Introduction
Heme oxygenase-1 (HO-1) has an important protective
role in various disease models due to its anti-inflammatory,
anti-apoptotic and anti-proliferative actions (1,2).
HO-1 also has an important role in the allograft immune response.
Following liver transplantation, various cell types induce HO-1
overexpression to prevent ischemia reperfusion injury (IRI) and
immune rejection (3–5). It is well established that Kupffer
cells (KCs) are among the most important resident macrophages of
the liver and account for ~20% of all hepatic non-parenchymal cells
(6). Increased attention has
focused on the potential roles and mechanisms of KCs in tolerance
induction following liver allografts. Wang et al (7) demonstrated that preconditioning with
nodosin perfusion induced HO-1 expression in KCs following
transplantation, and this upregulation was demonstrated to be
protective against IRI, a process which is thought to facilitate
immune rejection.
It is now appreciated that the function of mast
cells (MCs) is not limited to allergic disease or chronic immune
rejection. Recent studies have reported that active MCs degranulate
to induce IRI and acute immune rejection (8,9), and
these cells influence the tissue microenvironment via release of a
variety of pre-existing and cell-synthesized mediators, including
proteases, cytokines, chemokines and arachidonic acid metabolites
(10). A previous study reported
that MC degranulation may disrupt peripheral immune tolerance and
result in immune rejection (11),
and also suggests that MC degranulation may promote IRI in the rat
liver (12). Stabilizing MC
membranes may, therefore, alleviate immune rejection and IRI.
Takamiya et al (13)
demonstrated that HO-1 stabilizes MCs following exposure to the
anti-inflammatory compound bilirubin.
Dendritic cells (DCs) are one of the most potent
types of antigen-presenting cells and are known to be important in
triggering immunity to various types of antigens (14). Under normal circumstances, DCs are
immature in vivo, and co-stimulation of CD80, CD86 and major
histocompatibility complex class II at the surface of DCs is low
(15). Immature DCs migrate into
secondary lymphoid organs and differentiate into mature DCs that
are capable of triggering immune rejection following
transplantation. DCs express C-C motif chemokine receptor 1 (CCR1),
CCR7, CCR5 and CCR6 chemokine receptors, and exhibit chemotaxis
(16). Preventing DC migration to
secondary lymphoid organs may reduce the likelihood of immune
rejection following transplantation. Based on this information, the
current study hypothesized that HO-1 upregulation in KCs may
stabilize the MC membrane, decrease MC degranulation and prevent DC
migration to secondary lymphoid organs, and subsequently prevent
immune rejection.
Materials and methods
Animals
The experimental protocol was approved by the
institutional animal ethics committee of Shanghai Tenth People
Hospital of Tong Ji University (Shanghai, China).
A total of 18 male C57BL/6 mice, 8–10-weeks-old,
were purchased from Schleck Experimental Animals Co. (Shanghai,
China). All mice were housed in a pathogen-free facility,
maintained at 26°C under 12 h light/dark cycle and had access to
food and water ad libitum. They were used in accordance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals of the Chinese Academy of Sciences (5).
Antibodies and reagents
KIT proto-oncogene receptor tyrosine kinase
(CD117)-fluorescein isothiocyanate (FITC) (dilution 1:200; cat. no.
48-1171-80; eBioscience, Inc., San Diego, CA, USA), anti-mouse
F4/80-allophycocyanin (APC) (dilution 1:20; cat. no. 47-4801-80;
eBioscience, Inc.), anti-mouse CD11b-FITC (dilution 1:40; cat. no.
47-0118-41; eBioscience, Inc.), Fc fragment of IgE receptor Ia
(FCεRIα) -phycoerythrin (PE) (dilution 1:10; cat. no. ab124529;
Abcam, Cambridge, UK) and anti-mouse CCR7 (dilution 1:200; cat. no.
25-1971-63; eBioscience, Inc.) antibodies were used. The
metalloporphyrins, hemin (an HO-1 inducer) and zinc protoporphyrin
(Znpp; an HO-1 inhibitor), were purchased from Enzo Life Sciences,
Inc. (Farmingdale, NY, USA). Sodium cromoglicate (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) β-actin (1:2,000; cat. no.
A2228; Sigma-Aldrich; Merck Millipore) and antibody against HO-1
(1:1,000; cat. no. ab13248; Abcam).
Cell preparation
C57/BL6 mice were sacrificed by anesthesia with
intraperitoneal injection of ketamine (90 mg/kg) (Sigma-Aldrich;
Merck Millipore) and xylazine (10 mg/kg) (Sigma-Aldrich; Merck
Millipore) solution and test for loss of reflexes to ensure deep
narcotization. Then, non-parenchymal cell suspensions were acquired
from C57/BL6 mice using in situ collagenase perfusion of
liver and KCs were isolated by sedimentation in a two-step Percoll
gradient with selective adherence of cells to plastic flasks as
previously described (3). Cell
viability was determined by trypan blue exclusion, and the purity
of the KC fraction was determined using anti-mouse F4/80-APC
(dilution 1:20) and anti-mouse CD11b-FITC antibodies (dilution
1:40). Murine bone marrow-derived mast cells (BMMCs) and DCs
(BMDCs) were obtained as described previously (17,18).
The cells were collected 8×105 and centrifuged at 135 ×
g 5 min at 4°C, then the cells were resuspended in PBS and 2% fetal
bovine serum 200 µl. All cells were incubated with the antibody for
30 min on ice, then washed twice with PBS and 2% serum and
centrifuged at 1,200 × g for 5 min, analysis was performed using
FlowJo 7.6. The purity of BMMCs was assessed by measuring the
expression of CD117 and FCεRIα using flow cytometry. BMMCs were
used at a purity of 95%. The purity of DCs was analyzed by
measuring CD11c expression using flow cytometry.
RT-PCR and RT-qPCR
Total RNA was isolated from KCs using TRIzol
(Sigma-Aldrich; Merck Millipore) according to standard procedures.
