Introduction
Technologies for reprogramming differentiated cells
into a pluripotent cell line have provided a novel perspective into
regenerative medicine. Induced pluripotent stem cells (iPS)
represent a potential source for the development of
disease-modeling assays, drug testing assays and cell-based
replacement therapies. To date, iPS have been derived from a number
of species, including mouse (1),
human (2), rhesus monkey (3), rat (4), pig (5), dog (6), marmoset (7,8),
sheep (9,10), bovine (11), buffalo (Bubalus bubalis)
(12), equine (13) and bat (14). It has been recognized that iPS
technology is one of the most promising methods for cell-based
regenerative medicine therapies (2,15).
Thus, suitable animal models are required. Of the species that may
be useful, the guinea pig offers significant advantages.
Over the past several decades, the guinea pig has
been a widely used experimental animal model for the study of
infectious disease, particularly Mycobacterium (M.)
tuberculosis. The guinea pig is suitable for studies of M.
tuberculosis as it is susceptible to human tubercle bacilli.
There are a number of similarities between guinea pigs and humans,
including the following: i) Newborn guinea pigs, like human
infants, have a very mature lymphomyeloid complex; ii) hormone
secretion and immunological responses in guinea pigs are more
similar to humans than rodents; iii) guinea pigs, like humans,
require an exogenous supply of ascorbic acid (vitamin C) in their
diet; iv) guinea pigs, like humans and non-human primates, are
corticosteroid-resistant; and v) humans and guinea pigs have
similar physiological aspects of the pulmonary tract, particularly
with regard to lung tissue responses to inflammatory stimuli
(16). These similarities indicate
that the guinea pig is a particularly useful model of human
infectious disease.
Guinea pigs have been widely used in to assess
biological reagents and drugs utilized in tuberculosis (TB),
particularly in the biological standardization of tuberculins used
for skin testing. The development and preclinical testing of the
bacillus Calmette-Guérin vaccine was primarily based on guinea pig
models (16). Due to the response
of the guinea pig to anti-TB antibiotics, the species has been used
successfully to evaluate the efficacy of novel drugs and drug
combinations. With the development of multi drug-resistant strains
of M. tuberculosis, the guinea pig may be vital for the
identification of novel and efficient anti-myobacterial drugs
(16,17).
Proliferation of guinea pig embryonic fibroblasts
may be performed for only a limited number of passages (18); therefore, the successful generation
of guinea pig iPS would provide a model to facilitate research at
the cellular and molecular levels. To the best of our knowledge,
there have been no previous reports of guinea pig embryonic stem
cell (ESC) generation and, although ESCs have potential for future
cell therapy and regenerative medicine, iPS technologies would have
the specific advantages that: i) Derivatives of iPS may be suitable
for regenerative medicine, avoiding ethical concerns and potential
immune rejection; and ii) iPS may potentially be derived from donor
individuals of differing ages, health status and genetic
backgrounds. Therefore, the present study generated iPS from guinea
pigs as an initial step towards the development of experimental
cell therapy using autologous iPS from this species.
Materials and methods
Experimental animals
All animal procedures were approved by the Animal
Care and Use Committee of the Zhejiang Sci-Tech University
(Hangzhou, China). One guinea pig (Dunkin-Hartley; female; age, 1
year; 40 days pregnant; weight, 1,200 g) and 8 mice (BALB/c-nu;
female; age, 6–8 weeks; weight, 18–25 g) were provided by the
Laboratory Animal Center of Ningxia Medical University (Yinchuan,
China) and the Laboratory Animal Center of Zhejiang University
(Hangzhou, China). Animals were maintained at room temperature with
normal humidity, and were given free access to food and water under
a 12-h dark/light diurnal cycle.
Cell culture
Guinea pig iPS (giPS) were cultured on feeder layers
of mitomycin C-treated guinea pig fibroblast cells. All reagents
were purchased from Sigma Aldrich; Merck Millipore (Darmstadt,
Germany) unless otherwise indicated. giPS were passaged every 3
days. giPS were cultured in KnockOut™ Dulbecco's
modified Eagle's medium (DMEM) containing 0.5% glutamine, 1.0%
nonessential amino acids, 1 mM sodium pyruvate, 0.1 mM
β-mercaptoethanol, 15% ESC-qualified fetal bovine serum (FBS;
SH30406.02E; HyClone; GE Healthcare Life Sciences, Logan, UT, USA),
100 U/ml penicillin, 100 mg/ml streptomycin (15070–063; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1,000 U/ml
mouse leukemia inhibitory factor (LIF; ESG1106; EMD Millipore,
Billerica, MA, USA). To determine whether the giPS colonies could
grow under feeder-free culture conditions, giPS were alternatively
cultured in feeder-free culture conditions on gelatin-coated dishes
using ESGRO Complete Plus Clonal Grade medium (SF001-500P; EMD
Millipore) containing 15% FBS. giPS were cryopreserved in
ESC-qualified fetal bovine serum containing 10% dimethyl sulfoxide
(cell culture grade; A3672; AppliChem GmbH, Darmstadt, Germany).
The 293FT packaging cells (Invitrogen; Thermo Fisher Scientific,
Inc.) (19,20), which were used to produce
lentiviruses, were cultured in DMEM containing 10% FBS, 50 U/ml
penicillin and 50 mg/ml streptomycin.
To isolate guinea pig fetal fibroblasts, a
40-day-pregnant guinea pig was washed with phosphate-buffered
saline (PBS) followed by 75% alcohol. The animal was then
sacrificed and a fetus was collected, and their heads and visceral
tissues were removed. The remaining fetal tissues were washed in
fresh PBS, transferred into a 0.1 mM trypsin/1 mM EDTA solution (3
ml mixture/embryo) and incubated at 37°C for 20 min. Following
incubation, 3 ml 0.1 mM trypsin/1 mM EDTA solution was added to
each embryo, and embryos were incubated at 37°C for 20 min.
Following trypsinization, 6 ml DMEM containing 10% FBS was added to
each embryo, pipetting up and down a few times to dissociate cells.
Samples were incubated at room temperature for 5 min and
transferred into a new tube. Cells were collected by room
temperature centrifugation for 5 min at 1,000 × g, and then
resuspended in fresh medium. For the first passage,
1×106 cells were cultured on 100-mm dishes at 37°C with
5% CO2. The present study used guinea pig fibroblasts at
passages 3–5 to avoid replicative senescence.
Lentiviral infection and iPS cell
derivation
To obtain high-quality fetal fibroblasts, guinea pig
fibroblasts were isolated and morphologically characterized in
vitro. The lentiviral plasmid, FUW-OSKM (Addgene plasmid no.
