Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic and
progressive interstitial lung disease with unknown causes. IPF is
characterized by persistence of myofibroblasts in the lung, chronic
scar formation, and deposition of extracellular matrix proteins,
such as collagen. The median mortality of IPF patients is ~3 years
following diagnosis (1–4). At least five million individuals are
affected by IPF worldwide (5).
Currently, there are no effective treatments to improve the
survival rate of IPF patients. Thus, novel therapeutic strategies
are highly desirable.
Transforming growth factor-β (TGF-β) is considered
an important cytokine in the pathogenesis of IPF. The cytokine
interacts with a series of serine/threonine receptors, which are
part of a family of related receptor molecules termed activin
receptor-like kinases (ALKs) (6).
Inhibition of ALKs results in abrogation of the biological activity
of TGF-β. Since TGF-β signaling via the interaction with ALK-5
plays a fundamental role in mediating profibrotic responses in
healthy fibroblasts, pharmacologic inhibition of the receptor
kinase may represent a novel targeted approach for the control of
fibrosis.
Previous studies have shown that SB 431542, an ALK-5
inhibitor, is efficient in blocking TGF-β-mediated myofibroblast
differentiation and collagen expression (7–9). The
inhibitor significantly reduced the expression of fibrosis-related
genes in rat hepatic stellate cells (10). In this study, we aimed to determine
the effect of SB 431542 on pulmonary fibrosis, both in vitro
and in vivo, by using a TGF-β-induced cell model and a
bleomycin-induced mouse model, respectively.
The pulmonary reaction that follows intratracheal
administration of bleomycin in experimental animals has been
extensively used as a model of human pulmonary fibrosis (11), and administration of bleomycin is
typically associated with an increase in the synthesis and release
of TGF-β (12). The development of
pulmonary fibrosis is thought to comprise two phases: a persistent
inflammatory phase and a sequential fibrotic phase (13). The majority of previous studies on
animals have shown an attenuation of fibrosis upon administration
of agents at the beginning of the fibrotic process, but limited
studies exist testing drug intervention during established
progressive fibrosis, a situation more akin to the clinical
conditions of the disease in humans (14,15).
Chaudhary et al (14)
proposed that compounds administered during the early phase of
fibrosis should be considered as ‘preventive treatment’ whereas
‘true’ antifibrotic agents may be effective irrespective of timing,
particularly if administered during the ‘fibrotic’ phase of the
disease. In this context, we administered SB 431542 in two regimes:
the ‘preventive’ protocol concerned early treatment with SB 431542,
and the ‘therapeutic’ protocol concerned delayed treatment with SB
431542.
Materials and methods
Cell culture
The human lung fibroblast cell line IMR-90 was
purchased from the American Type Cell Collection (ATCC; Manassas,
VA). The cells were cultured in Invitrogen™ minimal essential
medium (MEM) (Thermo Fisher Scientific, Waltham, MA, USA)
supplemented with 10 mM sodium pyruvate (Sigma-Aldrich, St. Louis,
MO, USA), 10% Gibco® fetal bovine serum (FBS) and
Gibco® penicillin (100 IU/ml) and streptomycin (100
μg/ml) (Thermo Fisher Scientific, Roskilde, Danmark). The cells
were cultured at 37°C, in a humidified atmosphere of 95% air and 5%
CO2. IMR-90 cells between passages 12 and 20 were used
in the experiments.
Cell cytotoxicity determination by the
MTT assay
IMR-90 cells were seeded onto a 96-well plate at a
density of 3,000 cells/well, and were allowed to attach overnight.
The cells were then incubated with serial dilutions (6.25, 12.5, 25
and 50 μM) of SB 431542 (Sigma-Aldrich) for 72 h. Ten microliters
of 5 mg/ml MTT (Amresco, Solon, OH, USA) were then added into each
well, and incubated for 4 h at 37°C in a humidified atmosphere of
95% air and 5% CO2. Then the solution was replaced with
100 μl of dimethyl sulfoxide (Amresco), and the absorbance was
measured at 570 nm using an ELISA microplate reader (Dynex
Technologies Inc, Chantilly, VA, USA). The percentages of cell
viability were expressed relative to the cell viability of the
control, i.e., untreated IMR-90 cells.
Cell growth (proliferation) assay
IMR-90 cells were seeded onto a 24-well plate at a
density of 10,000 cells/well, and were allowed to attach overnight.
The MEM complete medium was then changed to serum-free medium.
Following a 24-h starvation in this medium, the cells were
stimulated with 10 ng/ml TGF-β1 (R&D Systems, Minneapolis, MN,
USA), and treated with 50 μM SB 431542. The cells were then
trypsinized and stained with 0.4% (w/v) trypan blue
(Sigma-Aldrich), and the cell number in each well was counted at
48, 72 and 96 h after treatment, under a phase contrast microscope
(Olympus, Tokyo, Japan) by using a haemacytometer.
α-smooth muscle actin (α-SMA) protein
detection
IMR-90 cells were seeded onto a 96-well plate (3,000
cells/well), and were allowed to attach overnight. The cells were
treated with TGF-β1 (10 ng/ml) alone or simultaneously with SB
431542 (0.1, 1 and 10 μM) for 72 h after 24 h of serum
starvation.
