Introduction
Colorectal cancer (CRC) is one of the most common
cancers worldwide, with an annual incidence rate of 1.2 million and
an annual mortality rate of over 600,000 individuals (1,2).
Despite considerable advances in surgical techniques and
neoadjuvant chemotherapy, the survival rate of CRC remains poor
(3). Antimetabolites, such as
5-fluorouracil (5-FU), have therapeutic properties for patients
with CRC; however, the side effects, which include myelotoxicity
and GI toxicities (such as diarrhea and stomatitis), limit their
long-term use. Thus, there is an urgent need for the development of
new drugs with more specific effects and low toxicity.
Natural products have been shown to be excellent and
reliable sources in pharmaceutical development of anticancer drugs
(4,5). Sinomenine (SIN), an alkaloid from
Sinomenium acutum, inhibits proliferation in SW1116
colorectal cancer cells by promoting G1 phase arrest,
with concomitant suppression of COX-2 expression (6). Bisleuconothine A, a bisindole
alkaloid, inhibits cell proliferation and induces apoptosis in
HCT116 and SW480 colorectal cancer cells, by increasing caspase
cleavage (7). Furthermore, it
dramatically suppresses Wnt target gene expression in an in
vivo HCT116 xenograft model, through upregulation of β-catenin
phosphorylation and subsequent Wnt signaling inhibition (7). Piperlongumine (PPLGM), an alkaloid
isolated from the long pepper (Piper longum L.), selectively
triggers cancer cell death in HCT116 colorectal cancer cells,
through activation of the JNK signaling pathway (8).
Hylomecon vernalis Maxim. has long been used
in traditional Chinese medicine for improving the local blood
supply, dissipating blood stasis, and relieving pain. Alkaloids
have multiple biological activities, including antitumor,
anti-inflammatory, and analgesic effects. In the present study, the
aim was to investigate the effect of HVMEE on viability and
apoptosis of HT-29 and SW620 human colorectal cancer cells and its
potential mechanism.
Materials and methods
Chemicals and reagents
MTT was purchased from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany). Polyclonal rabbit anti-human
cleaved caspase-3 (1:1,000; cat. no. 9661S), monoclonal rabbit
anti-human cleaved caspase-8 (1:1,000; cat. no. 9496S), polyclonal
rabbit anti-human cleaved caspase-9 (1:1,000; cat. no. 9505S),
monoclonal mouse anti-human BCL-2 (1:1,000; cat. no. 15071S),
polyclonal rabbit anti-human Bax (1:1,000; cat. no. 2772S),
monoclonal rabbit anti-human cyclin D1 (1:1,000; cat. no. 2978S),
monoclonal rabbit anti-human CDK4 (1:1,000; cat. no. 12790S),
monoclonal rabbit anti-human CDK6 (1:1,000; cat. no. 13331S) and
monoclonal rabbit anti-human p21 (1:1,000; cat. no. 2947S) primary
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). N-Benzyloxycarbonyl-Val-Ala-Asp (O-Me)
fluoromethyl ketone (Z-VAD-FMK) was purchased from Beyotime
Institute of Biotechnology (Haimen, China). The monoclonal mouse
anti-human β-actin primary antibody was obtained from Abcam
(1:1,000; cat. no. ab8226; Cambridge, UK). Goat anti-mouse and goat
anti-rabbit secondary antibodies were purchased from Thermo Fisher
Scientific, Inc., (1:5,000; cat. nos. A16072 and A16110,
respectively; Waltham, MA, USA).
Extraction of HVMEE
H. vernalis Maxim. was purchased from Shaanxi
Panlong Pharmaceutical Co., Ltd. (Shangluo, China). Briefly, the
dried root of H. vernalis Maxim. (10.0 kg) was extracted
with 70% ethanol three times. The extracts were combined,
concentrated, and dried at 80°C to obtain the HVMEE.
High-performance liquid chromatography (HPLC) in tandem with mass
spectrometry analysis was used to assess the main ingredients in
the extracts. HPLC was conducted in tandem with mass spectrometry
using an Agilent 1260 HPLC and AB SCIEX 4500Q trap triple
quadrupole mass spectrometer with ESI source: Mobile phase 0.1%
(v/v) (A) formic acid aqueous solution and (B) acetonitrile;
injection volume 5 µl; column temperature 35°C, using a gradient
elution mode. Run times from 0–10 min up to 15% B and from 11–20
min up to 27% B. The HPLC system consisted of a C18 column (3.9×300
mm, 10 µm) with 1 ml/min flow rate. The MassHunter (Agilent
Technologies, Inc., Santa Clara, CA, USA) system was used.
Cell culture
Human CRC cell lines HT-29 and SW620 were obtained
from the American Type Culture Collection (Manassas, VA, USA). The
cells were cultured in RPMI-1640 (Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal calf serum (Gibco; Thermo Fisher
Scientific, Inc.; cat. no. 10437-028), 100 U/ml penicillin, and 100
U/ml streptomycin in an atmosphere of 95% oxygen and 5%
CO2 at 37°C.
Cell viability assay
HT-29 and SW620 cells were seeded in 96-well plates
at a density of 2×104 cells/well for 24 h, then cells
were treated with 0.01, 0.03, 0.1, 0.3, 1, and 3 mg/ml HVMEE for 24
h in complete medium. Following treatment, 20 µl of MTT solution (5
mg/ml) was added to each well for 4 h. The cells were then washed
three times with PBS, and the resulting formazan was resuspended in
150 µl dimethyl sulfoxide. Absorbance was measured at 570 nm with a
Bio-Rad ELISA reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The experiment was repeated independently three times.
Flow cytometric assay for Annexin V
apoptosis detection
Cell apoptosis was detected using an Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit (BD Biosciences, Franklin Lakes, NJ, USA). Briefly,
HT-29 and SW620 cells were grown at a density of 5×105
per well in 6-well plates. In accordance with the IC50
values obtained from the MTT assay, the cells were treated with
0.01 or 0.05 mg/ml HVMEE for 24 h. Using the MTT assay results, the
IC50 values were 0.105±0.022 mg/ml for HT-29 and
0.146±0.013 mg/ml for SW620 cells. The authors identified that the
minimum effective concentration of HVMEE was 0.01 mg/ml for HT-29
and SW620 cells. Therefore, five times the minimum effective
concentration of HVEMEE was 0.05 mg/ml, which is a concentration
with a significant effect, whilst bearing an apoptosis rate (for
both HT-29 and SW620) of <50%.