20328; www.addgene.org/20328/), which is a
single polycistronic virus encoding octamer-binding transcription
factor 4 (Oct4), sex determining region Y-box 2 (Sox2),
Kruppel-like factor 4 (Klf4) and c-Myc, was donated by Dr. Rudolf
Jaenisch (Massachusetts Institute of Technology, Cambridge, MA,
USA) (15). giPS were generated
from fibroblasts and used at passage3-5, as described by Carey
et al (15). Briefly, 293FT
cells were plated at a density of 2×106 cells per 60-mm
dish. The following day, cells were transfected with 12 µg/ml
FUW-OSKM as well as 9 µg/ml PsPAX2 and 3.6 µg/ml PMD.2G (PMD.2G
encodes the viral protein V-G, and PsPAX2 is a packaging vector.
These two plasmids were donated by Dr. Peter Hornsby (University of
Texas Health Science Center at San Antonio) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Following transfection for 24 h, the supernatant of the
transfectant was collected and filtered using a 0.45-mm pore-size
Whatman® cellulose acetate filter (Sigma-Aldrich; Merck
Millipore) and concentrated by centrifugation at 10,000 × g for 3 h
at 4°C. Guinea pig fibroblasts were seeded at 4×105
cells per 35-mm dish and the following day the medium was replaced
with virus-containing supernatant supplemented with 8 µg/ml
polybrene (Nacalai Tesque, Inc., Kyoto, Japan) prior to incubation
for 24 h; this process was repeated three times. A total of 12 h
following the last infection, the medium was replaced with
fibroblast culture medium. Fibroblasts were passaged using trypsin
and plated at densities between 5×104 and
5×105 cells/10-cm on gelatin-coated dishes of guinea pig
fibroblast feeder layers, following infection for 5 days. For
reprogramming, the culture medium was replaced 24 h later by giPS
medium in the presence of 1 mM valproic acid and 10 µg/ml vitamin
C. The resultant giPS colonies were picked mechanically based on
morphology and maintained according to previously used mouse iPS
protocols (1). Colonies that were
compact with clear edges were manually selected and expanded on
guinea pig fibroblast feeder layers. One of the picked cell lines
was selected for further study and passaged >30 times.
Western blot analysis
Cells were prepared and lysed using Qproteome
Mammalian Protein Prep kit (Qiagen, Inc., Valencia, CA, USA), and
protein concentrations were determined using the Bradford protein
assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal
amounts of total protein (60 µg/lane) were boiled in denaturing
loading buffer (200 mM Tris-HCl at pH 6.8, 50% glycerol, 8% SDS,
400 mM DTT, 0.4% bromophenol blue), separated by 10% SDS-PAGE and
subsequently transferred to polyvinylidene difluoride (PVDF)
membranes (Bio-Rad Laboratories, Inc.). The membranes were blocked
in 5% non-fat milk powder in TBS containing 0.1% Tween-20 (TBST) at
pH 7.6 following the protocol of the antibody manufacturer. PVDF
membranes were incubated with the following primary antibodies
following a brief wash in TBST: Rabbit anti-Nanog (1:1,000;
14295-1-AP), rabbit polyclonal β-actin (1:1,000; 20536-1-AP),
rabbit anti-Sox2; 1:2,000; 11064-1-AP) and rabbit anti-Oct4; 1:500;
11263-1-AP), all purchased from ProteinTech Group, Inc., Chicago,
IL, USA. Antibodies were diluted in 2% non-fat milk powder in TBST,
and incubated overnight at 4°C. The membranes were subsequently
incubated with horseradish peroxidase-conjugated anti-rabbit
immunoglobulin (IgG) antibodies (1:2,000; SA00001-2; ProteinTech
Group, Inc.) for 60 min at room temperature, followed by detection
with an Enhanced Chemiluminescence reagent (GE Healthcare Life
Sciences, Chalfont, UK). β-actin served as a loading control.
Alkaline phosphatase staining and
immunocytochemistry for pluripotency markers
To assess alkaline phosphatase activity and
expression of pluripotency markers in giPS, cells were washed three
times in PBS, fixed in 4% paraformaldehyde at room temperature for
30 min and washed a further three times with PBS. For alkaline
phosphatase analysis, cells were subsequently incubated with
SIGMAFAST5-bromo-4-chloro-3-indolyl phosphate/nitro blue
tetrazolium (Sigma-Aldrich; Merck Millipore), according to the
manufacturer's protocol. For immunofluorescence, cells were
incubated with the following primary antibodies: Rabbit anti-Oct4
(1:200), mouse anti-stage-specific embryonic antigen 1 (SSEA1;
1:100; ab16285; Abcam, Cambridge, UK), and rabbit anti-Nanog
(1:200) and incubated at 4°C overnight. The following day, the
cells were incubated at room temperature for 60 min with the
following secondary antibodies: Goat anti-rabbit IgG
(H+L)-fluorescein isothiocyanate conjugated or donkey anti-mouse
IgG(H+L)-Alexa Fluor 555-labeled (A0562 and A0460; both from
Beyotime Institute of Biotechnology, Haimen, China). Cells were
counterstained with DAPI (C1005; Beyotime Institute of
Biotechnology). Images were captured using a fluorescence
microscope.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated using TRIzol®
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. RNA was prepared from guinea pig
fibroblasts and mouse induced pluripotent stem cells (donated by
Dr. Duanqing Pei; Guangzhou Institute of Biomedicine and Health,
Chinese Academy of Sciences) to serve as the control. A total of
500 ng RNA was treated using the DNaseI, RNase-free kit (Takara
Biotechnology Co., Ltd., Dalian, China) to remove any potential
genomic DNA contamination. The RevertAid First Strand cDNA
Synthesis kit (Takara Biotechnology Co., Ltd.) was used to
synthesize cDNA using a random hexamer primer, according to the
manufacturer's protocol. qPCR reactions were performed in duplicate
using the Power SYBR-Green Master mix (Takara Biotechnology Co.,
Ltd.), and the Bio-Rad iCycler iQ and iQ5 Real-Time PCR Detection
system (Bio-Rad Laboratories, Inc.). The thermocycling conditions
were as follows: Initial cycle at 95°C for 30 sec, followed by 40
cycles of 95°C denaturation for 5 sec, 60°C annealing for 30 sec
and 72°C extension for 15 sec. Expression values were normalized to
those of the β-actin housekeeping gene using the ΔΔCq method
(21). Primer sequences are
presented in Table I.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence
(5′-3′) | Size (bp) |
|---|
| Oct4 | F:
GCATACGAGTTCTGCGGAGGGAT | 191 |
|
| R:
GGTTCCACCTTCTCCAACTTCACG |
|
| Sox2 | F:
ACAGATGCAACCGATGCACCGC | 213 |
|
| R:
GCCCTGGAGTGGGAGGAAGAGGTAA |
|
| Nanog | F:
GCCCTACACCGTCCTTTCG | 170 |
|
| R:
GCCATTTCTTGCATTTCATTCTC |
|
| Tbx3 | F:
GGAGCAGTGGATGTCTAAAGTG | 169 |
|
| R:
AGTCCGAAATGTACTATAAGGGAG |
|
| β-actin | F:
AGACGAAGCCCAGAGCAAA | 273 |
|
| R:
CCAGAGGCATACAGGGACAG |
|
Karyotyping analysis
Karyotyping was performed using conventional
techniques as described by Wu et al (22). Briefly, chromosomes were analyzed
from actively proliferating cultures of giPS. Cells were treated
with colchicine at 37°C for 6 h, trypsinized, centrifuged for 5 min
at 1,000 × g at room temperature and incubated with 0.04 M KCI at
37°C for 20 min. The cells were subsequently fixed for 20 min using
ice-cold 1:3 (v/v) acetic acid/methanol and collected. The
resultant dispersed giPS suspension was smeared onto a cold slide,
dried, stained with 10% Giemsa for 20 min at room temperature and
observed microscopically.