The IMR-90 cells were fixed with absolute methanol
for 30 min, blocked with 1% bovine serum albumin (BSA; Amresco,
Solon, OH, USA) in Tris-buffered saline (pH 7.4; Thermo Fisher
Scientific) for 1 h, and incubated with a mouse anti-human
monoclonal anti-α-SMA conjugated with fluorescein isothiocyanate
(FITC) (1:200 dilution; Sigma-Aldrich) for 1 h. The cells were then
washed with 1% BSA/borate (pH 9.0), the plates were read at 485/20
nm (excitation) and 530/25 nm (emission) by using a Bio-Tek FL600™
microplate fluorescence reader (Bio-Tek Instruments, Inc.,
Winooski, VT, USA).
Subsequently, the cells were incubated with
propidium iodide (PI) solution containing 25 μg/ml RNase (Roche
Diagnostic Corp., Indianapolis, IN, USA) at 37°C for 30 min, to
allow staining of the nuclei. The plate was read at 485/40 nm
(excitation) and 590/25 nm (emission). The ratio of α-SMA-FITC to
PI values was then calculated.
Reverse transcription and quantitative
(q)PCR
To extract RNA from the IMR-90 cell line, cells were
seeded onto a 6-well plate at a density of 2×105
cells/well and were allowed to attach overnight. The cells were
treated with TGF-β1 (10 ng/ml) alone or simultaneously with SB
431542 (50 μM) for 72 h after 24 h of serum starvation. Total RNA
was then extracted using the TRI Reagent® (Molecular
Research Center, Inc., Cincinnati, OH, USA) and reverse-transcribed
into cDNA using the GoScript™ Reverse Transcription System
(Promega, Madison, WI, USA) according to the manufacturer’s
protocol.
The cDNA samples were analyzed with qPCR using the
GoTaq® Hot Start Green Master Mix kit (Promega) in order to
determine changes in the expression of the α-smooth muscle actin
gene. The primer sequences were: α-SMA forward (F),
5′-ACTGGGACGACATGGAAAAG-3′, and reverse (R),
5′-AGATGGGGACATTGTGGGT-3′; GAPDH F, 5′-ACCACAGTCCATGCCATCAC-3′, and
R, 5′-TCC ACCACCCTGTTGCTGTA-3′. The cycling conditions, applied on
a Rotor-Gene RG-300 instrument (Corbett Research, Sydney,
Australia) were the following: initial denaturation at 95°C for 2
minutes followed by 30 cycles of denaturation at 95°C for 40
seconds, annealing at 59°C for 30 seconds and extension at 72°C for
40 seconds. Melting curve analysis was performed from 75 to 90°C.
The amplification curves were analyzed with the Rotor-Gene Analysis
software 6.0. Cycle threshold (Ct) values for each
target gene were normalized to that of GAPDH, and the fold-changes
in mRNA expression were calculated relative to the control group
(16).
Animal model and protocols
ICR female mice (Harlan Laboratories, Indianapolis,
IN, USA) at 7–8 weeks of age were housed under pathogen-free
conditions in a satellite facility of the University Laboratory
Animal Resources of the Michigan State University (MSU; East
Lansing, MI, USA). The use of animals in this study was approved by
the Institutional Animal Care and Use Committee of MSU. To perform
the instillation of bleomycin or SB 431542, mice were anesthetized
by sodium pentobarbital. Fifty microliters of the treatment
solution were given to the animals. Immediately after, 300 μl of
air were instilled, to ensure delivery of the solution to the
distal airways.
The ICR mice were randomly divided into 4 groups.
Each of the groups contained 6 animals and received different
treatments: normal saline (NS; control group), bleomycin (1 U/kg),
bleomycin and SB 431542 (0.17 mg/kg), and SB 431542. SB 432542
groups were treated with two different regimes: i) the ICR mice in
the early treatment group received bleomycin simultaneously with SB
431542 on day 0, and the mice were sacrificed on day 14. This group
served for the determination of the preventive effect of the
inhibitor SB 431542. ii) the ICR mice in the delayed-treatment
group (for the determination of the therapeutic effect of the
inhibitor) received bleomycin on day 0, and SB 431542 was given to
the mice on days 5 and 10. The mice were sacrificed on day 14.
Immediately before sacrifice, animals were
intraperitoneally injected with sodium pentobarbital, and the
trachea was cannulated. The lungs were instilled with 4%
paraformaldehyde in phosphate-buffered saline at 20 cm, under
constant H2O pressure, and were carefully removed,
followed by immersion into the same fixative solution for 30 min.
The lungs were stored in 70% ethanol. The left lungs were embedded
in paraffin for histopathologic assessment, whereas the upper right
lobe was used for the hydroxyproline assay.
Measurement of hydroxyproline
content
For quantification of the total lung collagen, a
fixed weight of lung tissue was dried at 80°C. The dry tissue was
hydrolyzed in 6 N hydrochloric acid at 100°C, and was subjected to
the hydroxyproline assay, as descried earlier by Woessner (17). The hydroxyproline level in the
samples was determined by comparison to standard hydroxyproline
concentrations (Sigma-Aldrich).
