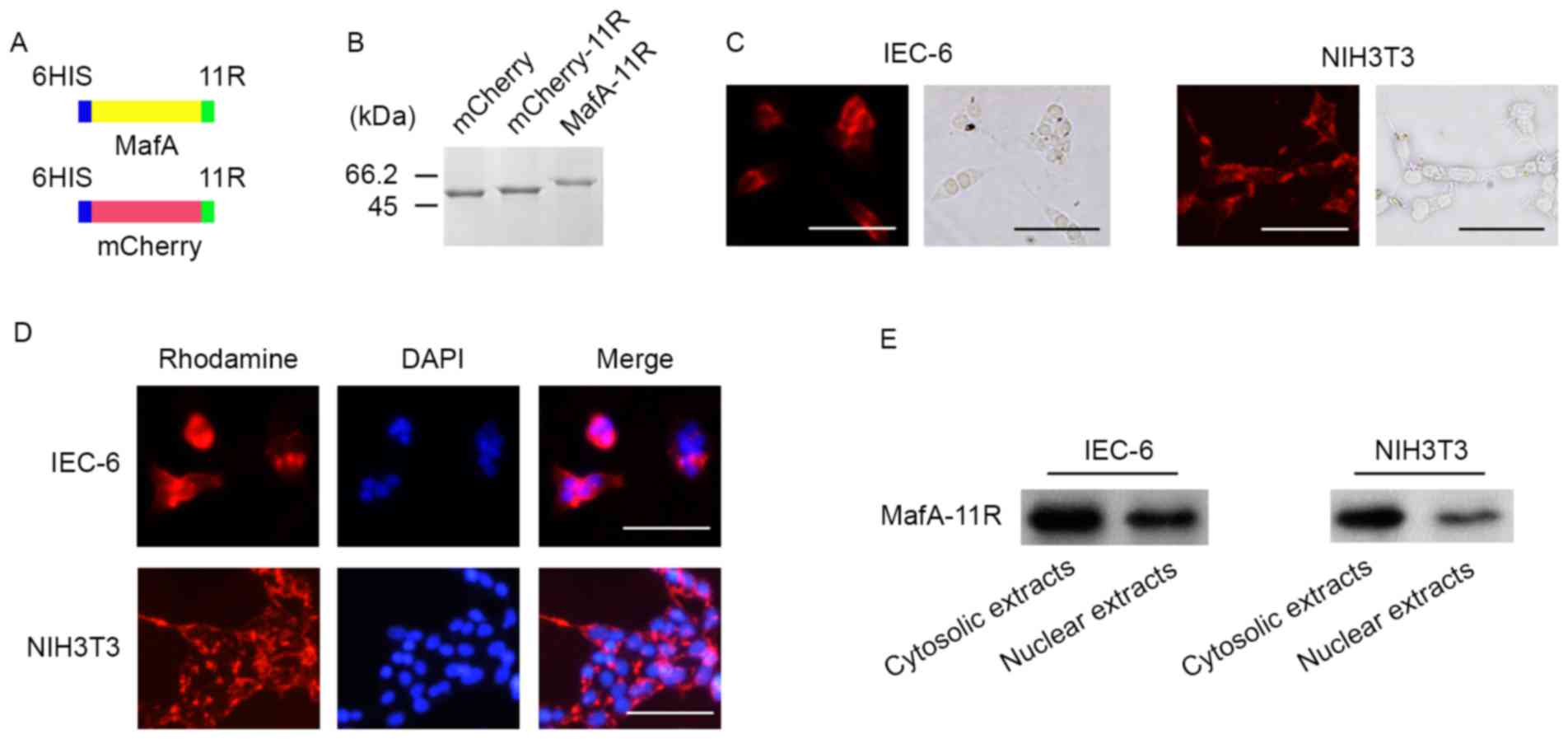
Introduction
Insulin gene expression is restricted to pancreatic
β-cells in adult mammals. Transcription of the insulin gene is
controlled by numerous factors, including pancreatic and duodenal
homeobox (Pdx) 1, neuronal differentiation (NeuroD) and musculo
aponeurotic fibrosarcoma BZIP transcription factor (Maf) A, which
bind to 5′-cis-regulator elements in the enhancer region (1). MafA is a basic leucine zipper
transcription factor, which controls expression of the insulin gene
via a rat insulin promoter element (RIPE) 3b regulatory element
(2). MafA deficient mice exhibit
age-dependent glucose intolerance and abnormal islet architecture,
suggesting that MafA is primarily involved in β-cell formation and
functionality (3). Ectopic
expression of MafA induces insulin production in various non-β-cell
lines (αTC6, AR42J and IEC-6) (4),
indicating that MafA exhibits the potential to induce
insulin-producing cells. However, the majority of strategies
involving the use of viruses as vectors for gene delivery result in
safety concerns.