Teratoma formation and hematoxylin and
eosin (H&E) staining
To assess the differentiation potential of giPS,
giPS were implanted into severe combined immunodeficient mice. A
total of 2×106 cells were suspended in 100 µl culture
medium containing 50% Matrigel (23,24)
and injected subcutaneously into the mice. Mice injected with
guinea pig fibroblast cells served as controls. A total of eight
weeks later, mice were sacrificed. Tumors were collected, fixed in
4% paraformaldehyde and examined using conventional histological
procedures (7). The H&E method
was used to stain the sections (6 µm).
Statistical analysis
Data were presented as the mean ± standard deviation
of three experiments with n=3 repeats. All statistical analyses
were performed using SPSS version 13.0 (SPSS, Inc., Chicago, IL).
One-way analysis of variance followed by Scheffe's post hoc test
was used for analysis of multiple groups. An independent samples
Student's t-test was used for comparison of two groups. P<0.01
was considered to indicate a statistically significant
difference.
Results
Reprogramming of guinea pig fetal
fibroblasts to giPS
Following the infection of guinea pig fibroblasts
with a single polycistronic virus encoding Oct4, Sox2, Klf4 and
c-Myc, cultures were maintained in ESC conditions in the presence
of valproic acid (25) and vitamin
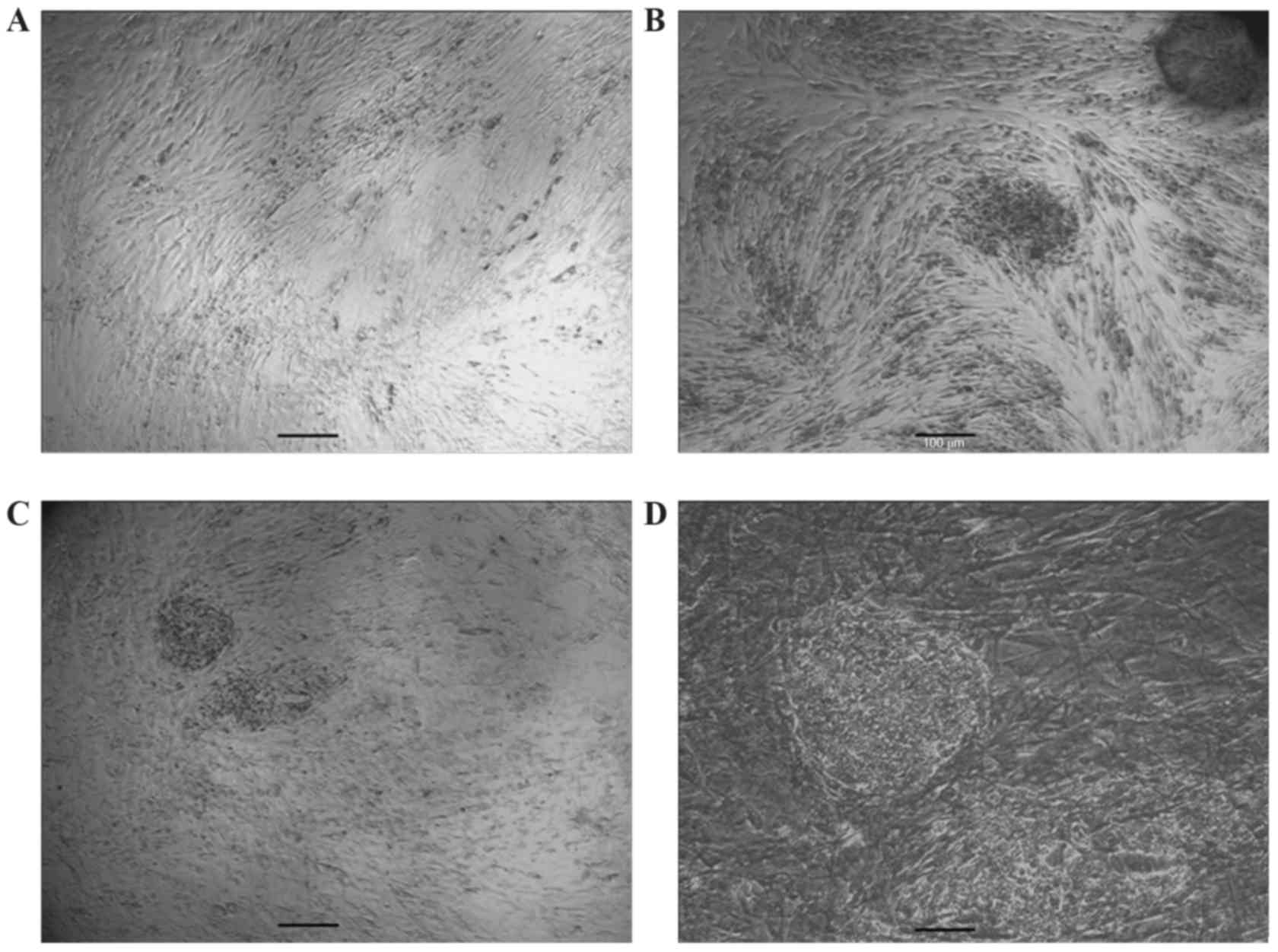
C. Following 10 to 14 days, a number of small colonies with altered
morphology appeared in the confluent fibroblast cultures. The cells