Thereafter, 2 µg of total RNA was reverse transcribed to cDNA using
the Superscript III Transcription kit (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with Genomic DNA Eraser (Takara
Biotechnology Co., Ltd., Dalian, China) according to manufacturer's
protocol. PCR was performed on a Px2 Thermal Cycler, using the
following conditions: 1 cycle of denaturation at 95°C for 5 min, 35
cycles of denaturation at 94°C for 30 sec, annealing at 55°C for 30
sec, and extension at 72°C for 40 sec and an additional cycle of
extension at 72°C for 10 min. Then 2% gel was used. GAPDH was used
as an internal control. qPCR was performed on a Chromo4 Four-Color
Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) using the SYBR Premix Ex Taq II (Tli RNaseH
Plus) kit (Takara Biotechnology Co., Ltd.). Using the following
conditions: Initial the cycle of denaturation at 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec, annealing 60°C for 30 sec
and extension 70°C for 15 sec. Data was normalized using the
2−ΔΔCq method (19).
PCR was performed on an ABI Prism 7700 (Applied Biosystems; Thermo
Fisher Scientific, Inc.). For linear amplification, GAPDH was used
as an internal control. The following PCR primers were synthesized
by Shanghai Sangon Biological Engineering Technology & Services
Co., Ltd. (Shanghai, China): HO-1, 5′-ACGCATATACCCGCTACCTG-3′
(forward) and 5′-TGCTGATCTGGGATTTTCCT-3′ (reverse); GAPDH,
5′-TCCCTCAAGATTGTCAGCAA-3′ (forward) and 5′-AGATCCACAACGGATACATT-3′
(reverse).
Protein extraction and western
blotting
Reagents were purchased from Sigma-Aldrich unless
otherwise indicated. Proteins were extracted from KCs and western
blotting was performed. Briefly, 2.5–6×106 cells were
incubated for 15 min on ice in lysis buffer [50 mM TrisHCl pH 8.0;
120 mM NaCl; 0.25% Nonidet P40; 0.1% SDS; and protease inhibitors
phenylmethylsulfonyl fluoride, aprotinin, leupeptin, and pepstatin
(Roche Diagnostics) at a final concentration of 10 ng/ml, 1 mM
DTT]. A total of 60 mg, of each protein sample was subjected to 15%
SDS-PAGE and blotted onto a nitrocellulose membrane (GE Healthcare
Life Sciences, Chalfont, UK). The protein quantification performed
using a BCA kit according to the manufacturer's protocol
(Sigma-Aldrich; Merck Millipore). Membranes were blocked with 5%
non-fat dry milk in TBS-Tween (0.5%) 4°C overnight and probed with
either rabbit anti-mouse HO-1 monoclonal antibody (2 mg/ml; Abcam)
or rabbit anti-mouse β-actin monoclonal antibody (1:2,000) followed
by horseradish peroxidase-conjugated anti-rabbit IgG antibody
(1:3,000; cat. no. RPN4301; GE Healthcare Life Sciences, Logan, UT,
USA). Immunoreactive protein bands incubated with the enhanced
chemiluminescence (ECL) reagent 30 sec according to the
manufacturer's protocol using an ECL detection kit (cat. no.
RPN998; GE Healthcare Life Sciences) and were visualized with image
lab 4.0 software.
MC degranulation assay
After treating KCs with PBS, dimethyl sulfoxide
(DMSO), 50 µM/l hemin or 50 µM/l Znpp for 8 h, cells were collected
and cultured in 24-well cell culture plates at a density of
2.5×105 cells per 200 µl, either with direct contact
with MCs or without MCs (5×105 cells), separated by a
Transwell chamber 0.4 µm. The 50 µM/l sodium cromoglicate was used
to pretreat the MC as the stabilization control. After 24 h, each
group of MCs were pre-incubated with anti-dinitrophenol (DNP)-IgE
(100 ng/ml) (1:1,000, cat. no. D8406; Sigma-Aldrich; Merck
Millipore) for 24 h and subsequently challenged using 100 ng/ml
dinitrophenol-human serum albumin DNP-HSA. After 1 h, the cell
supernatant of the co-culture system was collected. Following
solubilization with 0.5% Triton X-100 in Tyrode's buffer, the
enzymatic activity of β-hexosaminidase in supernatants and cell
pellets was measured using p-nitrophenyl-N-acetyl-β-D-glucosaminide
in 0.1 M sodium citrate, pH 4.5, at 37°C for 60 min. The reaction
was halted by addition of 0.2 M glycine (pH 10.7) and the amount of
p-nitrophenol released was measured by absorbance at a wavelength
of 405 nm using a spectrophotometer. The extent of degranulation
was calculated as the p-nitrophenol absorbance of the
supernatant/the total absorbance of the supernatant and
detergent-solubilized cell pellet.
Analysis of pretreated MC-DC
interaction and DC migration
MCs were cultured with KCs in 10% serum that were
pretreated with PBS, DMSO, hemin or Znpp 24 h, using 50 µM/l sodium
cromoglicate to pretreat the MCs as the stabilization control, then
MC degranulation was stimulated with anti-DNP-IgE associated with
DNP-HSA. DC migration was assessed using Transwell assays (8 µm
pore size). DCs were incubated in 10% serum in upper chambers at a
density of 5×105 cells per well, MCs by pretreated KCs
in the lower chamber. After 48 h, cells in the upper chamber were
discarded and DCs that had migrated to the lower chamber harvested.
Cells in the lower chamber were fixed with 10% methyl alcohol,
stained with 0.1% crystal violet for 20 min, washed with double
distilled H2O and counted under an inverted microscope,
at a magnification of ×200.
Additionally, DCs were cultured with stimulated MCs
for 24 h in Transwell plates Supernatants were harvested from lower
chamber of the co-culture system, and prostaglandin E2 (PGE2) (cat.
no. H6-KA0324; eBioscience USA), C-C motif chemokine ligand 19
(CCL-19) (cat. no. RK-KOA0264; eBioscience USA) and CCL21 (cat. no.
85-88-58214-22; eBioscience USA) were quantified by ELISA
(eBioscience, Inc.) according to the manufacturer's instructions.