Protein transduction domains (PTDs) are small
cationic peptides. Numerous PTD-fused full length functional
proteins have been demonstrated to transduce cells and tissues
(5,6). This ability of the PTDs to transport
intact proteins and allow them to penetrate the cell membrane,
provides an alternative route of protein therapy in diseases. Of
all the transduction peptides tested, tyrosine aminotransferase or
eleven arginine residues (11R) demonstrate the greatest
transduction efficiency (7,8).
Recombinant polyarginine-fused reprogramming factors have been used
to facilitate proteins to generate induced pluripotent stem cells
(9,10).
It has been previously demonstrated that MafA, in
conjuction with Pdx1 and NeuroD, may induce insulin gene expression
in the liver, however this was not observed when MafA was
administered alone (11).
Conversely, adenovirus transduction with MafA alone promotes
insulin production from intestinal cells (12), as the transcription factors Pdx1,
neurogenin (Neurog3), NeuroD, NK 2 homeobox 2 and Paired box (Pax)
4, which are essential for pancreatic β-cell differentiation, are
already expressed in the intestine (13,14).
The present study therefore delivered the MafA-11R recombinant
protein to mice with streptozotocin-induced diabetes and monitored
if the construct promoted the differentiation of intestinal cells
into insulin producing cells. mCherry-11R served as a control
protein, of which the distribution was observed via a small animal
imaging system.
Materials and methods
Plasmid construction
The synthetic 11R cDNA sequence was cloned into the
HindIII and XhoI sites of pET32a (EMD Millipore, Billerica, MA,
USA) and subsequently termed pET32a(+)-11R. Full-length mouse MafA
was amplified from mouse pancreas cDNA (cat. no. 9533; Takara
Biotechnology Co., Ltd., Dalian, China) and mCherry was amplified
from pmCherry-C1 (Clontech Laboratories, Inc., Mountainview, CA,
USA) by polymerase chain reaction (PCR). The primer sequences were
as follows: forward CGGGATCCATGGCCGCGGAGCTGGCGAT and reverse
CCCAAGCTTCAGAAAGAAGTCGGGTGCGC for mouse mafA; forward
CGGGATCCATGGTGAGCAAGGGCGAGGA and reverse
CCCAAGCTTCTTGTACAGCTCGTCCATGC for mCherry. PrimeSTAR HS DNA
polymerase (cat. no. R010A; Takara Biotechnology Co., Ltd.) was
used for PCR with the following conditions: 95°C for 5 min,
followed by 35 cycles of denaturation at 95°C for 15 sec and
annealing and extension at 58°C for 1 min. The PCR products were
cloned into double-digested (BamHI and HindIII) pET32a(+)-11R
vector. The resulting vectors were termed pET32a-MafA-11R and
pET32a-mCherry-11R. In brief, the vectors were tagged with a
6-histidine ladder, followed by MafA or mCherry cDNA, then 11-R
flanked by glycine and glutamic acid residues in the COOH terminal
(Fig. 1A). The insertion
efficiency of MafA, mCherry and 11R cDNA sequences into the pET32a
vector, and potential sequence mutations that appeared during the
process, were assessed via sequencing. The vector without 11R
served as the negative control.
Purification and identification of
recombinant proteins
The 11R fusion proteins were expressed and purified
as previously described (7). In
brief, the constructed plasmids were transformed into BL21(DE3) E.
coli that were then cultured with fresh Lysogeny broth (LB) (Amp+)
at 200 rpm at 37°C until optical density 600 reached 1.0, as
measured by a UV/VIS spectrophotometer.
Isopropyl-β-D-thiogalactopyranoside (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was added to a final concentration of 0.5 mM,
and the cells were then incubated for 12 h at 25°C. Cells were
sonicated, and the supernatants were recovered and applied to a
column of Ni-NTA agarose (Qiagen, Inc., Valencia, CA, USA). The
purified proteins were dissolved in buffer A (50 mM KH2PO4-HCl, 300
mM NaCl) and visualized by SDS-PAGE and Coomassie blue staining
(Fig. 1B).