expressed a high nuclear/cytoplasmic ratio and prominent nucleoli
as presented in Fig. 1. Colonies
that were compact with clear edges were manually selected and
expanded on guinea pig fibroblast feeder layers. One of the picked
cell lines was selected for further study and passaged >30
times. giPS at passage 30 with mouse ESC-like morphology are
presented in Fig. 2. When giPS
were cultured in media not containing LIF, their typical ESC
morphology and differentiation was altered. This indicated that
giPS were similar to mouse ESC. When such colonies were fixed and
stained for alkaline phosphatase activity analysis, they were
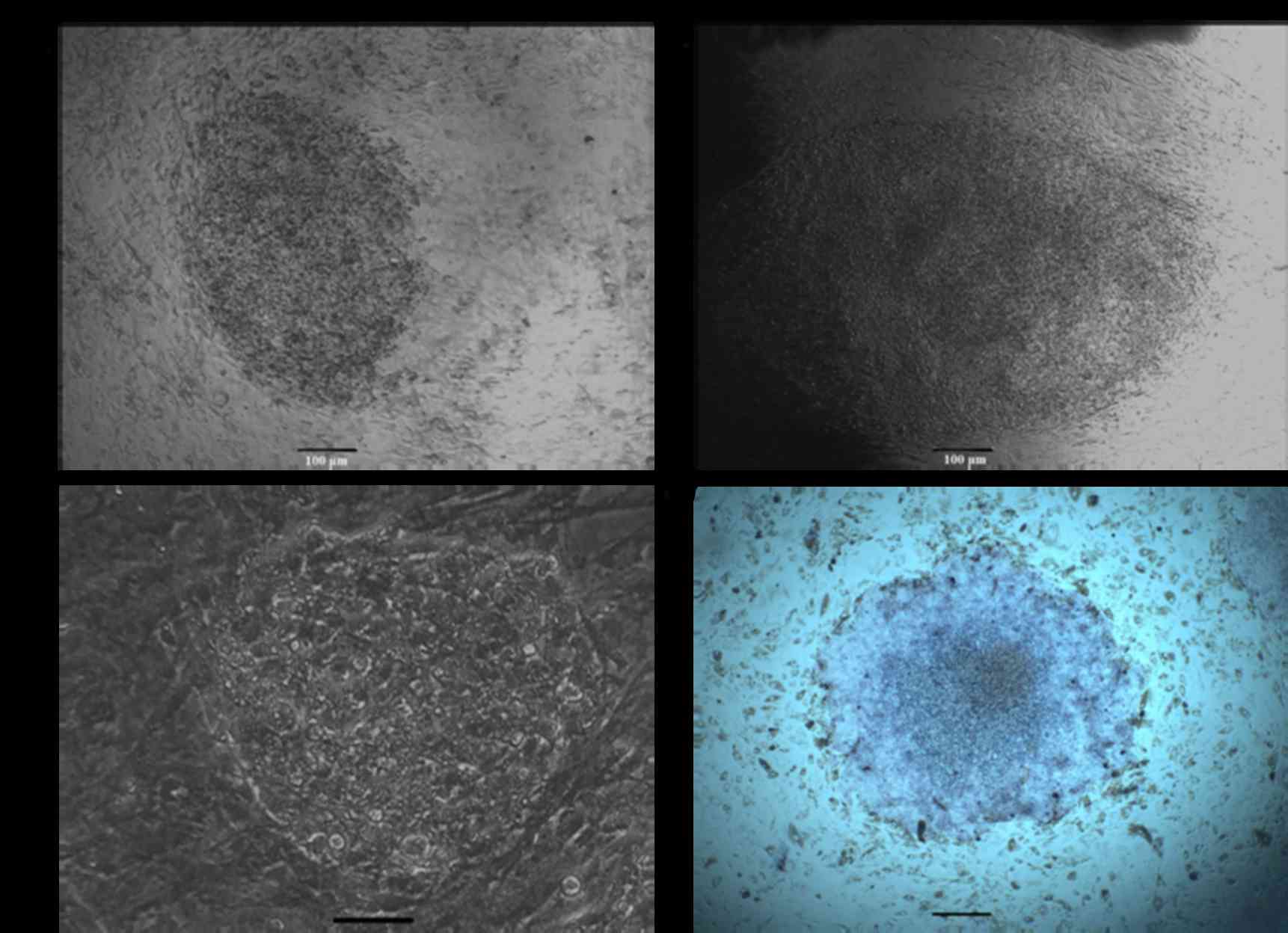
positive for alkaline phosphatase (Fig. 2). To determine whether colonies
could grow under feeder-free culture conditions, cells were
alternatively plated on gelatin-coated dishes in ESGRO Complete
Plus Clonal Grade Medium containing 15% FBS. As presented in
Fig. 3A, cells continued to grow
rapidly. In addition, alkaline phosphatase staining analysis
demonstrated that the dense patches of small rapidly dividing cells
were alkaline phosphatase positive (Fig. 3B). Clones used for detailed studies
in the present research have been grown continuously in culture at
normal growth rates for >30 passages.
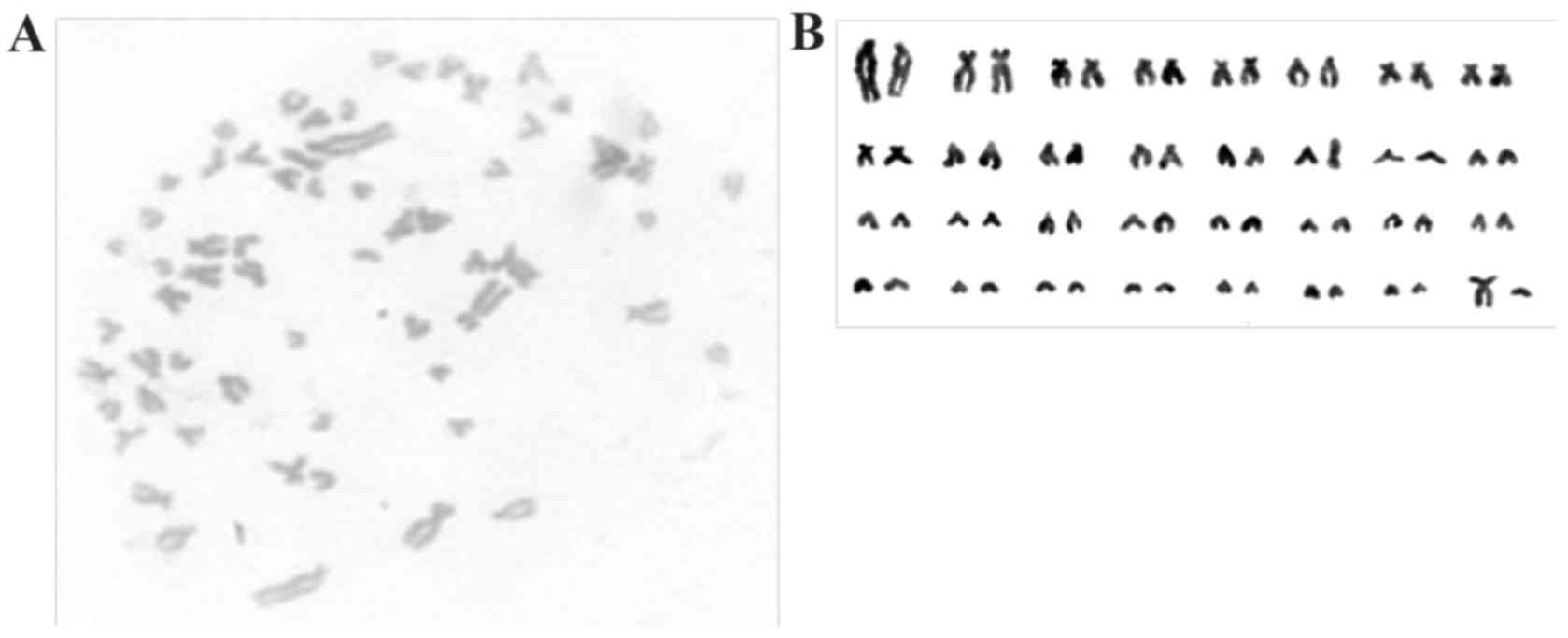
In karyotypic analysis performed at passage 30, giPS
exhibited a normal guinea pig karyotype of 64 XY, as presented in
Fig. 4.