DCs were incubated with immunofluorescent CD16/31 (dilution, 3 µl
for 106 cells) and anti-mouse CCR7 antibodies for 30 min
on ice then washed twice. Flow cytometry was performed using Flow
Jo 7.6 software.
Statistical analysis
All statistical analysis was carried out using SPSS
9.13 (SPSS, Inc., Chicago, IL, USA) and results are presented as
the mean ± standard deviation. One-way analysis of variance along
with Dunnett's post hoc test was performed to determine the
statistical significance of the data. P<0.05 was considered to
indicate a statistically significant difference.
Results
Hemin induces HO-1 mRNA and protein
expression in KCs
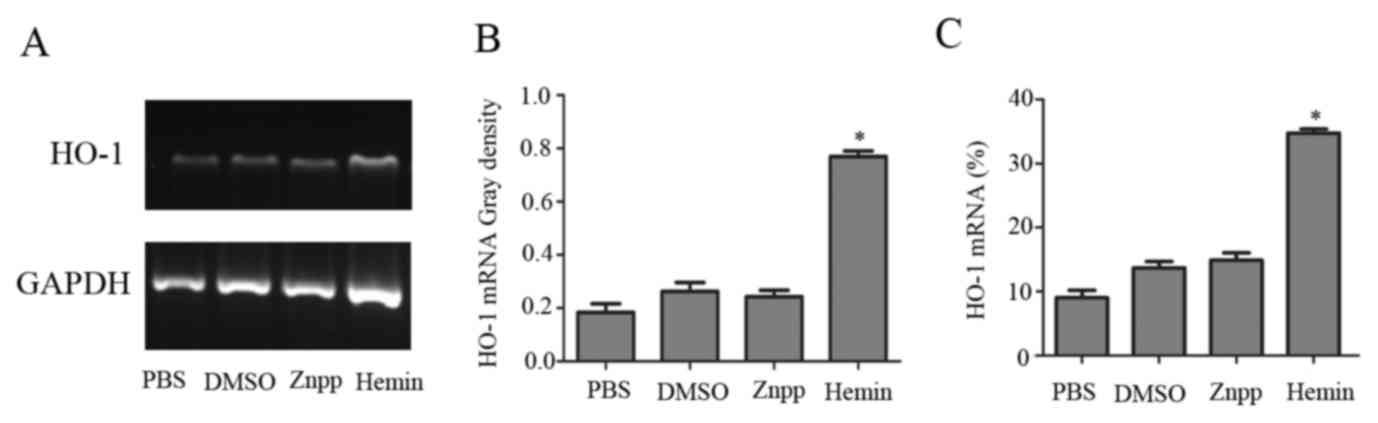
HO-1 mRNA and protein levels in KCs cultured with
PBS, DMSO, hemin or Znpp were measured by RT-PCR, RT-qPCR and
western blotting. HO-1 mRNA was significantly increased 8 h after
exposure to hemin compared with incubation with PBS, DMSO or Znpp
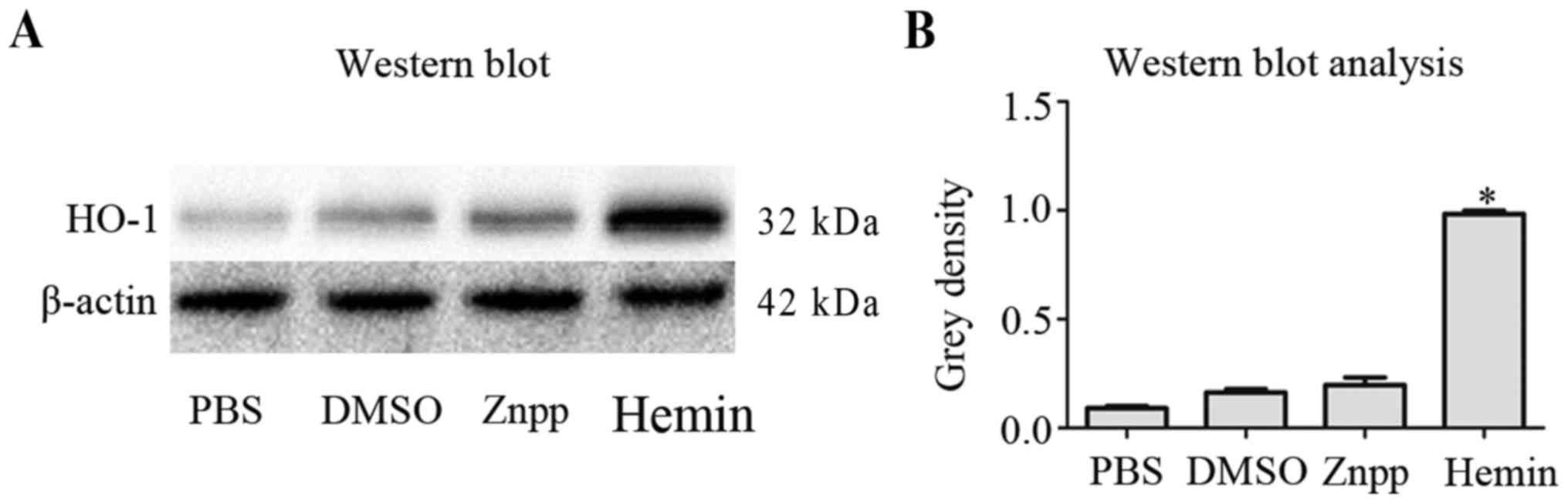
(Fig. 1). Consistent with these
results, western blot analysis demonstrated that HO-1 protein
levels were lower in PBS, DMSO and Znpp-treated groups after 8 h,
compared with the hemin-treated group (Fig. 2).
Upregulation of HO-1 expression in KCs
may inhibit mast cell degranulation
KCs were pretreated with PBS, DMSO, hemin or Znpp
for 8 h and cultured either in contact with MCs or separately
(Fig. 3). After 24 h, MC
degranulation was stimulated with anti-DNP-IgE and DNP-HSA, and
enzymatic activity of β-hexosaminidase was used to estimate the
level of MC degranulation usually. Following treatment with hemin,
a decrease in β-hexosaminidase release was observed in KCs that
were in contact with MCs, and also those that were separate from
MCs. There was no difference between the sodium cromoglicate group
and hemin group demonstrating that upregulation of HO-1 in KCs may
inhibit MC degranulation and stabilize MC membranes.