Histopathology assessment
For histological examination, the paraffin sections
were stained with hematoxylin and eosin (H&E), and
systematically observed under a light microscope (Olympus). The
severity of pulmonary fibrosis was compared among the groups by
using the Ashcroft score, calculated as in (18).
Statistical analysis
Group mean values and standard deviations (SD) were
calculated. Data were analyzed by a one-way analysis of variance
(ANOVA), followed by Student-Newman-Keuls post-hoc tests, using the
GraphPad InStat version 3.05 software (GraphPad Software, Inc., San
Diego, CA, USA). P<0.05 were considered to indicate
statistically significant differences.
Results
Effect of SB 431542 on IMR-90 cell
viability
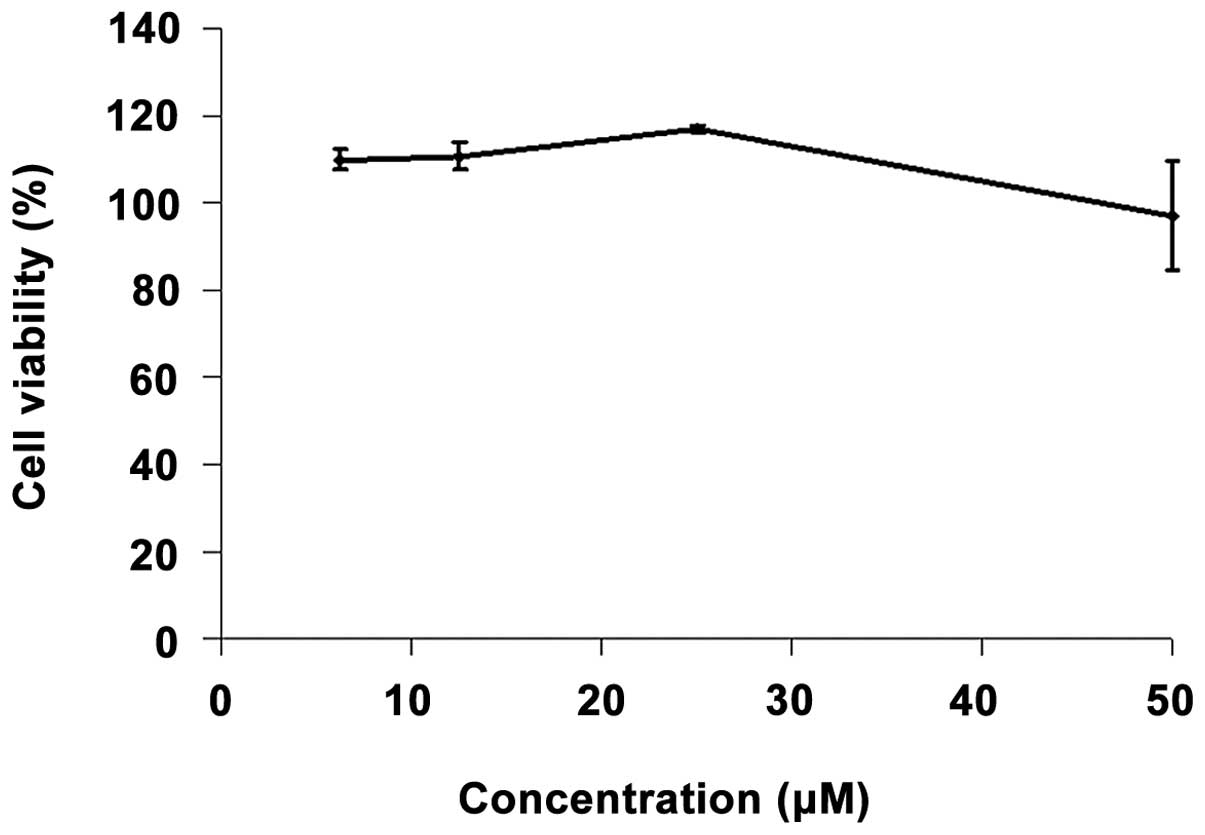
In order to determine the effect of SB 431542 on
lung cell viability, the MTT assay was performed on IMR-90 cells.
Nearly 100% cell viability was observed after 72 h of incubation
with various concentrations (6.25, 12.5, 25 and 50 μM) of SB
4321542 (Fig. 1).