Then, a total of 1×106 cells were
collected with centrifuge (700 × g) 3 min at room
temperature, washed with ice-cold PBS and resuspended in 1X binding
buffer [10 mM Hepes/NaOH (pH 7.4), 140 mM NaCl, 2.5 mM
CaCl2] at a concentration of 1×106 cells/ml.
Annexin V-FITC (5 µl of a 25 µg/ml solution) and PI (5 µl of a 250
µg/ml solution) were added to 100 µl of cell suspension. The cells
were then gently vortexed and incubated at room temperature in the
dark for 15 min. Then, 400 µl of ice-cold binding buffer was added
and mixed gently before the cell preparations were examined by flow
cytometry (FACSCalibur; BD Biosciences).
Flow cytometric assay for nuclear DNA
content distribution detection
To determine cell cycle distribution, cells were
collected with centrifuge (700 × g) 3 min at room
temperature, washed with ice-cold PBS and fixed for 2 h in 75%
ethanol at −20°C. The cells were then treated with 1% RNase A for 5
min at 37°C and stained with 50 mg/ml PI for 30 min. Fluorescence
intensity was examined by flow cytometry using a FACS Calibur
(FACSCalibur; BD Biosciences).
Caspase-3 activity
Caspase-3 activity in HT-29 and SW620 cells was
detected using the caspase-3 activity assay kit (Beyotime Institute
of Biotechnology), as per the manufacturer's instructions. HT-29
and SW-620 cells were plated in culture dish (diameter, 10 cm) at a
density of 1×106 cells and allowed to grow for 24 h. The
cells were then treated with HVMEE and Z-VAD-FMK (10 µM) for 24 h
or 48 h in complete medium with 37°C. Following drug treatment,
absorbance values were measured at 405 nm. Results were adjusted to
the total protein content, and activity was expressed as nmol of
p-Nitroaniline per mg of total protein.
Western blot analysis
HT-29 and SW-620 cells were plated in culture dish
(diameter, 10 cm) at a density of 10×106 cells and
allowed to grow for 24 h. Then, HT-29 cells and SW620 cells were
treated with 0.01 and 0.05 mg/ml HVMEE for 24 and 48 h. The cells
were then collected with a centrifuge (700 × g) 3 min at
room temperature, and resuspended in lysis buffer (Beyotime
Institute of Biotechnology) for 30 min in an ice bath. Following
centrifugation at 14,000 × g at 4°C for 30 min, the
supernatant was collected. Protein concentrations were determined
using the Bradford method. Equal protein amounts (30 µg) were
separated by 15% sodium dodecyl sulfate polyacrylamide gel
electrophoresis, transferred electrophoretically to nitrocellulose
membranes (Pall Corporation, New York, NY, USA) and blocked with
TBS buffer containing 0.05% Tween-20 and 10% non-fat milk for 2 h
at room temperature. The membranes were then incubated overnight at
4°C with various primary antibodies, followed by horseradish
peroxidase-conjugated secondary antibodies at room temperature for
1.5 h. Following washing the membranes three times for 5 min in TBS
buffer containing 0.05% Tween-20. Immunoreactive proteins on the
membrane were detected using the Immobilon Western HRP substrate
(EMD Millipore, Billerica, MA, USA). The bands were quantified
using Multi Gauge version 3.2 software (Fujifilm Holdings
Corporation, Tokyo, Japan). Experiments were repeated independently
3 times, and the relative expression of the target protein was
normalized to the level of β-actin in the same sample.
Statistical analysis
SPSS software (version, 13.0; SPSS, Inc., Chicago,
IL, USA) was used for the statistical analysis. Data were expressed
as the mean ± standard deviation. Statistical analyses were carried
out using a one-way analysis of variance and Fisher's least
significant difference (LSD) t-test to compare differences
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Results of mass spectrometry
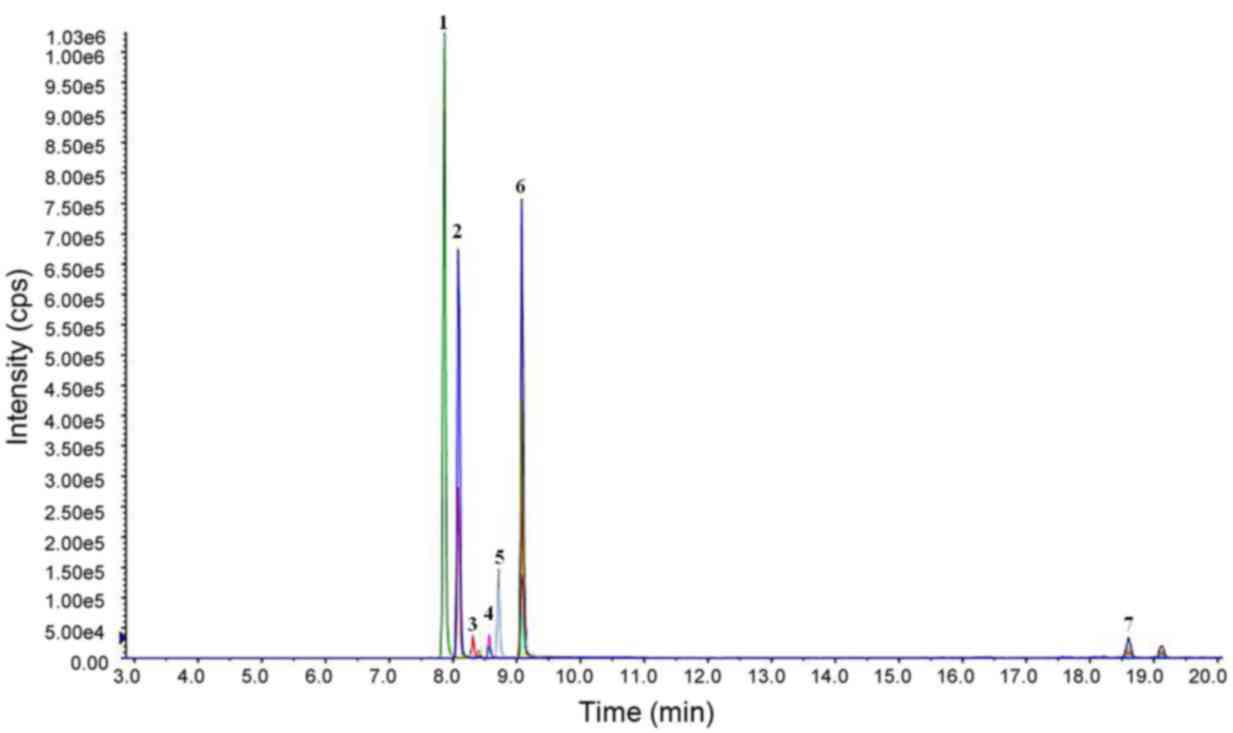
Ion flow results from HPLC analysis of the HVMEE are
demonstrated in Fig. 1. The
alkaloid content of the HVMEE was as high as 89.67%. The principal
alkaloids observed were allocryptopine, protopine, berberine,
sanguinarine, chelerythrine, tetrahydroberberine, and coptisine;
their relative contents were 13.48, 55.33, 0.16, 14.86, 5.81,
0.002, and 0.02%, respectively (Fig.