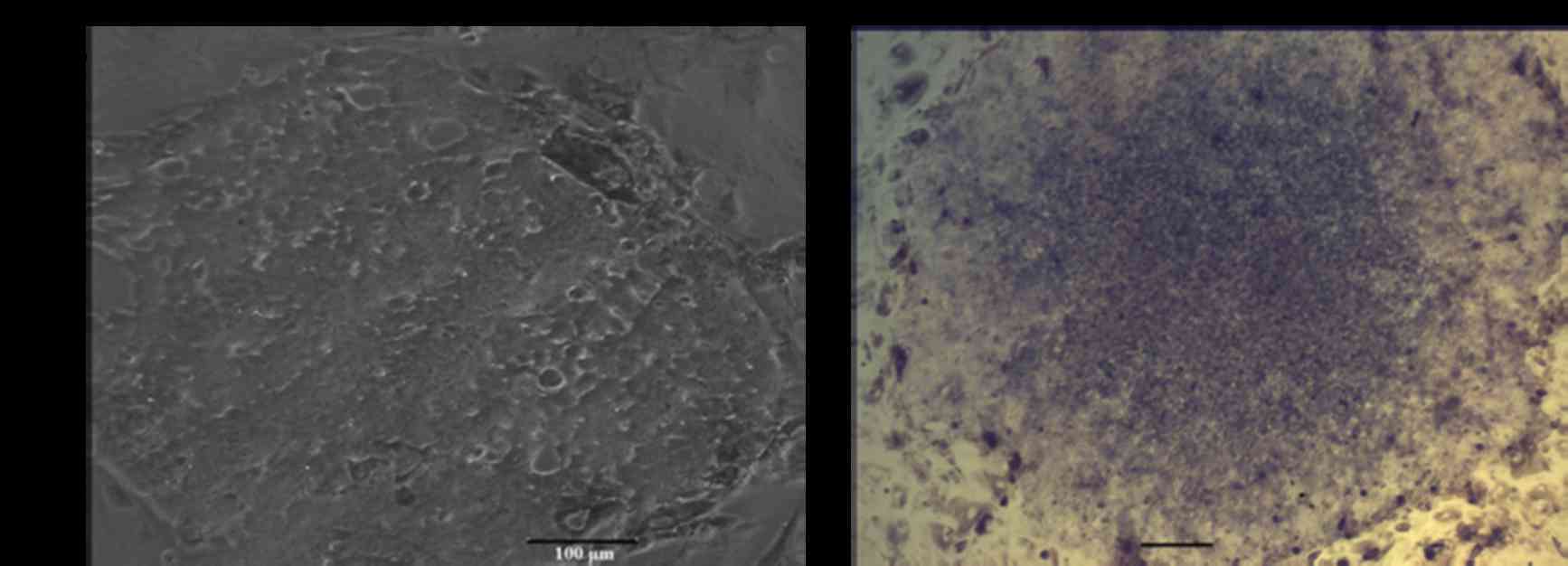
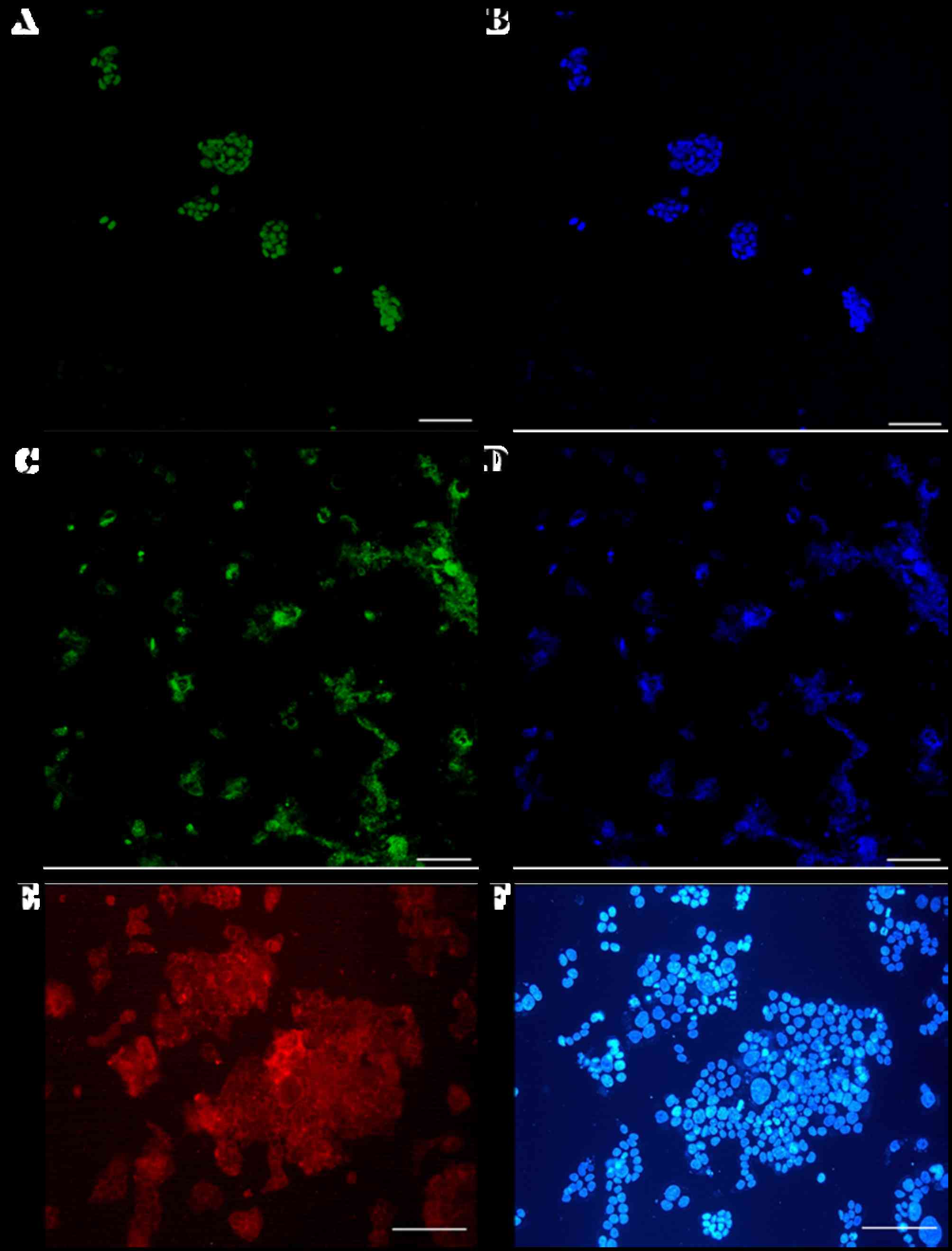
Markers of pluripotency in giPS
Immunostaining analyses demonstrated that
pluripotency markers of giPS were expressed at the protein level
(Fig. 5). giPS stained positive
for Nanog (Fig. 5A and B) and Oct4
(Fig. 5C and D). The cells were
additionally positive for the cell-surface marker SSEA1 (Fig. 5E and F), further verification that
giPS exhibited pluripotency.