Effect of SB 431542 on cell
proliferation
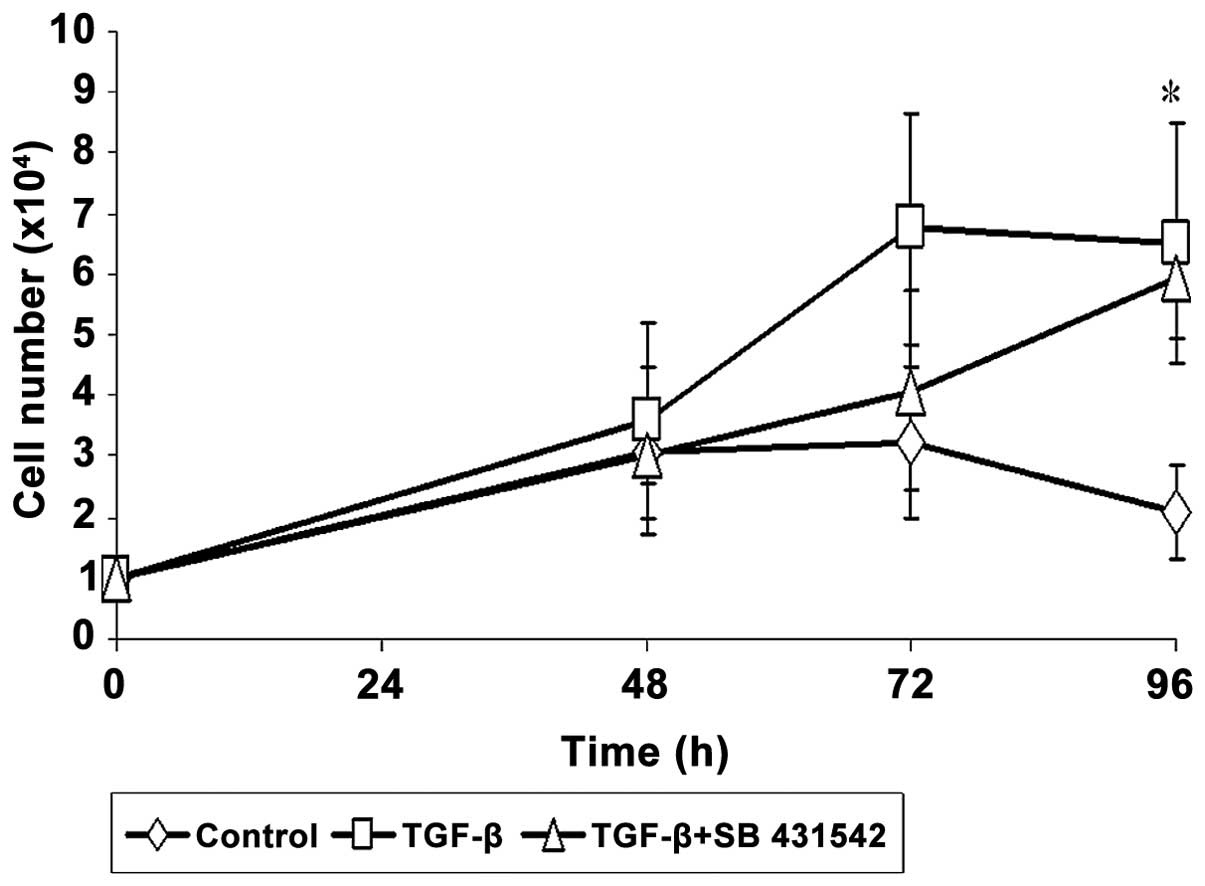
A cell growth assay was carried out to determine the
effect of SB 431542 on IMR-90 cell proliferation. TGF-β enhanced
IMR-90 cell proliferation, and a significant increase in cell
number was observed after 96 h of induction. The proliferation of
IMR-90 cells induced by TGF-β was reduced by SB 431542 (Fig. 2). However, this reduction was not
statistically significant.
Effect of SB 431542 on α-SMA expression
in the cells
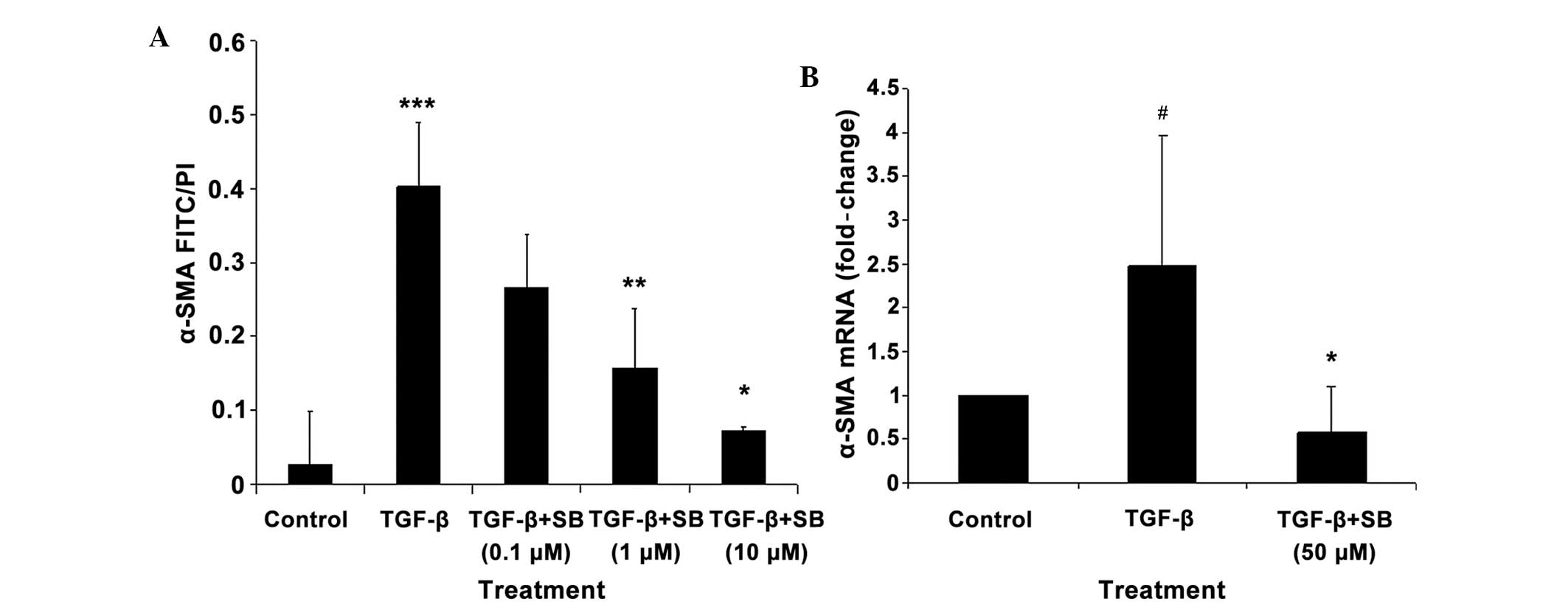
The expression of the α-SMA protein was
significantly increased by addition of TGF-β, but this effect was
significantly reduced by SB 431542 treatment at 1 μM (P<0.01)
and 10 μM (P<0.05). The effect of SB 431542 was dose-dependent
(Fig. 3A).