1).
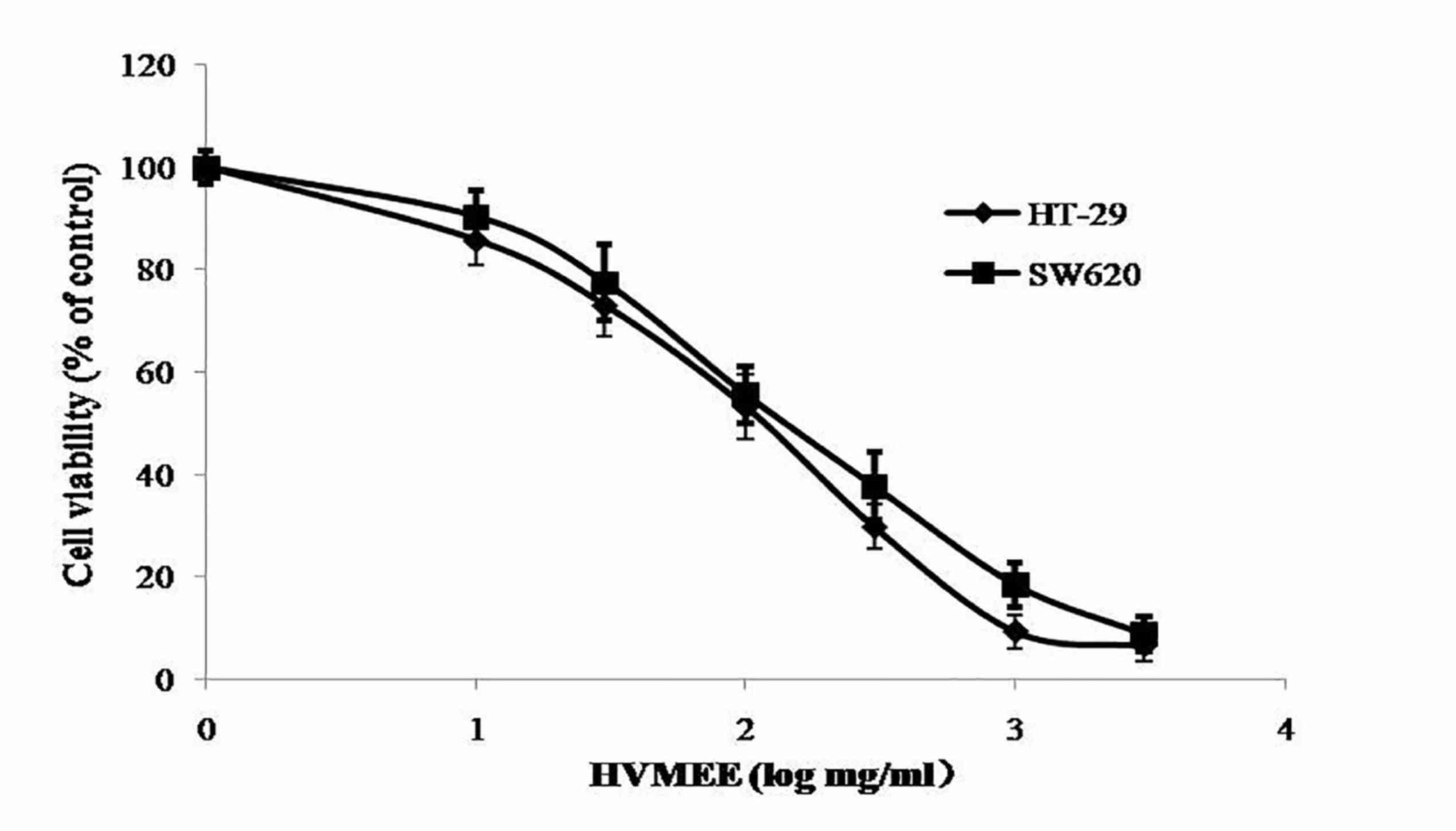
HVMEE decreases viability in HT-29 and
SW620 cells
The effect of HVMEE on cell viability was assessed
by MTT assay. As demonstrated in Fig.
2, HVMEE effectively decreased cell viability in HT-29 and
SW620 cells in a concentration-dependent manner. The
IC50 values were 0.105±0.022 mg/ml for HT-29 and
0.146±0.013 mg/ml for SW620 cells (Fig. 2).