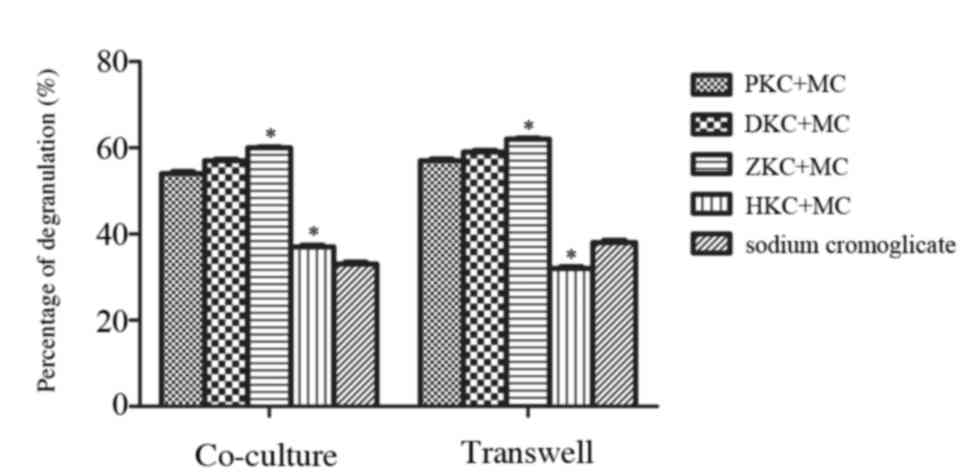 | Figure 3.Effect of heme oxygenase-1
upregulation in KCs on MC degranulation. KCs were pretreated with
PBS, dimethyl sulfoxide, hemin or zinc protoporphyrin for 8 h prior
to interaction with MCs. MC degranulation induced by monoclonal
anti-DNP-IgE and DNP-HSA was evaluated using 4-nitrophenyl
N-acetyl-β-D-glucosaminide. PKC+MC, DKC+MC and ZKC+MC groups
elicited MC degranulation, whereas the HKC+MC and sodium
cromoglicate groups inhibited the MC degranulation. The HKC+MC and
sodium cromoglicate groups were not significantly different.
Similar results were observed whether KCs and MCs were in contact
or separated by a Transwell. *P<0.05 HKC+MC group vs. ZKC+MC
group. KC, Kupffer cells; PKC, PBS-treated KCs; DKCs, dimethyl
sulfoxide-treated KCs; ZKCs, zinc protoporphyrin-treated KCs; HKCs,
hemin-treated KCs; MC, mast cell; DNP-HSA, dinitrophenol-human
serum albumin. |
MC degranulation stimulates CCR7
expression on the DC surface, and stabilizing the MC membrane
diminishes CCR7 expression
DC migration from peripheral tissues to secondary
immune organs, particularly lymph nodes, is a prerequisite for
initiating an effective immune response, and CCR7 expression at the
surface of DCs facilitates homing (20,21).
MC membranes were stabilized by hemin-pretreated KCs, whether
cultured in contact or separately as mentioned above, therefore MCs
cultured separately were selected for use in further experiments.
Degranulation was stimulated and they were co-cultured with DCs.
After 24 h, expression of CCR7 at the DC surface was decreased in
hemin and sodium cromoglicate-treated groups compared with PBS,
DMSO and Znpp-treated groups (Fig.
4), and there was no difference between the sodium cromoglicate
group and hemin group.
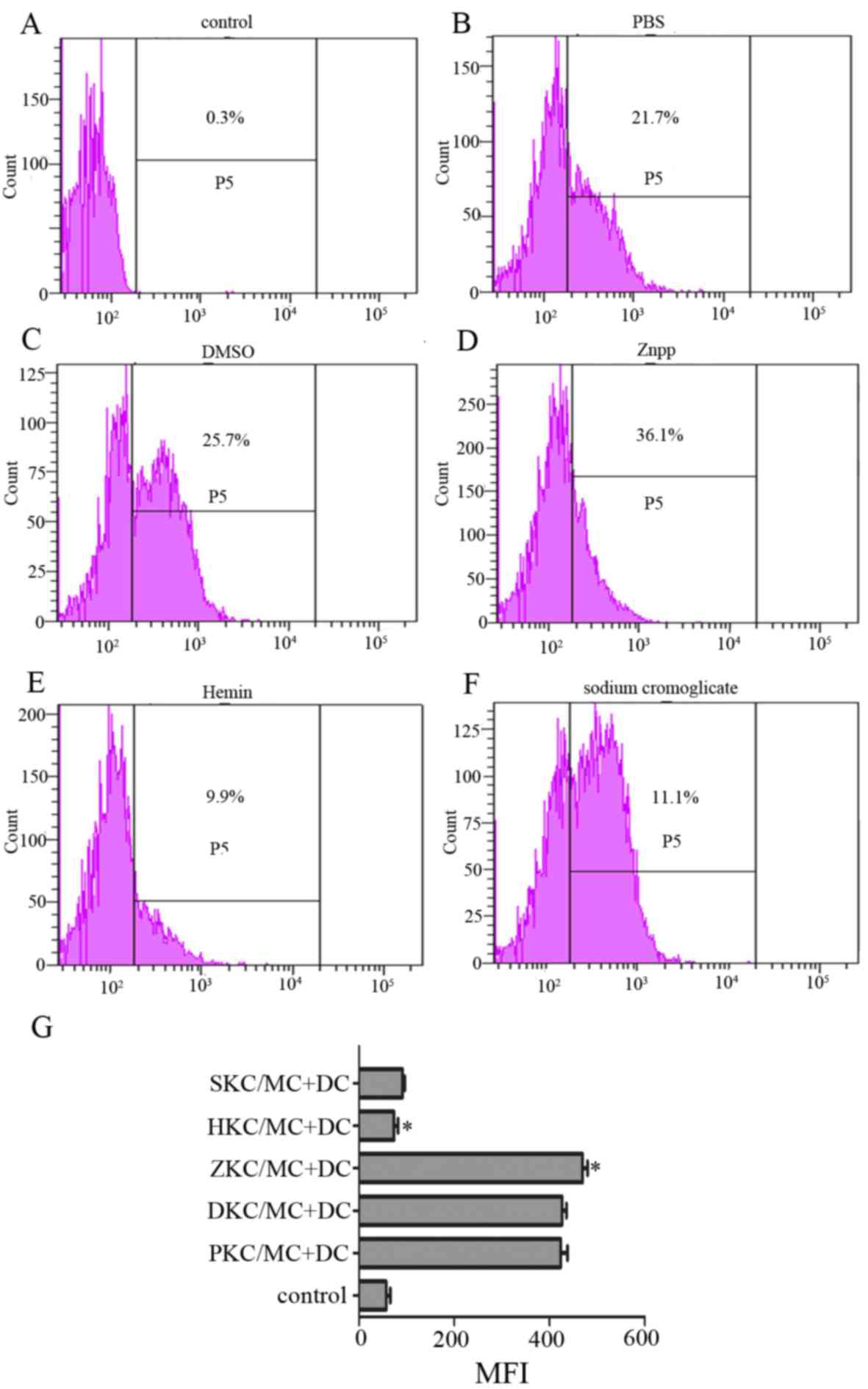 | Figure 4.Expression of chemokine receptor CCR7
on the surface of DCs. Levels of CCR7 on DCs, that were co-cultured
with activated MCs interacting with KCs that had been pretreated
with (A) control, (B) PBS, (C) DMSO, (D) Znpp, (E) hemin and (F)