In addition, a 2.5-fold increase in the expression
of the α-SMA mRNA was observed following addition of TGF-β
into the IMR-90 cells, but again, this effect was significantly
(P<0.05) reduced by SB 431542 treatment (Fig. 3B).
Effect of SB 431542 on lung
histopathology
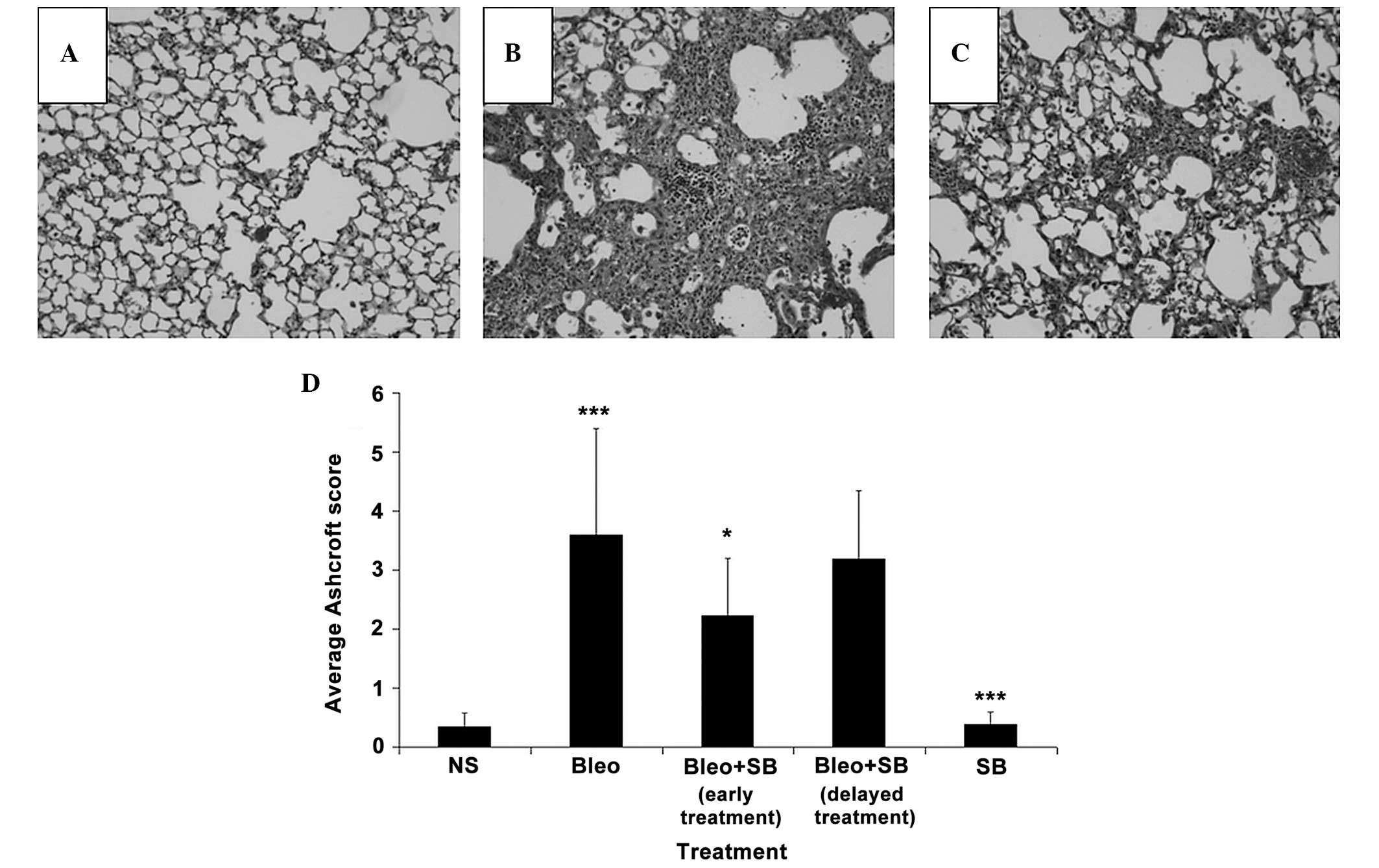
Changes in several lung regions, consisting of
thickened alveolar septa and dense intra-alveolar fibrosis,
accompanied by an increase in the number of alveolar macrophages,
were observed in the lung sections of the bleomycin-treated group
(Fig. 4B). These changes were not
detected in the lungs of NS-treated mice (Fig. 4A). Attenuation of fibrosis was
observed in the SB 431542-treated groups (Fig. 4C). The Ashcroft scores of the
early-treatment group were significantly (P<0.05) reduced
compared to those of the bleomycin-treated group (Fig. 4D). The administration of SB 431542
alone did not cause any marked histopathologic changes.