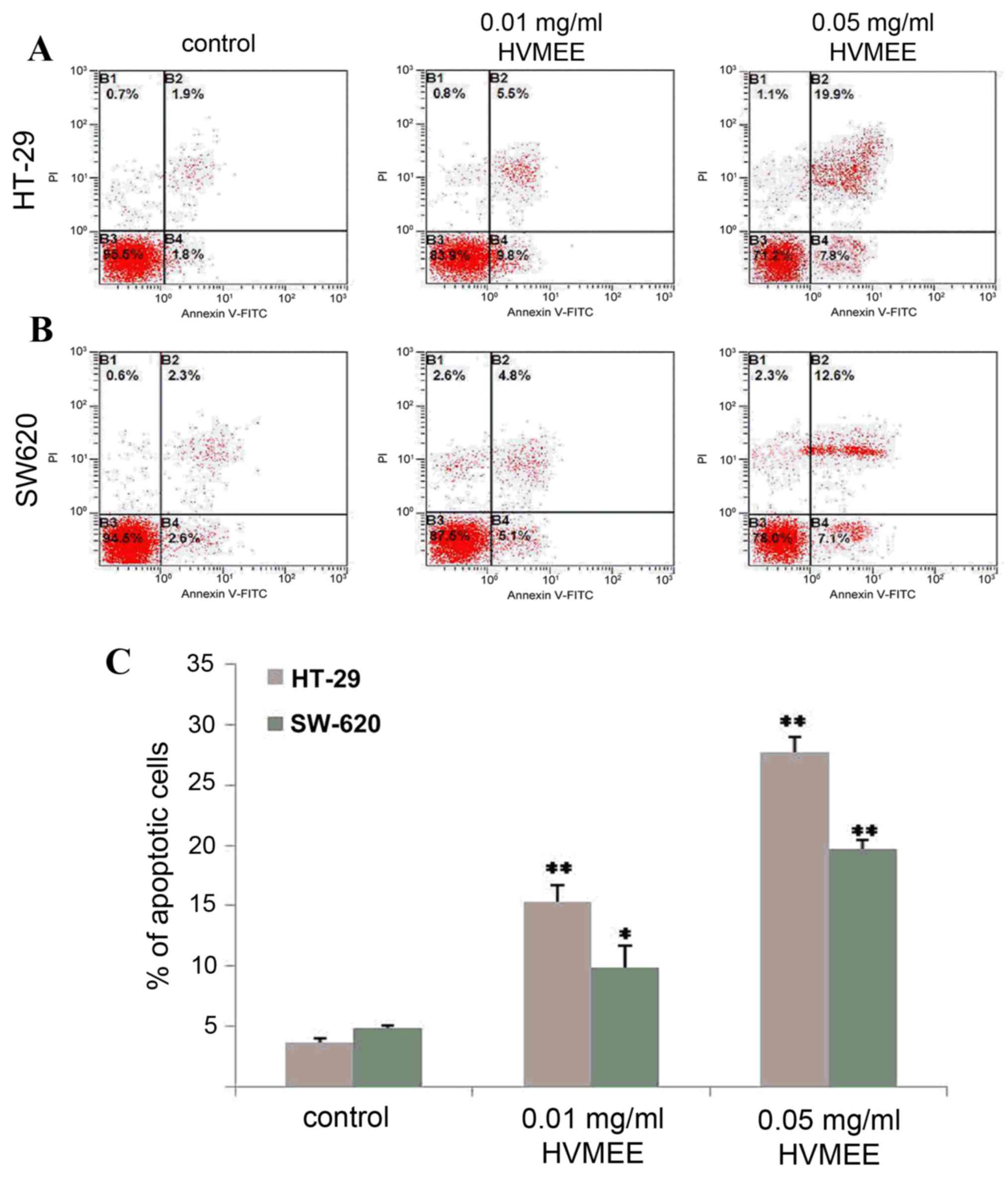
HVMEE treatment triggers cell
apoptosis
Apoptosis of HVMEE colorectal cancer cells was
assessed by flow cytometry analysis. As demonstrated in Fig. 3, the percentages of apoptotic cells
(the sum of B2 and B4 quadrants of the flow cytometry plots) in the
untreated control HT-29 (Fig. 3A)
and SW620 cells (Fig. 3B) were
3.7±0.3% (Fig. 3A and C) and
4.9±0.2% (Fig. 3B and C),
respectively. When exposed to 0.01 mg/ml HVMEE for 24 h, the
percentage of apoptotic cells increased to 15.3±1.4% in the HT-29
cells (P<0.01; Fig. 3A and C)
and 9.9±1.8% in the SW620 cells (P<0.05; Fig. 3B and C). When exposed to 0.05 mg/ml
of HVMEE for 24 h, the percentages of apoptotic cells increased to
27.7±1.3% in the HT-29 cells (P<0.01; Fig. 3A and C) and 19.7±0.7 in the SW620
cells (P<0.01; Fig. 3B and C).
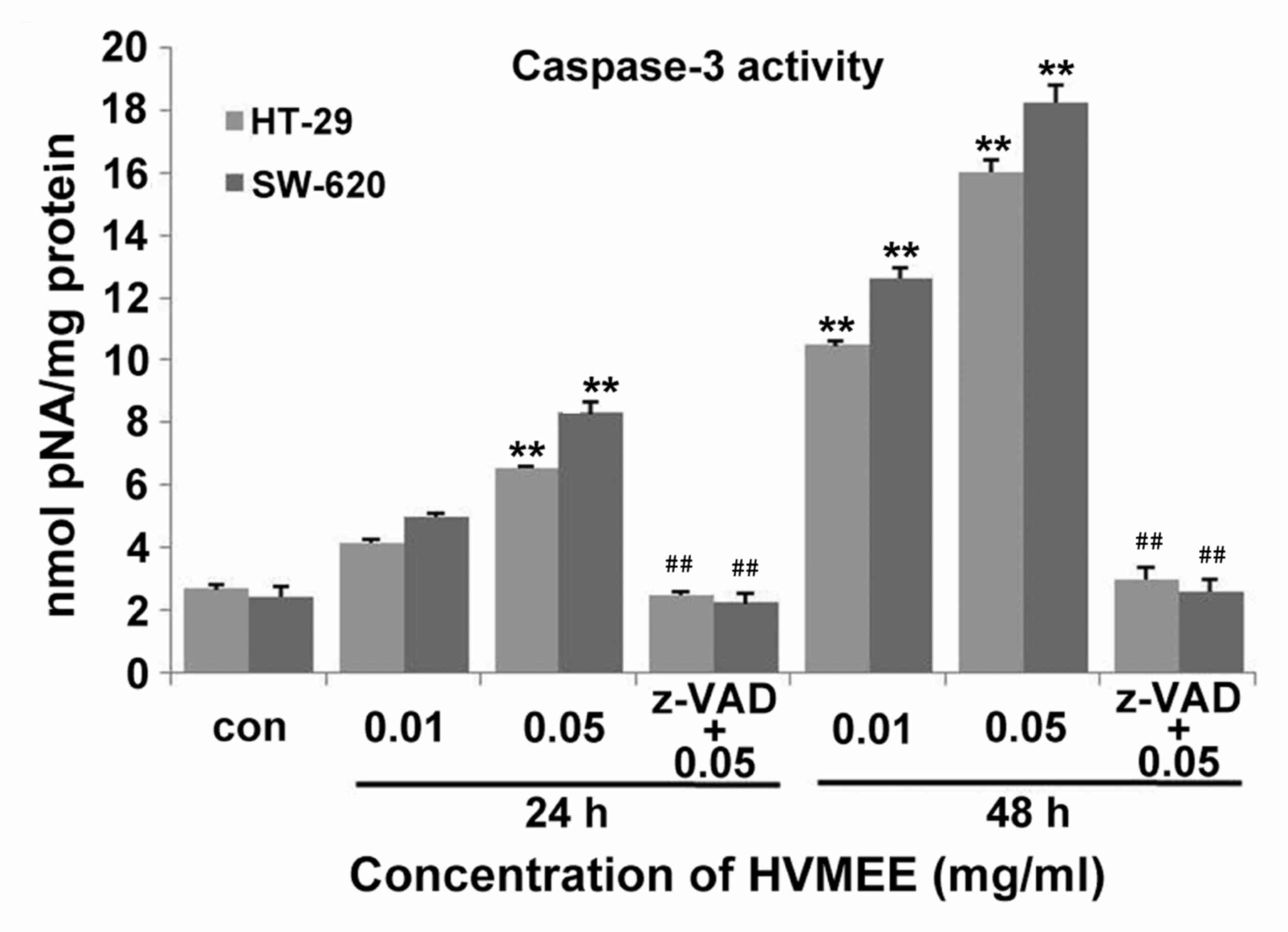
In addition, treatment of HT-29 and SW620 cells with 0.01 and 0.05
mg/ml HVMEE resulted in a significant increase in caspase-3
activation in both cell lines compared with untreated control
(Fig. 4). The HVMEE-induced
caspase-3 activity was significantly blocked by pretreatment with a
general caspase inhibitor, Z-VAD-FMK (Fig. 4).