Protein transduction and cytotoxicity
assays
The cytotoxicity of MafA-11R was evaluated via WST-1
assay kit (Roche Applied Science, Mannheim, Germany), according to
the manufacturer's protocol, prior to commencing of animal
experiments in vivo. IEC-6 rat intestinal epithelial cells
and NIH3T3 mouse embryonic fibroblast cells were obtained from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
calf serum, 100 U/ml penicillin, and 100 µg/ml streptomycin (all
from Hyclone; GE Healthcare Life Sciences, Logan, UT, USA). Cells
were incubated with 0.5 mmol/l mCherry-11R or MafA-11R at 37°C for
24 h followed by 10% of WST-1 solution for 30 min. The product in
the resulting colored solution was measured using a microplate
reader at a wavelength of 450 nm.
Animals
All animal studies were approved by the Ethics
Committee for Animal Experimentation and the Animal Welfare at the
Fuzhou General Hospital (Fuzhou, China). Male C57BL/6 mice (age, 8
weeks; weight, ~23 g; provided by and bred at the Shanghai
Laboratory Animal Center, Shanghai, China) were randomly assigned
to the following experiments.
Small animal imaging of mCherry-11R
protein
The distribution of the mCherry-11R protein was
examined, which consisted of a 6-histidine tag and a set of
consecutive 11R. In brief, 0.1 mg mCherry-11R protein was injected
into the caudal vein of mice. Recombinant mCherry protein without
11R was used as the negative control (0.1 mg). Animals were
sacrificed 12 h following injection of protein. An IVIS 200 series
system with a cooled charge-coupled-device camera (Caliper Life
Sciences, Waltham, MA, USA) was utilized to image major organs.
Acquisition times were from 1 sec to 1 min with varying binning
factors depending on the light emission. Regions of interest were
created around specific anatomic sites and light emission was
measured as photons/sec/cm2/sr (photon flux) using
Living Image Software Version 4.3.1 (PerkinElmer, Inc., Waltham,
MA, USA).
MafA-11R protein in vivo tissue
distribution
Mice were injected intravenously with 0.1 mg
MafA-11R. Following 12 h, heart, liver, muscle, intestine, kidney
and pancreas tissues from MafA-11R-treated mice were harvested.
Cell extracts were prepared by lysing the tissues with CytoBuster
Protein Extraction Reagent (cat. no. 71009; Millipore; Merck KGaA).
Total protein quantification was performed using a bicinchoninic
acid assay kit (Pierce; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Protein samples (50 µg) were resolved on 10% SDS-PAGE
gels and then transferred to polyvinylidine fluoride membranes
(Immobilon-P PVDF Membrane; EMD Millipore). Membranes were blocked
with 5% bovine serum albumin (BSA; Sangon Biotech Co., Ltd.,
Shanghai, China) in TBST (50 mmol/l Tris-HCl, 150 mmol/l NaCl, and
0.1% Tween-20) at room temperature for 30 min and then incubated
with primary antibody against polyclonal anti-6-histidine (1:2,000;
cat. no. 000000011922416001; Sigma-Aldrich, Merck KGaA) at 4°C
overnight. Following this, the membranes were incubated with
anti-mouse horseradish peroxidase-conjugated secondary IgG
antibodies (1:1000; cat. no. 7076S, Cell Signaling Technology) at
room temperature for 1 h and developed using a SuperSignal West
Pico chemiluminescent kit (Pierce; Thermo Fisher Scientific,
Inc.).
Diabetic mouse model preparation and
treatment with protein
C57BL/6 male mice (n=30) received an intraperitoneal
injection with streptozotocin (STZ; 50 mg/kg; Sigma Aldrich; Merck
KGaA) to induce diabetes for 5 consecutive days (15). Animals with fasting blood glucose
levels of 16 mM for two consecutive readings (n=22) received
protein treatment. The diabetic mice were injected with 0.1 mg
MafA-11R or 0.1 mg mCherry-11R protein into the tail vein, 10 days
post-STZ-injection. Following protein injection, non-fasting blood
glucose levels were measured regularly.
Intraperitoneal glucose tolerance
test
The normal, MafA-11R, and mCherry-11R-treated mice
fasted overnight. They were administered glucose (1 mg/g body
weight) intraperitoneally. Blood glucose levels were measured prior
to and following glucose administration. Blood samples were
obtained from the tail vein and analyzed using a glucometer.