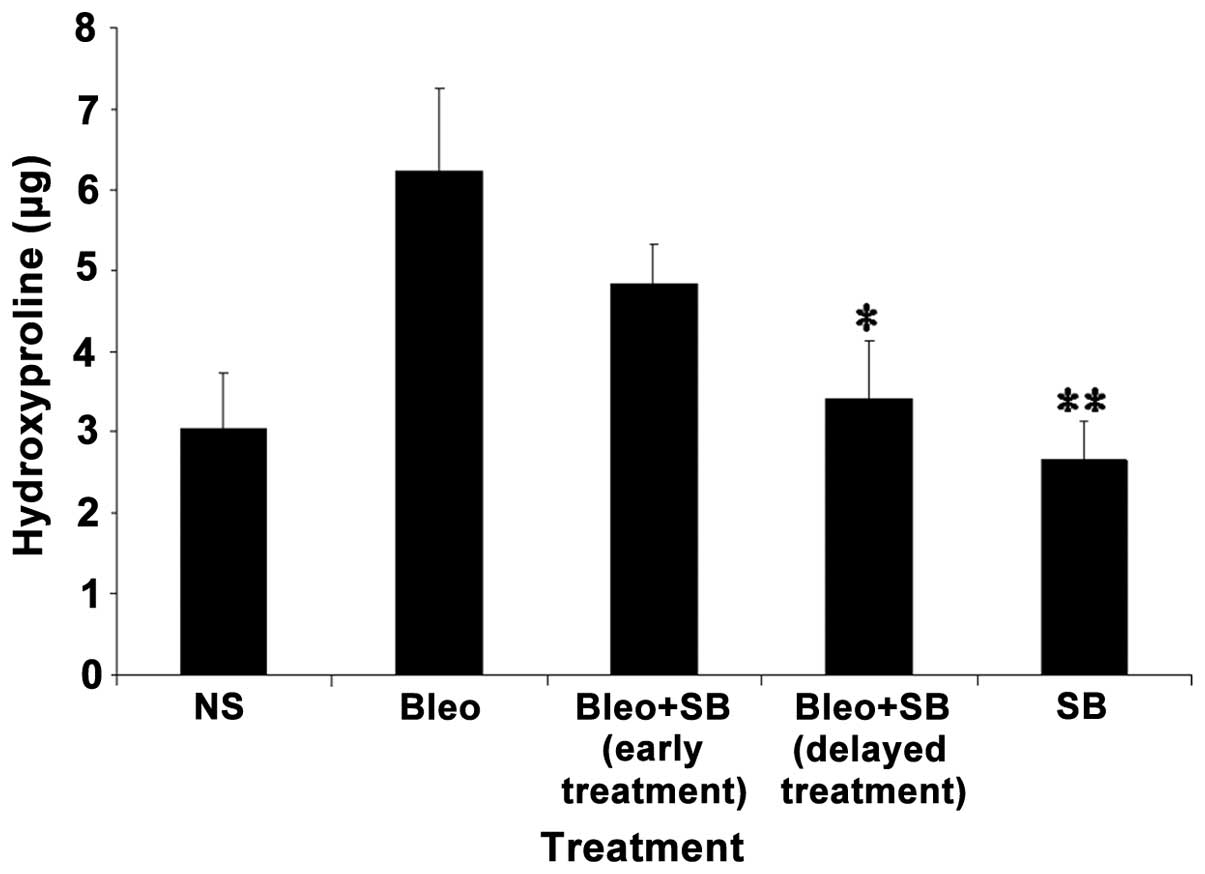
Effect of SB 431542 on the hydroxyproline
content of lung tissues
The concentration of hydroxyproline following
instillation of bleomycin was significantly higher compared to the
NS-treated group. In both groups receiving either early or delayed
SB 431542 treatment, the hydroxyproline content was reduced
compared to the bleomycin-treated group. However, a significant
(P<0.05) difference was observed in the delayed-treatment group.
The hydroxyproline expression in SB 431542 alone treatment group
was similar to the control group (Fig.
5).
Discussion
IPF is a disease caused by the scarring process in
the lung. Currently, there is a lack of treatment options for IPF.