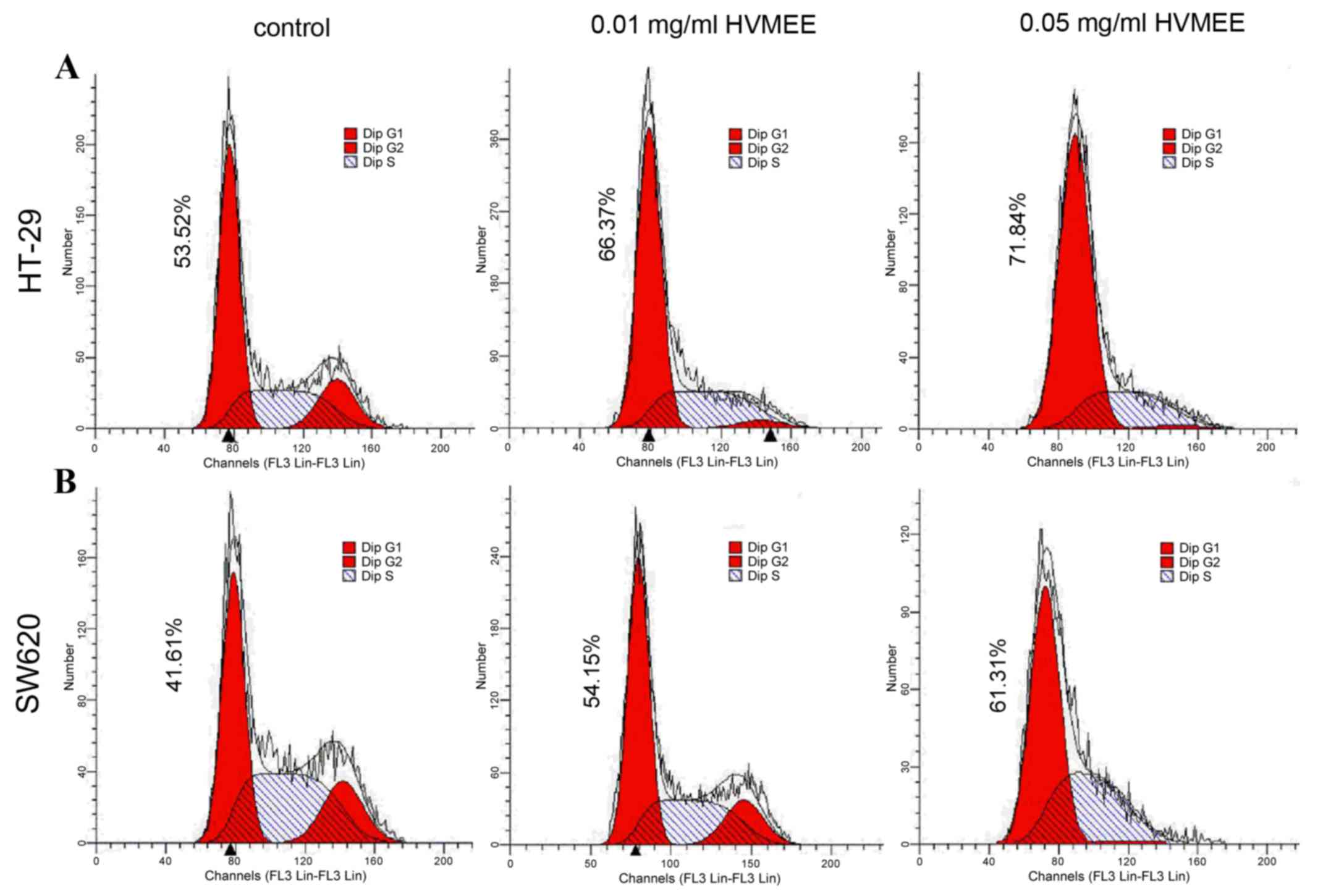
HVMEE induces cell cycle arrest at the
G1 phase in HT-29 and SW620 cells
The cell cycle phase distribution in HT-29 (Fig. 5A) and SW620 (Fig. 5B) cells was examined following
treatement with 0.01 and 0.05 mg/ml HVMEE for 24 h. Flow cytometric
analysis of nuclear DNA distribution demonstrated a
concentration-dependent increase of G1 proportions in
HVMEE-treated cells, compared with untreated control cells
(Fig. 5).