sodium cromoglicate, were detected. Expression of CCR7 on the DC
surface was decreased by hemin and sodium cromoglicate. (G)
Expression of CCR7 on the DC surface is presented as MFI, which was
measured by flow cytometry after 24 h of incubation (with isotype
control subtracted). Data are presented as the percentage MFI of
control group, which was set as 100%. *P<0.05 HKC/MC+DC group
vs. ZKC/MC+DC group. DCs, dendritic cells; MCs, mast cells; KCs,
Kupffer cells; DMSO, dimethyl sulfoxide; Znpp, zinc protoporphyrin;
MFI, mean fluorescence intensity; SKC, sodium cromoglicate-treated
KCs; HKC, hemin-treated KCs; ZKC, Znpp-treated KCs; DKC,
DMSO-treated KCs; PKC, PBS-treated KCs; CCR7, C-C motif chemokine
receptor 7. |
MC degranulation stimulates DC
migration
Transwell plates were used to investigate DC
migration (Fig. 5), and five areas
were chosen randomly for cell counting under the inverted
microscope at magnification of ×200. Pretreatment of KCs with hemin
(Fig. 5D) upregulated HO-1,
stabilized MC membranes and decreased migration of DCs to the lower
Transwell chamber, compared with KCs pretreated with PBS (Fig. 5B), DMSO (Fig. 5C) or Znpp (Fig. 5E). Again, there was no difference
between the sodium cromoglicate group (Fig. 5F) and hemin group, confirming that
MC degranulation stimulated DC migration.
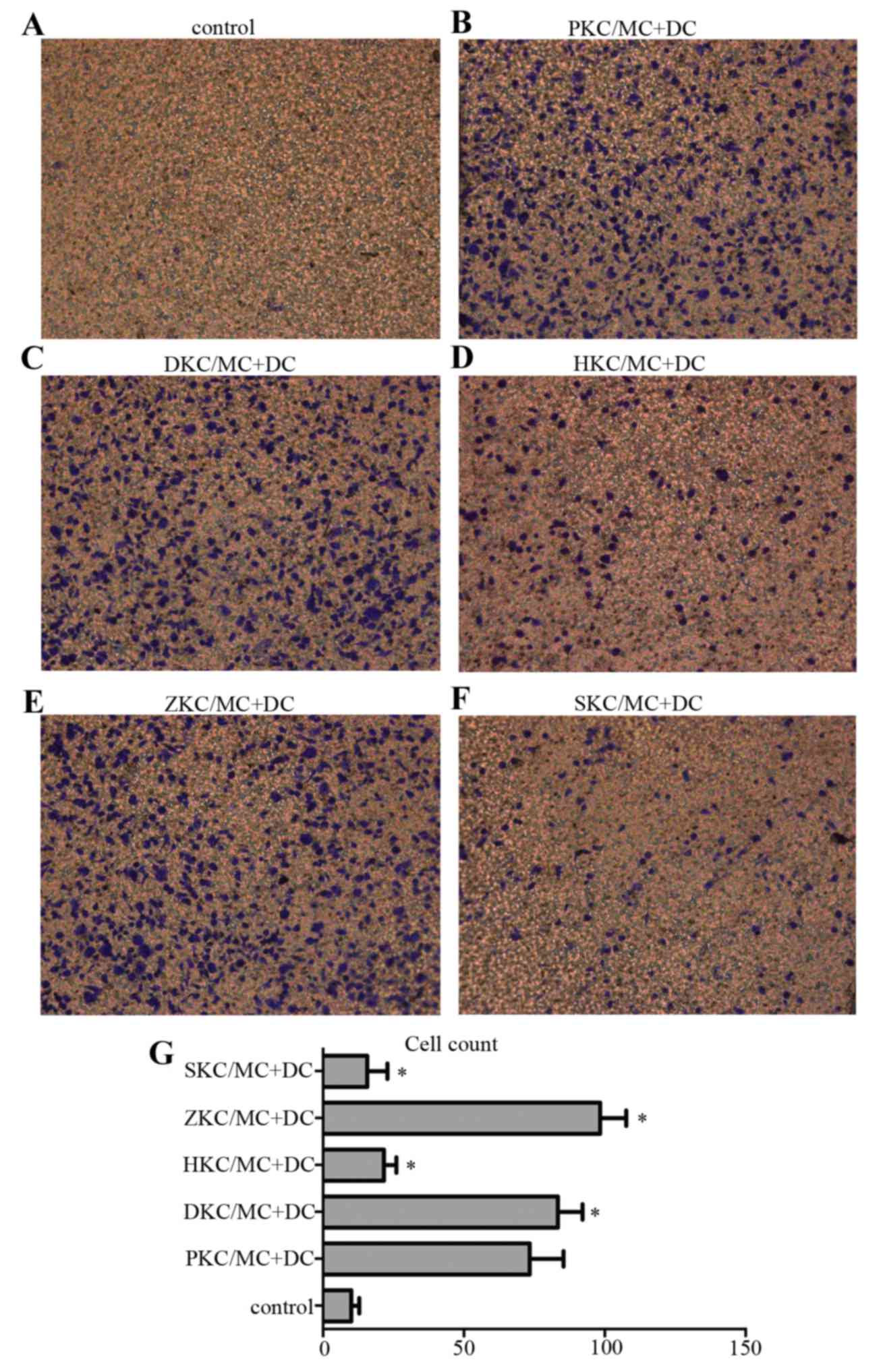 | Figure 5.DC migration in vitro. DC
migration was tested using a chemotaxis assay in a 24-well
Transwell chamber. Lower wells were loaded with activated MCs
interacting with pretreated KCs or medium only (control), and upper
wells were loaded with 5×105 DCs/well/condition and incubated for
48 h at 37°C. The cell number was 80–100/field for (B:PKC/MC+DC),
(C:DKC/MC+DC) and (E:ZKC/MC+DC), and 10–30/field for (A:control),
(D:HKC/MC+DC) and (F:SKC/MC+DC). (G) Presents the number of cells
in each group. (n=5/group). *P<0.05 vs. control. DC, dendritic
cells; MC, mast cells; KC, Kupffer cells; SKC, sodium
cromoglicate-treated KCs; HKC, hemin-treated KCs; ZKC, zinc
protoporphyrin-treated KCs; DKC, dimethyl sulfoxide-treated KCs;