Various studies have been carried out with the aim of identifying a
new cure for the disease. In the present study, SB 431542, an ALK-5
(or TGF-β receptor) inhibitor was used to inhibit the fibrotic
responses in vitro. The inhibitor was also administrated
into mice lungs using both a preventive and a therapeutic regime to
evaluate its antifibrotic effect in bleomycin-induced IPF mice.
Fibroblast proliferation plays a critical role in
the fibrogenic process (19–21),
and TGF-β is a major inducer of proliferation. In accordance with
these, our study showed that addition of TGF-β onto IMR-90 cells
enhances cell proliferation, with a significant increase in cell
number observed following a 96-h incubation with the cytokine. SB
431542 treatment was found to slightly reduce cell growth, although
this reduction was not statistically significant. Hence, our
results suggested that inhibition of the TGF-β receptor by SB
431542 may not be useful in inhibiting cell proliferation in lung
fibrosis.
One of the characteristics of human fibrotic
diseases is the persistence of myofibroblasts, which leads to
extensive tissue architectural remodeling and progressive fibrosis
(22). Expression of the α-SMA
protein is a main feature of the myofibroblasts (23). Thus, differentiation of the
fibroblasts to myofibroblasts can be determined by assessing the
expression of α-SMA. A previous study reported strong expression of
α-SMA in lung cells following TGF-β stimulation (24). In our study, the TGF-β-induced
increase in the level of the α-SMA protein was significantly
reduced by SB 431542 treatment. The TGF-β-induced α-SMA mRNA
expression was also inhibited by SB 431542 treatment. Inhibition of
the TGF-β receptor by SB 431542 was accompanied by decreased α-SMA
production; hence, targeting ALK-5 may have beneficial effects in
the context of treatment of fibrotic diseases.
To exclude the possibility that the reduction in
cell proliferation and α-SMA production in cells treated with TGF-β
and SB 431542 is due to cell death, the MTT assay was performed.
There was no evidence of cellular toxicity when the IMR-90 cells
were incubated with SB 431542 at concentrations of up to 50 μM, and
up to 72 h after treatment. This result allowed to exclude the
cytotoxic effect of SB 431542 as a cause for the reduction in cell
growth and in the expression of the myofibroblast
development-related protein α-SMA.
In the present study, bleomycin-treated mice showed
severe lung morphology alterations. These changes were not observed
in lungs of NS-treated (control) mice. A significantly higher
Ashcroft score was estimated for the bleomycin group compared to
the control group. A significantly higher hydroxyproline expression
was also observed in the bleomycin, compared with the control
group. Reduced Ashcroft scores and lung tissue hydroxyproline
contents were observed following both early and delayed
administration of SB 431542 into the bleomycin-treated mice. A
previous report showed that administration of bleomycin increases
the synthesis and release of TGF-β (12), which is important for IPF
pathogenesis. The inhibitory effect of SB 431542 on the severity of
pulmonary fibrosis and collagen expression suggests that blocking
ALK-5 can prevent and arrest the progression of fibrosis.
Taken together, our results indicate that SB 431542
treatment of IMR-90 cells is associated with a marked reduction of
TGF-β-induced cellular responses involved in fibrogenesis, while
the inhibitor has no detectable cytotoxicity. Inhibition of ALK-5
by SB 431542 reduced the fibrotic progression in vitro, and
this suggests that the inhibitor may have a therapeutic effect on
pulmonary fibrosis in in vivo models as well.
Early treatment with SB 431542 showed a more
prominent effect in inhibiting the severity of lung injury compared
to the delayed treatment regime, while delayed treatment with SB
431542 had a more significant effect on the inhibition of collagen
production. These data suggest that SB 431542 prevents the
progression of pulmonary fibrosis by reducing the early lung injury
caused by inflammatory processes, and that the inhibitor may have
anti-inflammatory properties in vivo. On the other hand,
early treatment with SB 431542 did not exert a significant effect
on the inhibition of collagen expression, which was only noted at a
later stage, during the ‘true’ fibrotic phase; this suggests that
SB 431542 may have a transient effect on the animals. By contrast,
a significant decrease in the hydroxyproline level was observed
when repeated administration of the inhibitor was performed during
the ‘true’ fibrotic phase. This result suggests that this approach
may be promising as a therapeutic antifibrotic intervention,
although one should note that no significant inhibition on lung
injury was observed with this treatment.
SB 431542 is a specific and potent inhibitor of
TGF-β signaling in vitro. Using this inhibitor, we were able
to significantly inhibit TGF-β-induced fibrosis in lung
fibroblasts. Furthermore, we provide data demonstrating that
blocking ALK-5 via this inhibitor strongly inhibits the
bleomycin-induced initiation of lung fibrosis, and arrests the
progression of established pulmonary fibrosis. In summary, our
study suggests that inhibition of ALK-5 may have a role in chronic
fibrotic diseases such as IPF. Moreover, considering that fibrosis
tends to be an organ-restricted disorder, short-term local
administration of this drug directly into the lungs by
intratracheal instillation may prove highly beneficial.
Acknowledgements
This study was supported by the E-Science grant no.
5450187 (Ministry of Science, Technology and Innovation, Malaysia).