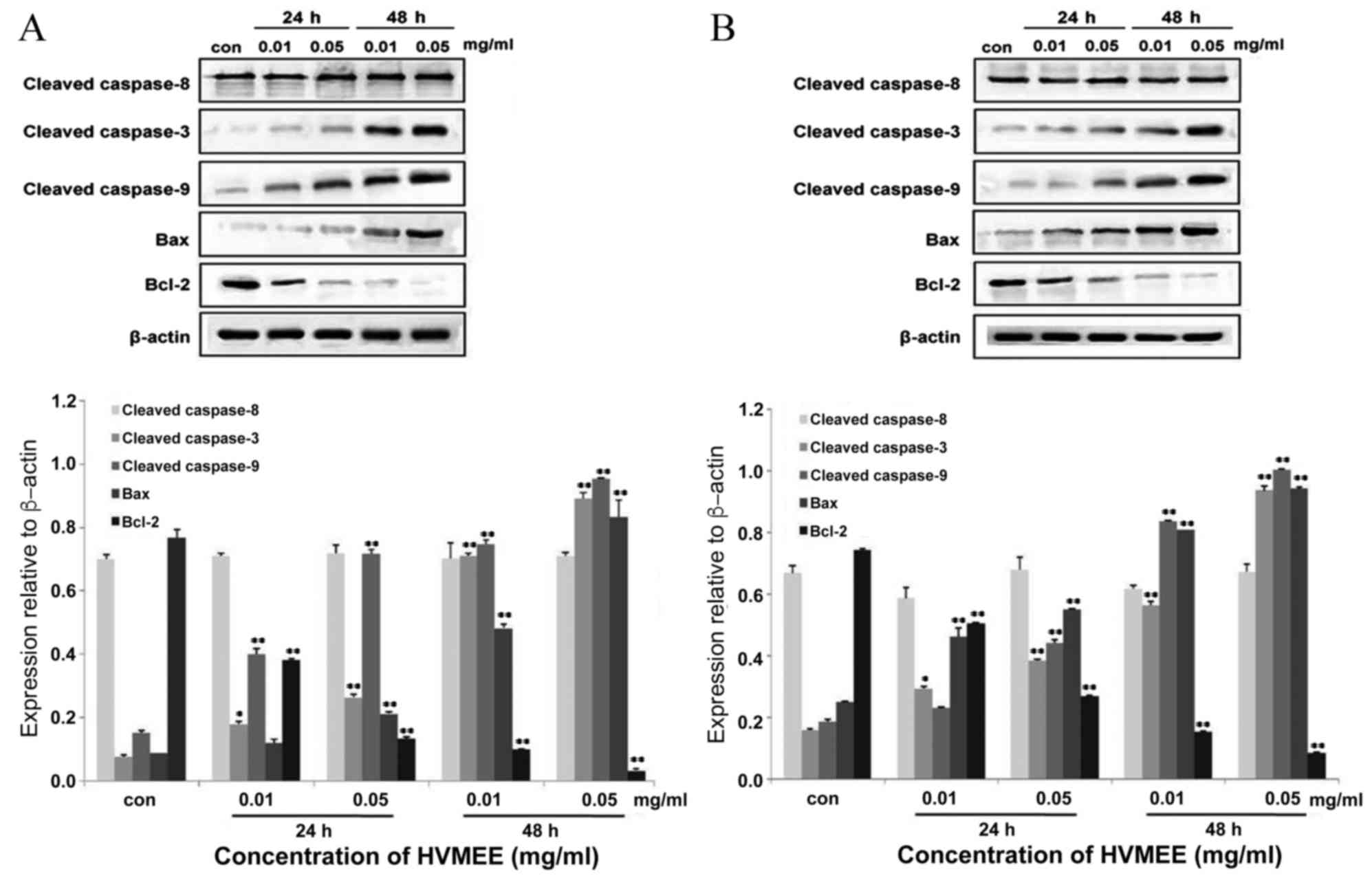
HVMEE affects apoptosis-related
protein expression
Following 24 and 48 h of 0.01 and 0.05 mg/ml HVMEE
treatment in HT-29 and SW620 cells, expression levels of several
apoptosis-related proteins were examined by western blotting
(Fig. 6A and B, respectively).
Expression levels of pro-apoptotic proteins cleaved caspase-3,
cleaved caspase-9, and Bax increased in HVMEE-treated cells
compared with untreated cells (Fig.
6). Expression levels of cleaved caspase-8 presented no
significant change (Fig. 6). By
contrast, expression levels of Bcl-2, an apoptosis inhibitor and a
central regulator of caspase activation, significantly decreased in
the HVMEE-treated cells compared with untreated cells (Fig. 6).
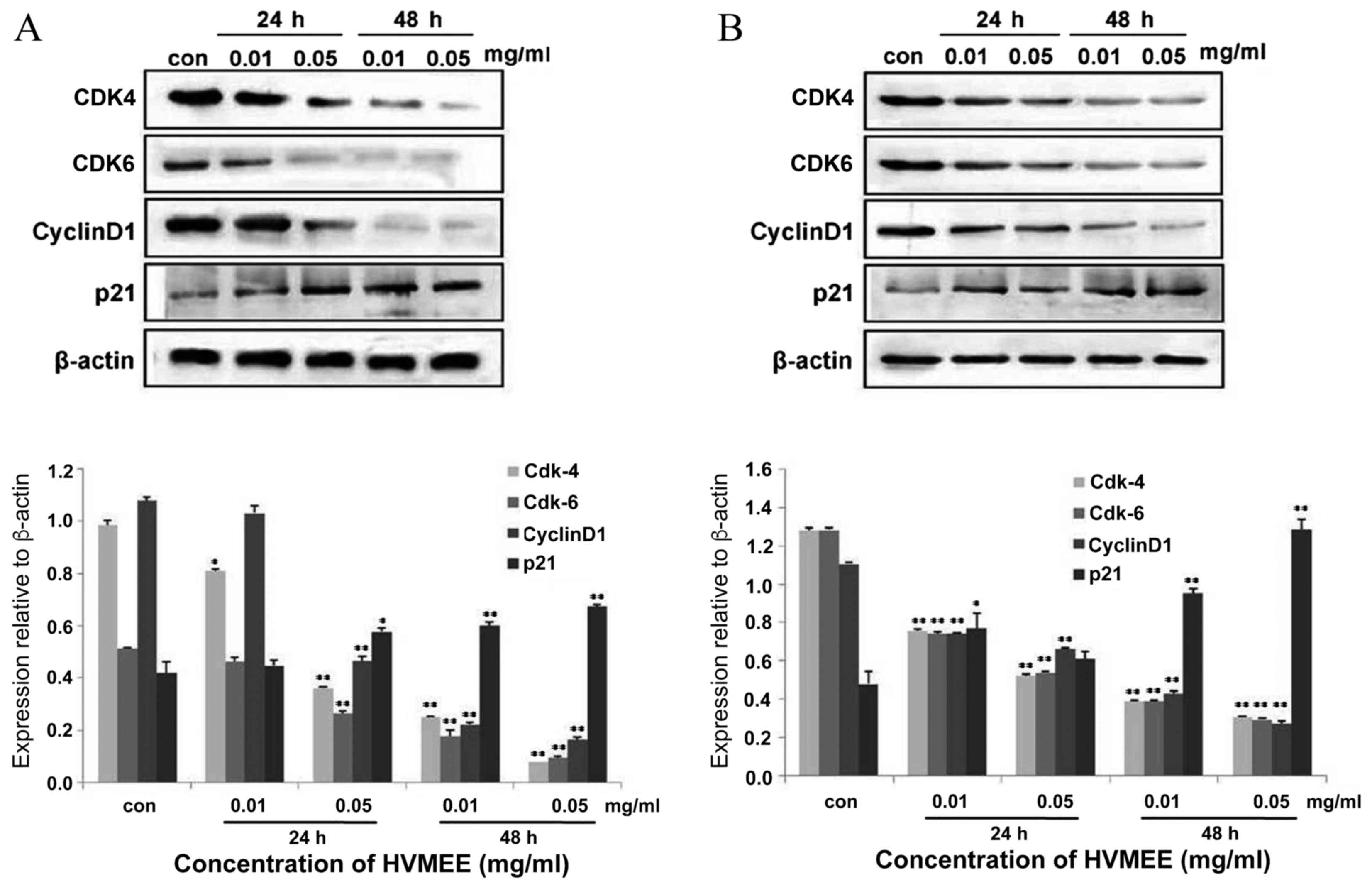
HVMEE affects cell cycle-related
protein expression
To test the mechanisms of HVMEE-induced cell cycle
arrest, expression of cell cycle-related proteins was further
assessed by western blotting. Following treatment of HT-29 and
SW620 cells with 0.01 and 0.05 mg/ml HVMEE for 24 and 48 h, the
expression levels of cyclin D1, cyclin dependent kinase (CDK) 4,
and CDK6 decreased significantly in HVMEE-treated cells compared
with the untreated cells (Fig. 7).
By contrast, protein expression levels of cyclin dependent kinase
inhibitor 1A (known as p21) were significantly upregulated in the
HVMEE-treated cells compared with the untreated cells (Fig. 7). Since induction of p21 leads to
CDK inhibition and cell cycle arrest at the G1/S
checkpoint (9–11), these data indicate that the
HVMEE-induced G1 phase arrest may be due to inhibition
of CDK4/6-cyclin D1 complexes by p21 upregulation.
Discussion
Apoptosis is an important process to maintain tissue
homeostasis by eliminating potentially deleterious cells.
Deregulated apoptotic cell death causes diseases, such as cancer.
In cancer cells, the incidence of apoptosis and the rate of cell
proliferation are uncontrolled (12,13).
Therefore, inducing apoptosis is a potential method of treating
cancer. Several chemotherapy drugs, including 5-FU, irinotecan, and
oxaliplatin, display anticancer effects partly by inducing
apoptosis in cancer cells (14–16).
In addition, many alkaloids, such as
6-Acetonyldihydrochelerythrine, piperine, and monoterpene bisindole
alkaloids, exert antitumor functions by affecting apoptosis
(14–16). In the present study, flow cytometry
was used to analyze the effects of HVMEE on apoptosis of colon
cancer cell lines HT-29 and SW620. The HT-29 cell line originated
from the primary tumor tissue of a colorectal cancer patient, while
the SW620 cell line originated from a lymph node metastatic site of
a colorectal cancer patient with a Dukes' C classification
(17,18). Therefore, these two cell lines were
selected to test the effects of HVMEE in order to represent the two
different states of colorectal cancer, at the primary and the
metastatic stage, respectively. The findings from the MTT and flow