Serum insulin measurements via
enzyme-linked immunosorbent assay (ELISA)
The normal, MafA-11R and mCherry-11R-treated mice
fasted overnight and blood samples were collected at 15 min
intervals following intraperitoneal glucose (1 mg/g body weight)
administration. Serum insulin levels were determined via an ELISA
kit (cat. no. EZRMI-13K; EMD Millipore).
Reverse transcription (RT) PCR
Total RNA from tissues was extracted using the
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and cDNA was synthesized by reverse transcription using
ReverTra Ace (Toyobo Co., Ltd., Osaka, Japan). The oligonucleotide
primer sequences were as follows: Forward, GTCCCTCACCCTCCCAAAAG and
reverse, GCTGCCTCAACACCTCAACCC for mouse actin and forward,
CAGCAAGCAGGAAGCCTATC and reverse, GGAACCACAAAGGTGCTGCT for mouse
insulin 2. The PCR was run for 35 cycles with 20 pmol primer under
the following conditions: 94°C for 30 sec, 56°C for 30 sec and 72°C
for 30 sec.
Quantitative RT-PCR (RT-qPCR)
RT-qPCR was performed as previously described
(16). The primer sequences used
were as follows: Forward, CAACCGTGTAAATGCCACTG and reverse,
TGCTACGGATGGACTGTTTG for mouse insulin 1; forward,
AATGGTCGCCTCATTCTTTG and reverse, ATCAAGAGGGCTCCAGTCAA for mouse
(glucose transporter) glut2; forward, CATCTCCCCATACGAAGTGC and
reverse, ACGGGTCCTCTTGTTTTCCT for mouse pdx-1; forward
GCTCCAGGGTTATGAGATCG and reverse, CTCTGCATTCATGGCTTCAA for mouse
neuroD; forward, TCTACCAGCCAATCCCACAG and reverse,
ATCATAACTCCGCCCATTCA for mouse pax6; forward, GGCTGCTGGTATCTTTGCTC,
and reverse, CCAGAGCTTTGGCATTTAGC for mouse proprotein convertase
(pc)1/3. The primers for β-actin and mouse insulin 2 were used as
above. Relative fold changes in mRNA expression were calculated
using the formula 2-ΔΔCq (17).
Immunofluorescence assay
The mice were sacrificed, and small intestine
tissues were harvested, and fixed overnight with 4%
paraformaldehyde. The fixed tissues were paraffin embedded and 5 µm
sections were cut and mounted onto slides. For insulin detection,
the sections were rehydrated by bathing 3 times each in xylenes
followed by isopropyl alcohol. Slides were then rinsed in distilled
water. Antigen retrieval was performed with heated 10 mM sodium
citrate buffer, pH 6.0. A hydrophobic pen was used to outline the
tissue. Tissue was blocked with PBS containing 5% BSA (Sangon
Biotech Co., Ltd.) and 0.05% Tween-20 for 30 min at room
temperature. Mouse anti-6-histidine (1:500; cat. no. MA1-21315;
Thermo Fisher Scientific, Inc.) or anti-insulin antibodies (1:250;
cat. no. sc9168; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
were diluted in blocking buffer and applied to the slides overnight
at 4°C. The sections were then incubated with rhodamine-conjugated
goat anti-mouse or Dylight 594-conjugated donkey anti-rabbit IgG
secondary antibodies (1:100; cat. nos. 115-025–075 and 715-585-150;
Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) and
counterstained with DAPI (Sigma-Aldrich, Merck KGaA). Slides were
analyzed using an epifluorescence microscope (X81; Olympus
Corporation, Tokyo, Japan).
Statistical analysis
Statistical analyses were performed using SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. Data were analyzed
using one- or two-way analysis of variance, followed by
Bonferroni's multiple comparison test. P<0.05 was considered to
indicate a statistically significant difference.
Results
11R fusion proteins efficiently
transduce cells in vitro
mCherry has previously been demonstrated to act as a
useful monomeric red fluorescent protein, and therefore the
mCherry-11R recombinant protein was used to visualize if 11R
transduced fused proteins into the cells of interest. The IEC-6 and
NIH3T3 cells were treated with mCherry-11R. Following 4 h
incubation, a fluorescent signal was observed in the two cell lines
(n=12, each; Fig. 1C). To assess
if the recombinant MafA-11R transduced into cells, IEC-6 and NIH3T3
were treated with His-tagged MafA-11R fusion protein.