PKC, PBS-treated KCs. |
MC degranulation stimulates DC
migration via release of PGE2, CCL19 and CCL21
Stimulation of MC degranulation may result in the
release of cytokines that influence DC migration. The levels
cytokines PGE2, CCL19 and CCL21 were measured in supernatants from
co-cultures using ELISA (Fig. 6).
Compared with membrane-stabilized MCs, represented by hemin and
sodium cromoglicate-treated groups, degranulated MCs produced
significantly increased levels of all three cytokines. Due to the
fact that increased MC degranulation led to increased DC migration,
these cytokines therefore potentially contributed to the changes
observed in DC migration.
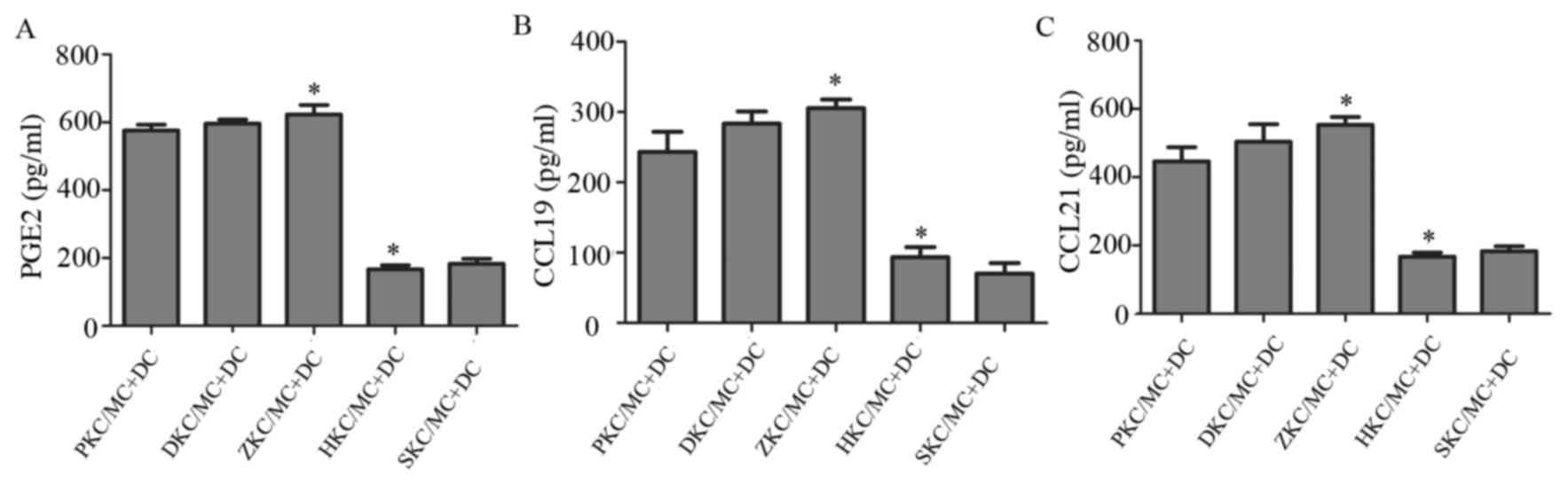 | Figure 6.Changes in cytokine levels in cell
supernatants. (A) PGE2, (B) CCL19 and (C) CCL21 levels in the
supernatant of co-cultured cells of each group. *P<0.05
ZKC/MC+DC vs. HKC/MC+DC. PGE2, prostaglandin E2; CCL, C-C motif
chemokine ligand; DC, dendritic cells; MC, mast cells; KC, Kupffer
cells; SKC, sodium cromoglicate-treated KCs; HKC, hemin-treated
KCs; ZKC, zinc protoporphyrin-treated KCs; DKC, dimethyl
sulfoxide-treated KCs; PKC, PBS-treated KCs. |
Discussion
HO-1 catabolizes heme into carbon monoxide,
biliverdin and free iron, which helps to protect cells against a
variety of potential oxidative stimuli (22). Recent studies have demonstrated
that HO-1 may confer a protective effect in organ transplantation,
since HO-1 and its byproducts may protect the allograft from IRI
and the immune response following a liver transplant (23). In rat liver, HO-1 is highly
expressed in KCs (24),
liver-resident macrophages that have an important role in the acute
and chronic responses of the liver to toxic compounds. Our previous
study demonstrated that preconditioning donor liver with nodosin
perfusion reduces IRI in rats, and this occurs via upregulation of
HO-1 that may then prime KCs, which go on to suppress the immune
response (25). The understanding
of the role of KCs in IRI and the immune response is incomplete.