cytometry assays demonstrated that HVMEE exhibited a more potent
effect on HT-29 cells than SW620 cells, indicating that HVMEE may
be more efficient in treating primary rather than metastatic
colorectal cancer.
Caspases, a family of cysteine proteases, are
central regulators of apoptosis. Caspase-8 is involved in the death
receptor apoptotic pathway and caspase-9 is involved in the
mitochondrial apoptotic pathway. Following activation, they cleave
and activate downstream effectors, such as caspase-3, which
subsequently cleave cytoskeletal and nuclear proteins (19). Western blot analysis demonstrated
that HVMEE treatment increased the expression levels of cleaved
caspase-3 and −9, but had no significant effect on expression of
cleaved caspase-8. HVMEE-induced caspase-3 cleavage was prevented
by pretreatment of HT-29 and SW620 cells with a pan-caspase
inhibitor, Z-VAD-FMK. These data suggest that HVMEE induced
apoptosis in CRC cells in a caspase-dependent manner.
The Bcl-2 protein family, which is important in the
mitochondrial apoptotic pathway and whose abnormal expression is
related to the development of CRC (20), is divided into two functional
subfamilies: Anti-apoptotic proteins [(Bcl-2 and BCL2-like 1
(Bcl-xL)] and pro-apoptotic proteins [(Bax and BH3 interacting
domain death agonist (Bid)] (21,22).
HVMEE greatly reduced Bcl-2 expression and increased Bax expression
in a time and concentration-dependent manner, suggesting that HVMEE
triggered apoptosis in CRC cells by regulating the Bax/Bcl-2
protein ratio.
The transition from one cell cycle phase to another
occurs in an orderly fashion and is regulated by two types of
important components: Cyclins and CDKs. Different cyclins reach
their maximum activity levels during different phases of the cell
cycle (23,24). In the present study, HVMEE
treatment was demonstrated to trigger G1 phase arrest.
In order to explore the mechanism, expression of proteins
associated with G1 phase transition was further
investigated. Cyclin D1 is overexpressed in many human cancers,
including CRC, and it binds to CDK4 and CDK6 to form a
CDK4/6-cyclin D1 complex, which is essential for cells to enter the
G1 phase (9). Western
blot analysis demonstrated that HVMEE treatment decreased
expression levels of cyclin D1, CDK4, and CDK6 compared to
untreated cells. p21, which prevents the replication of damaged
DNA, is important in CDK inhibition and G1 phase arrest
(10,11). HVMEE treatment increased p21
expression compared with untreated cells. Therefore, HVMEE may
induce G1 arrest in CRC cells partly through
upregulation of p21 expression. Of note, p53 expression levels in
HVMEE-treated HT-29 and SW620 cells exhibited no significant change
compared with the untreated cells (data not shown). HT-29 cells
have a mutant p53 gene which suggests that p53 may not be as
significant in cell cycle and apoptosis regulation in HT-29 cells
(25). Taken together, these
findings imply that HVMEE induced cell cycle apoptosis and arrest
in a p53-independent manner.