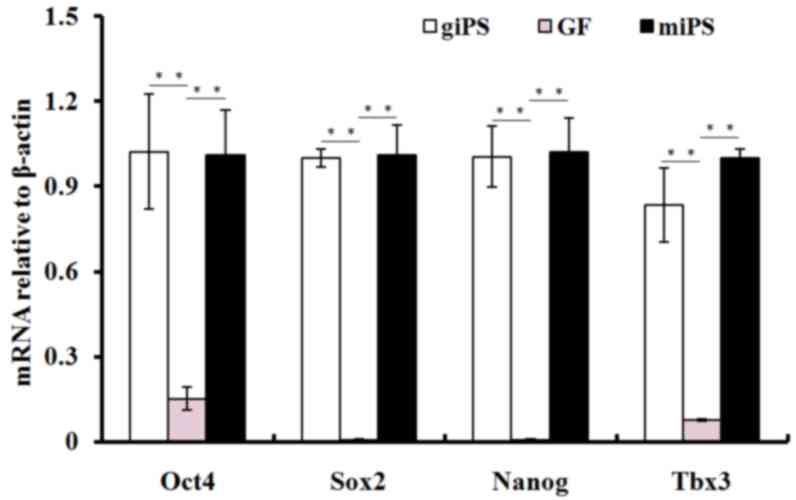
RT-qPCR was performed to assess the expression of
pluripotency genes in giPS, using guinea pig fibroblasts as a
control. Relative gene expression levels for pluripotency genes
Oct4, Sox2, T-box 3 (Tbx3) and Nanog are presented in Fig. 6. The mRNA expression levels of
Oct4, Sox2, Tbx3 and Nanog increased significantly (P<0.01) in
giPS compared with guinea pig fibroblasts, and were similar to
those of mouse iPS.
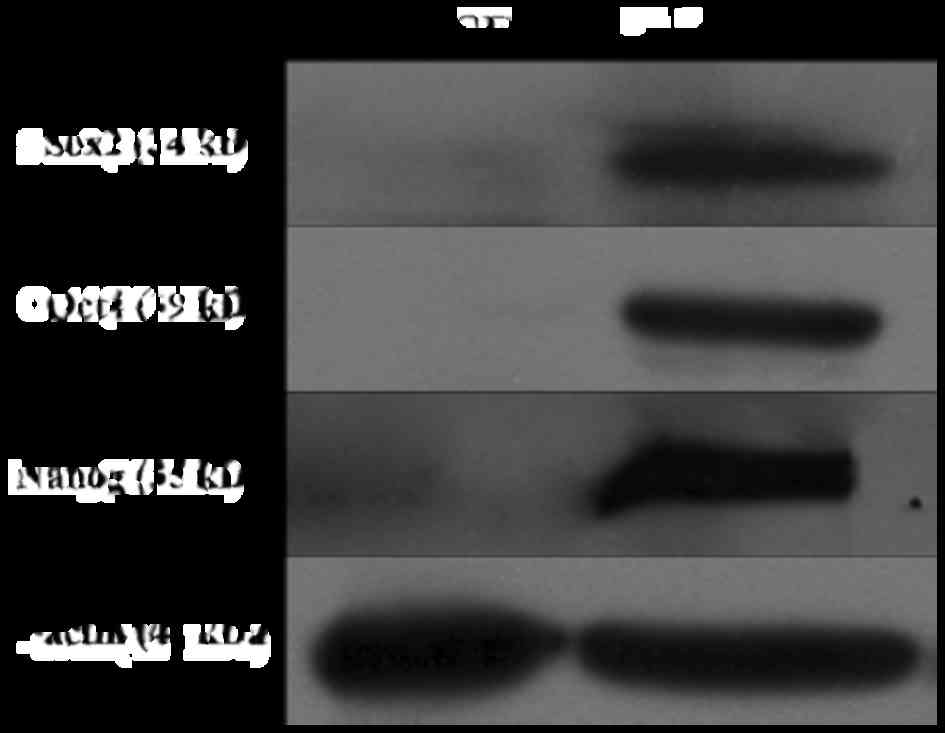
To further assess protein expression of pluripotency
factors in giPS, western blot analyses were performed. The results
revealed that Oct4, Sox2 and Nanog were expressed in giPS, but not
in guinea pig fibroblasts, which further confirmed the pluripotency
of giPS (Fig. 7).