Upregulation of HO-1 may alleviate IRI and decrease MC
degranulation, whereas increased MC degranulation promoted IRI in
rat liver (12). MCs are known to
produce various factors responsible for the allergic response,
including histamine and inflammatory proteins (26). These cells function in the innate
(27) and adaptive immune system
(28). MCs may release cytokines
that influence the diseased state, and inhibition of MC
degranulation by HO-1 disrupted DC maturation in vitro
(29). This indicates that for MCs
to perform their function in the adaptive immune system, DC
maturation and migration may be required. DCs reside in an immature
state in peripheral blood and tissues until activated by
inflammatory cytokines or antigens. Following activation, DCs are
transported via the afferent lymphatic system into the draining
lymph node before initiating an immune response (30). DC migration is influenced by CCRs
on the cell surface, and CCR7 is the most important receptor in
numerous diseases (31).
Pahne-Zeppenfeld et al (32) reported that cervical cancer cells
suppress the induction of CCR7 in phenotypically mature DCs, which
impairs their migration toward the lymph node that is required for
the adaptive immune response. CCR7 and its ligands, CCL19 and
CCL21, control a diverse array of migratory events during adaptive
immunity (33). Blocking CCR7 or
its ligands was effective in promoting graft survival in animal
models of heart or islet allotransplantation (34). Expression of CCR7 is influenced by
PGE2, and PGE2 antagonists downregulate CCR7 expression (35,36).
Torres et al (37)
determined that MC degranulation caused PGE2 release, which
inhibited asthma.
Our previous study revealed that the upregulation
the HO-1 expression of liver tissue may inhibit MC degranulation
and the HO-1 expression in the KC was intracellular. In the present
study HO-1 was upregulated in KCs when they were pretreated with
hemin, then co-cultured with MC, the MC membranes were stabilized.
Co-culturing DCs with membrane-stabilized MCs resulted in
downregulation of CCR7 on the surface of DCs. Furthermore, the
levels of the cytokines PGE2, CCL9 and CCL21 were decreased in the
supernatants of co-cultured DCs. Membrane-stabilized MCs also
impaired DC migration. The present study demonstrates a potential
mechanism of DC homing in vitro and may explain a possible
mechanism that MC degranulation would induce immune rejection. The
relevance of this potential mechanism in vivo requires
further investigation.
Acknowledgements
National Natural Science Foundation of China (grant
nos. 81270555; 81470897 and 81472501) and Program for New Century
Excellent Talents in University (grant no. NECT-13-0422).
References
|
1
|
Waller HL, Harper SJ, Hosgood SA, Bagul A,
Kay MD, Kaushik M, Yang B, Bicknell GR and Nicholson ML:
Differential expression of cytoprotective and apoptotic genes in an
ischaemia-reperfusion isolated organ perfusion model of the
transplanted kidney. Transplant Int. 20:625–631. 2007. View Article : Google Scholar
|
|
2
|
Ke BB, Buelow R, Shen XD, Melinek J,
Amersi F, Gao F, Ritter T, Volk HD, Busuttil RW and
Kupiec-Weglinski JW: Hemeoxygenase 1 gene transfer prevents
CD95/Fas ligand-mediated apoptosis and improves liver allograft
survival via carbon monoxide signaling pathway. Hum Gene Ther.
13:1189–1199. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeng Z, Huang HF, Chen MQ, Song F and
Zhang YJ: Heme oxy-genase-1 protects donor livers from
ischemia/reperfusion injury: The role of Kupffer cells. World J
Gastroenterol. 16:1285–1292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yue LH, Zhao YL, Chen J and Lu DR: Effect
of fusion protein TAT and heme oxygenase-1 on liver sinusoidal
endothelial cells apoptosis during preservation injury. Chin Med J
(Engl). 123:68–73. 2010.PubMed/NCBI
|
|
5
|
Jung ID, Lee JS, Lee CM, Noh KT, Jeong YI,
Park WS, Chun SH, Jeong SK, Park JW, Son KH, et al: Induction of
indoleamine 2,3-dioxygenase expression via heme
oxygenase-1-dependant pathway during murine dendritic cell
maturation. Biochem Pharmacol. 80:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomson AW and Knolle PA:
Antigen-presenting cell function in the tolerogenic liver
environment. Nat Rev Immunol. 11:753–766. 2010. View Article : Google Scholar
|
|
7
|
Wang CF, Wang ZY, Tao SF, Ding J, Sun LJ,
Li JY and Quan ZW: Preconditioning donor liver with Nodosin
perfusion lessens rat ischemia reperfusion injury via heme
oxygenase-1 upregulation. J Gastroenterol Hepatol. 27:832–840.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Vries VC, Wasiuk A, Bennett KA, Benson
MJ, Elgueta R, Waldschmidt TJ and Noelle RJ: Mast cell
degranulation breaks peripheral tolerance. Am J Transplant.
9:2270–2280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iwasaki W, Kume M, Kudo K, Uchinami H,
Kikuchi I, Nakagawa Y, Yoshioka M and Yamamoto Y: Changes in the
fatty acid composition of the liver with the administration of N-3
polyunsaturated fatty acids and the effects on warm
ischemia/reperfusion injury in the rat liver. Shock. 3:306–314.
2010. View Article : Google Scholar
|
|
10
|
Vliagoftis H and Befus AD: Mast cells at
mucosal frontiers. Curr Mol Med. 6:573–589. 2005. View Article : Google Scholar
|
|
11
|
Choi AM and Alam J: Heme oxygenase-1:
Function, regulation, and implication of a novel stress-inducible
protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol.
