and rural Africa. Proc
Natl Acad Sci USA. 107:14691–14696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Macfarlane GT, Steed H and Macfarlane S:
Bacterial metabolism and health-related effects of
galacto-oligosaccharides and other prebiotics. J Appl Microbiol.
104:305–344. 2008.PubMed/NCBI
|
|
4
|
Bertozzi CR and Kiessling LL: Chemical
glycobioloy. Science. 291:2357–2364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pang PC, Chiu PC, Lee CL, et al: Human
sperm binding is mediated by the sialyl-Lewis(x) oligosaccharide on
the zona pellucida. Science. 333:1761–1764. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mau JL, Tsai SY, Tseng YH and Huang SJ:
Antioxidant properties of hot water extracts from Ganoderma tsugae
Murrill. LWT - Food Sci Technol. 38:589–597. 2005. View Article : Google Scholar
|
|
7
|
Kinsella JE, Frankel EN, German JB and
Kanner J: Possible mechanisms for the protective role of
antioxidants in wine and plant foods. Food Technol. 47:85–89.
1993.
|
|
8
|
Fan WW and Huang HH: Advances on Hericium
erinaceus Polysaccharides. Food science. 29:355–358. 2008.
|
|
9
|
Staub AM: Removal of protein - Sevag
method. Methods in Carbohydrate Chemistry Volume 5: General
Polysaccharides. Whistler RL: Academic Press; New York, NY: pp.
5–6. 1965
|
|
10
|
Dubois M, Gillis KA, Hamilton JK, Rebers
PA and Smith F: Colorimetric method for determination of sugars and
related substances. Anal Chem. 28:350–356. 1956. View Article : Google Scholar
|
|
11
|
Yamamoto Y, Nunome T, Yamauchi R, Kato K
and Sone Y: Structure of an exocelluluar polysaccharide of
Lactobacillus helveticus TN-4, a spontaneous mutant strain of
Lactobacillus helveticus TY1–2. Carbohydr Res. 275:319–332. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu RM, Yin Y, Yang W, Ma WL, Yang L, Chen
XJ, Zhang Z, Ye B and Song LY: Structural elucidation and
biological activity of a novel polysaccharide by alkaline
extraction from cultured Cordyceps militaris. Carbohydr Polym.
75:166–171. 2009. View Article : Google Scholar
|
|
13
|
Partridge SM: Aniline hydrogen phthalate
as spraying reagent for chromatography of sugars. Nature.
164:4431949. View
Article : Google Scholar
|
|
14
|
Auddy B, Ferreira M, Blasina F, Lafon L,
Arredondo F, Dajas F, Tripathi PC, Seal T and Mukherjee B:
Screening of antioxidant activity of three Indian medicinal plants,
traditionally used for the management of neurodegenerative
diseases. J Ethnopharmacol. 84:131–138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao W, Li XQ, Liu L, Wang M, Fan HT, Li C,
et al: Structural analysis of water-soluble glucans from the root
of Angelica sinensis (Oliv. ) Diels Carbohydr Res. 341:1870–1877.
2006. View Article : Google Scholar
|
|
16
|
Braca A, De Tommasi N, Di Bari L, Pizza C,
Politi M and Morelli I: Antioxidant principles from Bauhinia
terapotensis. J Nat Prod. 64:892–895. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smirnoff N and Cumbes QJ: Hydroxyl radical
scavenging activity of compatible solutes. Phytochemistry.
28:1057–1060. 1989. View Article : Google Scholar
|
|
18
|
Barker SA, Bourne EJ, Stacey M and Whiffen
DH: Infra-red spectra of carbohydrates. Part I Some derivatives of
D-glucopyranose. J Chem Soc. 1954:171–176. 1954. View Article : Google Scholar
|
|
19
|
Wang ZJ, Luo DH and Liang ZY: Structure of
polysaccharides from the fruiting body of Hericium erinaceus Pers.
Carbohydr Poly. 57:241–247. 2004. View Article : Google Scholar
|
|
20
|
Chen Y, Xie MY, Nie SP, Li C and Wang YX:
Purification, composition analysis and antioxidant activity of a
polysaccharide from the fruiting bodies of Ganoderma atrum. Food
Chem. 107:231–241. 2008. View Article : Google Scholar
|
|
21
|
Ye H, Wang KQ, Zhou CH, Liu J and Zeng XX:
Purification, antitumor and antioxidant activities in vitro of
polysaccharides from the brown seaweed Sargassum pallidum. Food
Chem. 111:428–432. 2008. View Article : Google Scholar
|