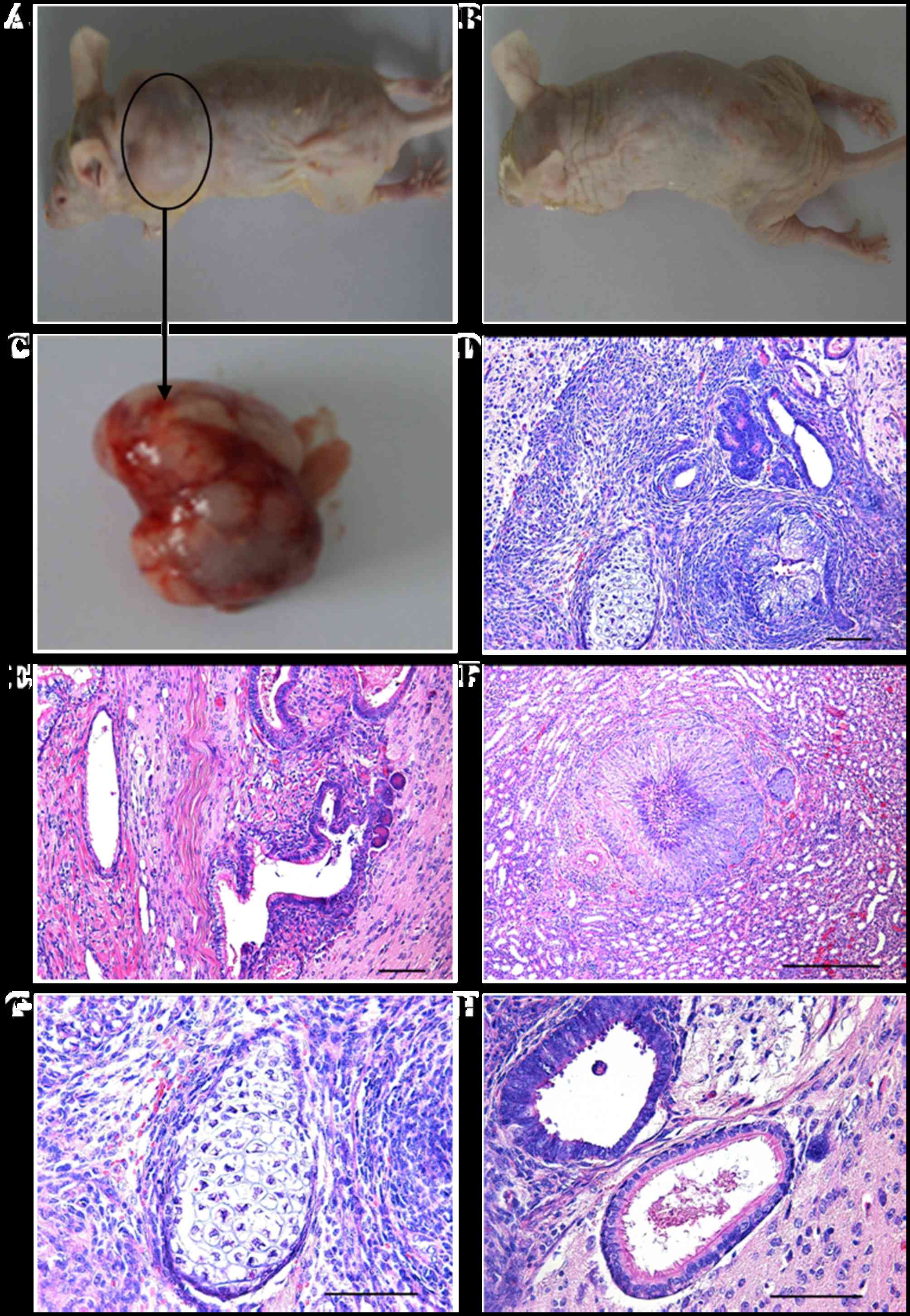
Differentiation of giPS in vivo
To assess whether giPS have the ability to
differentiate into all three germ layer cell types in vivo,
giPS were transplanted into immunodeficient mice by subcutaneous
injection. Teratomas developed at the subcutaneous sites. H&E
staining demonstrated that the teratomas were comprised of a number
of different tissue types, which represented all three germ layers,
including the ectoderm (neural rosettes), mesoderm (cartilage and
muscle) and endoderm (gland like cells), as presented in Fig. 8.
Discussion
With the development of iPS technology, these cells
have become a valuable tool for cell therapy and regenerative
medicine (7,22). iPS have the potential to
differentiate into numerous cell types. For differentiated cells,
derived from individual specific iPS, to be transplanted
successfully into individuals, translational proof of principle
that such procedures are safe and effective is required.
Consequently, appropriate studies are required in suitable
mammalian models. Although rodents and other species are excellent
cell sources for reprogramming, it is generally accepted that, with
the rise of multi drug-resistant strains of M. tuberculosis
(17), the guinea pig is the most
appropriate mammalian model for developing new and efficient
antimycobacterial drugs.
The present study derived lines of giPS from guinea
pig fetal fibroblasts and established a suitable cell culture
system. These giPS exhibited similar characteristics to mouse iPS.
They had typical ESC morphology, proliferated rapidly, expressed
pluripotent genes and markers at the protein and mRNA levels and
grew in an LIF-dependent manner. Teratoma analysis has commonly
been used to determine pluripotency and the in vivo
differentiation ability of iPS (1); it is considered to be the gold
standard for characterizing new iPS lines. The results of the
present study indicated that guinea pig cells have the ability to
differentiate in vivo.
Retroviral and lentiviral vectors were the earliest
established methods to deliver reprogramming factors and have been
widely used. Despite their tendency to randomly and stably
integrate into chromosomes, which may increase the risk of
tumorigenesis (26), they have a
high reprogramming efficiency when compared to other methods
(14).
The methodologies applied in the present study were
similar to those typically performed; however, some were modified
significantly. It is commonly recognized that using feeder-free
conditions for iPS and ESC growth results in significant advantages
for subsequent differentiation and cell transplantation (7). In the majority of previous
reprogramming studies, iPS have been generated and grown on feeder
layers. In certain studies, cells were later transferred to
feeder-free conditions (7,22). In the present study, giPS were
adapted to feeder-free conditions at an early stage. giPS grew well
under these conditions, as they were expanded and subsequently
cryopreserved.
In certain circumstances, the transfer of human ESCs
to feeder-free culture conditions, following routine growth on
feeder layers, has resulted in abnormal karyotypes (27). Karyotypic abnormalities
characterize iPS as ‘low quality’, thereby restricting further use
of such cells. In the present study, giPS continued to exhibit a
normal karyotype following transfer to feeder-free culture
conditions. In addition, they maintained typical iPS/ESC
morphology. These cells were alkaline phosphatase positive and
expressed markers of pluripotency.
Vitamin C may suppress cell senescence (28), and valproic acid (25) has been reported to promote
reprogramming. Therefore, to enhance giPS generation, vitamin C was
mixed with valproic acid in the ESC medium. This served an
important role in suppressing the potential adverse consequences of
transgene expression, particularly of c-Myc (29). The viral based methodology of iPS
generation is valuable for basic and translational experiments in
animals. For clinical use, however, reprogramming methods must be
established in such a way that the reprogramming vectors may be
excised from the cells prior to therapeutic application (30,31);
alternatively, methods may avoid genetic modification completely
(32,33). Thus, the generation of giPS
requires further study, particularly to generate insertion-free
giPS. However, the relative efficiency of reprogramming makes the
viral vector method attractive for solving basic issues. Once these
problems are solved, relatively safer methods may be developed. The
characterization of giPS in the present study may provide proof of
principle of iPS generation for this species and support the future
generation of individual-specific iPS for proof of principle
experiments on autologous cell therapy in guinea pigs.
Acknowledgements
The authors thank Dr William D. Hohenboken (Oregon
State University) for critical evaluation of the manuscript and
helpful comments. The present study was supported by the National
Natural Science Foundation of China (grant nos. 31260287, 31201867
and 31460585), the National Key Basic Research Program of China
(973 Program; grant no. 2012CB518801), the Zhejiang Provincial
Natural Science Foundation of China (grant no. LY17C120001) and the
Science Foundation of Zhejiang Sci-Tech University (grant nos.
11612932611535 and 11610431251501).