15:9–19. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang MQ, Ma YY, Tao SF, Ding J, Rao LH,
Jiang H and Li JY: Mast cell degranulation promotes ischemia
ereperfusion injury in rat liver. J Surg Res. 186:170–178. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takamiya R, Murakami M, Kajimura M, Goda
N, Makino N, Takamiya Y, Yamaguchi T, Ishimura Y, Hozumi N and
Suematsu M: Stabilization of mast cells by heme oxygenase-1: An
anti-inflammatory role. Am J Physiol Heart Circ Physiol.
3:H861–H870. 2002. View Article : Google Scholar
|
|
14
|
Bakdash G, Schneider LP, Van Capel TM,
Kapsenberg ML, Teunissen MB and de Jong EC: Intradermal application
of vitamin D3 increases migration of CD14+ dermal dendritic cells
and promotes the development of Foxp3+ Regulatory T cells. Hum
Vaccin Immunother. 2:250–258. 2013. View
Article : Google Scholar
|
|
15
|
Chen M, Huang L, Shabier Z and Wang J:
Regulation of the lifespan in dendritic cell subsets. Mol Immunol.
44:2558–2565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schaeuble K, Hauser MA, Rippl AV, Bruderer
R, Otero C, Groettrup M and Legler DF: Ubiquitylation of the
chemokine receptor CCR7 enables efficient receptor recycling and
cell migration. J Cell Sci. 125:4463–4474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kageyama-Yahara N, Suehiro Y, Yamamoto T
and Kadowaki M: IgE-induced degranulation of mucosal mast cells is
negatively regulated via nicotinic acetylcholine receptors. Biochem
Biophys Res Commun. 377:321–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho NH, Cheong TC, Min JH, Wu JH, Lee SJ,
Kim D, Yang JS, Kim S, Kim YK and Seong SY: A multifunctional
core-shell nanoparticle for dendritic cell-based cancer
immunotherapy. Nat Nanotechnol. 6:675–682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
MartIn-Fontecha A, Sebastiani S, Höpken
UE, Uguccioni M, Lipp M, Lanzavecchia A and Sallusto F: Regulation
of dendritic cell migration to the draining lymph node: Impact on T
lymphocyte traffic and priming. J Exp Med. 198:615–621. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verdijk P, Aarntzen EH, Punt CJ, de Vries
IJ and Figdor CG: Maximizing dendritic cell migration in cancer
immunotherapy. Expert Opin Biol Ther. 8:865–874. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Katana E, Skoura L, Giakoustidis D,
Takoudas D, Malisiovas N and Daniilidis M: Association between the
heme oxygenase-1 promoter polymorphism and renal transplantation
outcome in Greece. Transplant Proc. 42:2479–2485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salahudeen AA, Jenkins JK, Huang H,
Ndebele K and Salahudeen AK: Overexpression of heme oxygenase
protects renal tubular cells against cold storage injury: Studies
using hemin induction and HO-1 gene transfer. Transplantation.
72:1498–1504. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tamura T, Kondo T, Ogawa K, Fukunaga K and
Ohkohchi N: Protective effect of heme oxygenase-1 on hepatic
ischemia-reperfusion injury through inhibition of platelet adhesion
to the sinusoids. J Gastroenterol Hepatol. 28:700–706. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang M, Xu S, Han Y and Cao X: Apoptotic
cells attenuate fulminant hepatitis by priming Kupffer cells to
produce interleukin-10 through membrane-bound TGF-β. Hepatology.
53:306–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beaven MA: Our perception of the mast cell
from Paul Ehrlich to now. Eur J Immuno. 39:11–25. 2009. View Article : Google Scholar
|
|
27
|
Leslie M: Mast cells show their might.
Science. 317:614–616. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McLachlan JB, Hart JP, Pizzo SV, Shelburne
CP, Staats HF, Gunn MD and Abraham SN: Mast cell-derived tumor
necrosis factor induces hypertrophy of draining lymph nodes during
infection. Nat Immunol. 4:1199–1205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma YY, Yang MQ, Wang CF, Ding J and Li JY:
Inhibiting mast cell degranulation by HO-1 affects dendritic cell
maturation in vitro. Inflamm Res. 63:527–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gouwy M, Struyf S, Leutenez L, Pörtner N,
Sozzani S and Van Damme J: Chemokines and other GPCR ligands
synergize in receptor-mediated migration of monocyte-derived
immature and mature dendritic cells. Immunobiology. 219:218–229.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Förster R, Davalos-Misslitz AC and Rot A:
CCR7 and its ligands: Balancing immunity and tolerance. Nat Rev
Immunol. 8:362–371. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pahne-Zeppenfeld J, Schröer N,
Walch-Rückheim B, Oldak M, Gorter A, Hegde S and Smola S: Cervical
cancer cell-derived interleukin-6 impairs CCR7-de pendent migration
of MMP-9-e xpressingDendritic cells. Int J Cancer. 134:2061–2073.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Comerford I, Harata-Lee Y, Bunting MD,
Gregor C, Kara EE and McColl SR: A myriad of functions and complex
regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive
immune system. Cytokine Growth Factor Rev. 24:269–283. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ziegler E, Gueler F, Rong S, Mengel M,
Witzke O, Kribben A, Haller H, Kunzendorf U and Krautwald S:
CL19-IgG prevents allograft rejection by impairment of immune cell
trafficking. J Am Soc Nephrol. 17:2521–2532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luft T, Jefford M, Luetjens P, Toy T,
Hochrein H, Masterman KA, Maliszewski C, Shortman K, Cebon J and
Maraskovsky E: Functionally distinct dendritic cell (DC)
populations induced by physiologic stimuli: Prostaglandin E(2)
regulates the migratory capacity of specific DC subsets. Blood.
100:1362–1372. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pan MR, Hou MF, Chang HC and Hung WC:
Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling
pathways to enhance lymphatic invasion of breast cancer cells. J
Biol Chem. 283:11155–11163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Torres R, Picado C and de Mora F: The
PGE2–EP2–mast cell axis: An antiasthma mechanism. Mol Immunol.
63:61–68. 2015. View Article : Google Scholar : PubMed/NCBI
|