In conclusion, HVMEE was obtained from Hylomecon
vernalis and demonstrated to exhibit a potent growth inhibitory
effect in HT-29 and SW620 colorectal carcinoma cells. The possible
mechanism of HVMEE antitumor effect may be related to inducing
apoptosis and G1 phase arrest. These data suggest that
HVMEE may have potential in the clinical prevention and treatment
of colon cancer.
Acknowledgements
The present investigation was supported by The
Project of Science and Technology Research and Development of
Shaanxi Province (grant nos. 2012SF2-07 and 2013KJXX-71).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Winawer SJ: The multidisciplinary
management of gastrointestinal cancer. Colorectal cancer screening.
Best Pract Res Clin Gastroenterol. 21:1031–1048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baron JA, Cole BF, Sandler RS, Haile RW,
Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R,
Burke CA, et al: A randomized trial of aspirin to prevent
colorectal adenomas. N Engl J Med. 348:891–899. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang CH, Liu LF, Shiu LY, Huang YS, Chang
LC and Kuo KW: Action of solamargine on TNFs and
cisplatin-resistant human lung cancer cells. Biochem Biophys Res
Commun. 322:751–758. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: Preclinical and clinical studies.
Anticancer Res. 24:2783–2840. 2004.PubMed/NCBI
|
|
6
|
Yang H, Yin P, Shi Z, Ma Y, Zhao C, Zheng
J and Chen T: Sinomenine, a COX-2 inhibitor, induces cell cycle
arrest and inhibits growth of human colon carcinoma cells in vitro
and in vivo. Oncol Lett. 11:411–418. 2016.PubMed/NCBI
|
|
7
|
Kong LM, Feng T, Wang YY, Li XY, Ye ZN, An
T, Qing C, Luo XD and Li Y: Bisleuconothine A, a bisindole
alkaloid, inhibits colorectal cancer cell in vitro and in vivo
targeting Wnt signaling. Oncotarget. 7:10203–10214. 2016.PubMed/NCBI
|
|
8
|
Li W, Wen C, Bai H, Wang X, Zhang X, Huang
L, Yang X, Iwamoto A and Liu H: JNK signaling pathway is involved
in piperlongumine-mediated apoptosis in human colorectal cancer
HCT116 cells. Oncol Lett. 10:709–715. 2015.PubMed/NCBI
|
|
9
|
Sherr CJ: G1 phase progression: Cycling on
cue. Cell. 79:551–555. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ko LJ and Prives C: p53: Puzzle and
paradigm. Genes Dev. 10:1054–1072. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siliciano JD, Canman CE, Taya Y, Sakaguchi
K, Appella E and Kastan MB: DNA damage induces phosphorylation of
the amino terminus of p53. Genes Dev. 11:3471–3481. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rudolf E, Kralova V, Rudolf K and John S:
The role of p38 in irinotecan-induced DNA damage and apoptosis of
colon cancer cells. Mutat Res. 741–742. 27–34. 2013.
|
|
13
|
Arango D, Wilson AJ, Shi Q, Corner GA,
Arañes MJ, Nicholas C, Lesser M, Mariadason JM and Augenlicht LH:
Molecular mechanisms of action and prediction of response to
oxaliplatin in colorectal cancer cells. Br J Cancer. 91:1931–1946.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mansoor TA, Borralho PM, Luo X, Mulhovo S,
Rodrigues CM and Ferreira MJ: 6-Acetonyldihydrochelerythrine is a
potent inducer of apoptosis in HCT116 and SW620 colon cancer cells.
J Nat Prod. 77:1825–1830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yaffe PB, Coombs MR Power, Doucette CD,
Walsh M and Hoskin DW: Piperine, an alkaloid from black pepper,
inhibits growth of human colon cancer cells via G1 arrest and
apoptosis triggered by endoplasmic reticulum stress. Mol Carcinog.
54:1070–1085. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mansoor TA, Borralho PM, Dewanjee S,
Mulhovo S, Rodrigues CM and Ferreira MJ: Monoterpene bisindole
alkaloids, from the African medicinal plant Tabernaemontana
elegans, induce apoptosis in HCT116 human colon carcinoma cells. J
Ethnopharmacol. 149:463–470. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abdolahad M, Shashaani H, Janmaleki M and
Mohajerzadeh S: Silicon nanograss based impedance biosensor for
label free detection of rare metastatic cells among primary
cancerous colon cells, suitable for more accurate cancer staging.
Biosens Bioelectron. 59:151–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jordan A, Scholz R, Schüler J, Wust P and
Felix R: Arrhenius analysis of the thermal response of human
colonic adenocarcinoma cells in vitro using the multi-target,
single-hit and the linear-quadratic model. Int J Hyperthermia.
13:83–88. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li XF, Liu XF, Yang YY, Liu AY, Zhang MY,
Bai XF, Gao H and Guo XG: Correlation study of Bcl-2, B7-H1, EGFR,
VEGF and colorectal cancer. Am J Cancer Res. 5:2277–2284.
2015.PubMed/NCBI
|
|
21
|
Koehler BC, Scherr AL, Lorenz S, Urbanik
T, Kautz N, Elssner C, Welte S, Bermejo JL, Jäger D and
Schulze-Bergkamen H: Beyond cell death-antiapoptotic Bcl-2 proteins
regulate migration and invasion of colorectal cancer cells in
vitro. PLoS One. 8:e764462013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hector S and Prehn JH: Apoptosis signaling
proteins as prognostic biomarkers in colorectal cancer: A review.
Biochim Biophys Acta. 1795:117–129. 2009.PubMed/NCBI
|
|
23
|
Rowinsky EK, Cazenave LA and Donehower RC:
Taxol: A novel investigational antimicrotubule agent. J Natl Cancer
Inst. 82:1247–1259. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamilton G, Klameth L, Rath B and
Thalhammer T: Synergism of cyclin-dependent kinase inhibitors with
camptothecin derivatives in small cell lung cancer cell lines.
Molecules. 19:2077–2088. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harper JW: Cyclin dependent kinase
inhibitors. Cancer Surv. 29:91–107. 1997.PubMed/NCBI
|