We thank the Department of Physiology and Biomedical Sciences,
Michigan State University, USA, for partially funding this
study.
References
|
1
|
Bjoraker JA, Ryu JH, Edwin MK, Myers JL,
Tazelaar HD, Schroeder DR and Offord KP: Prognostic significance of
histopathologic subsets in idiopathic pulmonary fibrosis. Am J
Respir Crit Care Med. 157:199–203. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nicholson AG, Colby TV, du Bois RM,
Hansell DM and Wells AU: The prognostic significance of the
histologic pattern of interstitial pneumonia in patients presenting
with the clinical entity of cryptogenic fibrosing alveolitis. Am J
Respir Crit Care Med. 162:2213–2217. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Daniil ZD, Gilchrist FC, Nicholson AG,
Hansell DM, Harris J, Colby TV and du Bois RM: A histologic pattern
of nonspecific interstitial pneumonia is associated with a better
prognosis than usual interstitial pneumonia in patients with
cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med.
160:899–905. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Douglas WW, Ryu JH, Swensen SJ, Offord KP,
Schroeder DR, Caron GM and DeRemee RA: Colchicine versus prednisone
in the treatment of idiopathic pulmonary fibrosis. A randomized
prospective study Members of the Lung Study Group. Am J Respir Crit
Care Med. 158:220–225. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gribbin J, Hubbard RB, Le Jeune I, Smith
CJ, West J and Tata LJ: Incidence and mortality of idiopathic
pulmonary fibrosis and sarcoidosis in the UK. Thorax. 61:980–985.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dennler S, Goumans MJ and ten Dijke P:
Transforming growth factor beta signal transduction. J Leukoc Biol.
71:731–740. 2002.PubMed/NCBI
|
|
7
|
Tojo M, Hamashima Y, Hanyu A, et al: The
ALK-5 inhibitor A-83-01 inhibits Smad signaling and
epithelial-to-mesenchymal transition by transforming growth
factor-beta. Cancer Sci. 96:791–800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mori Y, Ishida W, Bhattacharyya S, Li Y,
Platanias LC and Varga J: Selective inhibition of activin
receptor-like kinase 5 signaling blocks profibrotic transforming
growth factor beta responses in skin fibroblasts. Arthritis Rheum.
50:4008–4021. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishida W, Mori Y, Lakos G, et al:
Intracellular TGF-beta receptor blockade abrogates Smad-dependent
fibroblast activation in vitro and in vivo. J Invest Dermatol.
126:1733–1744. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu XJ, Ruan CM, Gong XF, Li XZ, Wang HL,
Wang MW and Yin JQ: Antagonism of transforming growth factor-Beta
signaling inhibits fibrosis-related genes. Biotechnol Lett.
27:1609–1615. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borzone G, Moreno R, Urrea R, Meneses M,
Oyarzun M and Lisboa C: Bleomycin-induced chronic lung damage does
not resemble human idiopathic pulmonary fibrosis. Am J Respir Crit
Care Med. 163:1648–1653. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoyt DG and Lazo JS: Alterations in
pulmonary mRNA encoding procollagens, fibronectin and transforming
growth factor-beta precede bleomycin-induced pulmonary fibrosis in
mice. J Pharmacol Exp Ther. 246:765–771. 1988.PubMed/NCBI
|
|
13
|
Osborne ML, Vollmer WM, Linton KL and
Buist AS: Characteristics of patients with asthma within a large
HMO: a comparison by age and gender. Am J Respir Crit Care Med.
157:123–128. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chaudhary NI, Schnapp A and Park JE:
Pharmacologic differentiation of inflammation and fibrosis in the
rat bleomycin model. Am J Respir Crit Care Med. 173:769–776. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bouros D and Antoniou KM: Current and
future therapeutic approaches in idiopathic pulmonary fibrosis. Eur
Respir J. 26:693–702. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Woessner JF Jr: The determination of
hydroxyproline in tissue and protein samples containing small
proportions of this imino acid. Arch Biochem Biophys. 93:440–447.
1961. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ashcroft T, Simpson JM and Timbrell V:
Simple method of estimating severity of pulmonary fibrosis on a
numerical scale. J Clin Pathol. 41:467–470. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Flaherty KR, Toews GB, Lynch JP, et al:
Steroids in idiopathic pulmonary fibrosis: a prospective assessment
of adverse reactions, response to therapy, and survival. Am J Med.
110:278–282. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pardo A and Selman M: Idiopathic pulmonary
fibrosis: new insights in its pathogenesis. Int J Biochem Cell
Biol. 34:1534–1538. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Selman M and Pardo A: The
epithelial/fibroblastic pathway in the pathogenesis of idiopathic
pulmonary fibrosis. Am J Respir Cell Mol Biol. 29:S93–97.
2003.PubMed/NCBI
|
|
22
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gabbiani G, Ryan GB and Majne G: Presence
of modified fibroblasts in granulation tissue and their possible
role in wound contraction. Experientia. 27:549–550. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chaudhary NI, Roth GJ, Hilberg F, et al:
Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis.
Eur Respir J. 29:976–985. 2007. View Article : Google Scholar : PubMed/NCBI
|