References
|
1
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu H, Zhu F, Yong J, Zhang P, Hou P, Li
H, Jiang W, Cai J, Liu M, Cui K, et al: Generation of induced
pluripotent stem cells from adult rhesus monkey fibroblasts. Cell
stem cell. 3:587–590. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liao J, Cui C, Chen S, Ren J, Chen J, Gao
Y, Li H, Jia N, Cheng L, Xiao H and Xiao L: Generation of induced
pluripotent stem cell lines from adult rat cells. Cell stem cell.
4:11–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ezashi T, Telugu BP, Alexenko AP, Sachdev
S, Sinha S and Roberts RM: Derivation of induced pluripotent stem
cells from pig somatic cells. Proc Natl Acad Sci USA. 106:pp.
10993–10998. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimada H, Nakada A, Hashimoto Y, Shigeno
K, Shionoya Y and Nakamura T: Generation of canine induced
pluripotent stem cells by retroviral transduction and chemical
inhibitors. Mol Reprod Dev. 77:22010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Y, Zhang Y, Mishra A, Tardif SD and
Hornsby PJ: Generation of induced pluripotent stem cells from
newborn marmoset skin fibroblasts. Stem Cell Res. 4:180–188. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Y, Mishra A, Qiu Z, Farnsworth S,
Tardif SD and Hornsby PJ: Nonhuman primate induced pluripotent stem
cells in regenerative medicine. Stem Cells Int:. 2012:7671952012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J, Balehosur D, Murray B, Kelly JM,
Sumer H and Verma PJ: Generation and characterization of
reprogrammed sheep induced pluripotent stem cells. Theriogenology.
77:338–346.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bao L, He L, Chen J, Wu Z, Liao J, Rao L,
Ren J, Li H, Zhu H, Qian L, et al: Reprogramming of ovine adult
fibroblasts to pluripotency via drug-inducible expression of
defined factors. Cell Res. 21:600–608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han X, Han J, Ding F, Cao S, Lim SS, Dai
Y, Zhang R, Zhang Y, Lim B and Li N: Generation of induced
pluripotent stem cells from bovine embryonic fibroblast cells. Cell
Res. 21:1509–1512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng Y, Liu Q, Luo C, Chen S, Li X, Wang
C, Liu Z, Lei X, Zhang H, Sun H, et al: Generation of induced
pluripotent stem cells from buffalo (Bubalus bubalis) fetal
fibroblasts with buffalo defined factors. Stem Cells Dev.
21:2485–2494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagy K, Sung HK, Zhang P, Laflamme S,
Vincent P, Agha-Mohammadi S, Woltjen K, Monetti C, Michael IP,
Smith LC and Nagy A: Induced pluripotent stem cell lines derived
from equine fibroblasts. Stem Cell Rev. 7:693–702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mo X, Li N and Wu S: Generation and
characterization of bat-induced pluripotent stem cells.
Theriogenology. 82:283–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carey BW, Markoulaki S, Hanna J, Saha K,
Gao Q, Mitalipova M and Jaenisch R: Reprogramming of murine and
human somatic cells using a single polycistronic vector. Proc Natl
Acad Sci USA. 106:pp. 157–162. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta UD and Katoch VM: Animal models of
tuberculosis. Tuberculosis (Edinb). 85:277–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Collins FM: Tuberculosis: The return of an
old enemy. Crit Rev Microbiol. 19:1–16. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mehrabani D, Mahboobi R, Dianatpour M,
Zare S, Tamadon A and Hosseini SE: Establishment, culture, and
characterization of Guinea pig fetal fibroblast cell. Vet Med Int.
2014:5103282014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y, Melton DW, Zhang Y and Hornsby PJ:
Improved coinfection with amphotropic pseudotyped retroviral
vectors. J Biomed Biotechnol. 2009:9010792009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morita S, Kojima T and Kitamura T: Plat-E:
An efficient and stable system for transient packaging of
retroviruses. Gene Ther. 7:1063–1066. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Y, Shu J, He C, Li M, Wang Y, Ou W and
He Y: ROCK inhibitor Y27632 promotes proliferation and diminishes
apoptosis of marmoset induced pluripotent stem cells by suppressing
expression and activity of caspase 3. Theriogenology. 85:302–314.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hentze H, Soong PL, Wang ST, Phillips BW,
Putti TC and Dunn NR: Teratoma formation by human embryonic stem
cells: Evaluation of essential parameters for future safety
studies. Stem Cell Res. 2:198–210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prokhorova TA, Harkness LM, Frandsen U,
Ditzel N, Schrøder HD, Burns JS and Kassem M: Teratoma formation by
human embryonic stem cells is site dependent and enhanced by the
presence of Matrigel. Stem Cells Dev. 18:47–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huangfu D, Osafune K, Maehr R, Guo W,
Eijkelenboom A, Chen S, Muhlestein W and Melton DA: Induction of
pluripotent stem cells from primary human fibroblasts with only
Oct4 and Sox2. Nat Biotechnol. 26:1269–1275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okita K, Nakagawa M, Hyenjong H, Ichisaka
T and Yamanaka S: Generation of mouse induced pluripotent stem
cells without viral vectors. Science. 322:949–953. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hasegawa K, Fujioka T, Nakamura Y,
Nakatsuji N and Suemori H: A method for the selection of human
embryonic stem cell sublines with high replating efficiency after
single-cell dissociation. Stem Cells. 24:2649–2660. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Esteban MA, Wang T, Qin B, Yang J, Qin D,
Cai J, Li W, Weng Z, Chen J, Ni S, et al: Vitamin C enhances the
generation of mouse and human induced pluripotent stem cells. Cell
Stem Cell. 6:71–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakagawa M, Koyanagi M, Tanabe K,
Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N
and Yamanaka S: Generation of induced pluripotent stem cells
without Myc from mouse and human fibroblasts. Nat Biotechnol.
26:101–106. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soldner F, Hockemeyer D, Beard C, Gao Q,
Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, et al:
Parkinson's disease patient-derived induced pluripotent stem cells
free of viral reprogramming factors. Cell. 136:964–977. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang CW, Lai YS, Pawlik KM, Liu K, Sun
CW, Li C, Schoeb TR and Townes TM: Polycistronic lentiviral vector
for ‘hit and run’ reprogramming of adult skin fibroblasts to
induced pluripotent stem cells. Stem Cells. 27:1042–1049. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin
T, Trauger S, Bien G, Yao S, Zhu Y, et al: Generation of induced
pluripotent stem cells using recombinant proteins. Cell Stem Cell.
4:381–384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim D, Kim CH, Moon JI, Chung YG, Chang
MY, Han BS, Ko S, Yang E, Cha KY, Lanza R and Kim KS: Generation of
human induced pluripotent stem cells by direct delivery of
reprogramming proteins. Cell Stem Cell. 4:472–476. 2009. View Article : Google Scholar : PubMed/NCBI
|