Immunostaining of His-tagged MafA protein revealed transduction of
the MafA-11R protein (n=6; Fig.
1D). The transduced MafA-11R protein was detected in the
cytosolic and nuclear extracts of protein treated cells via western
blot analysis, using anti-6-histidine antibody (n=6; Fig. 1E). These data demonstrated that 11R
acted as a functional PTD and the recombinant proteins were capable
of penetrating the cell membrane.
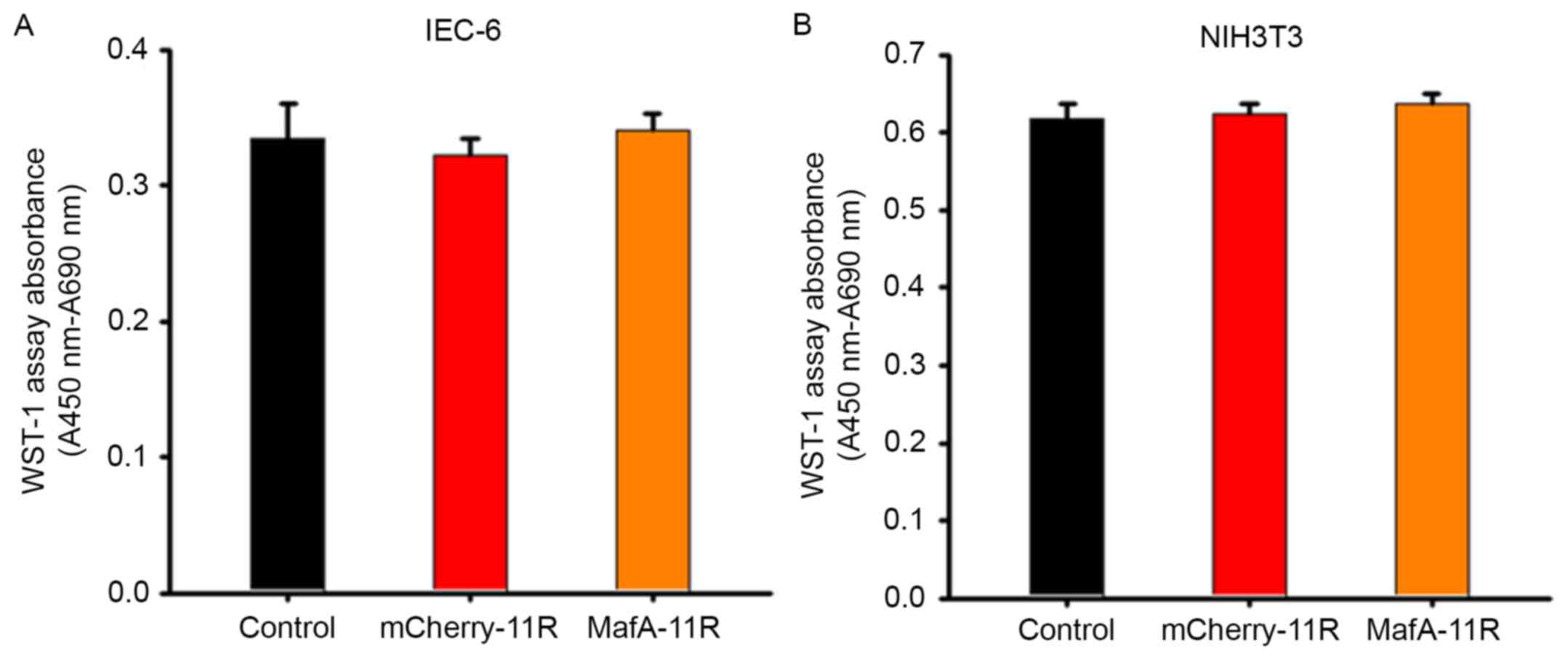
Cell viability assay
Prior to the commencement of the animal experiments
in vivo, a cell viability assay was performed in
vitro. The results demonstrated that the addition of
mCherry-11R or MafA-11R fusion proteins did not inhibit the cell
growth of the IEC-6 and NIH3T3 cell lines, which indicated that the
proteins exhibited a low cytotoxicity (n=48 each; Fig. 2).
11R recombinant protein primarily
present in liver and intestine cells in vivo
Firstly, as mCherry was easily observed by a small
animal imaging system (17), the
present study used it to detect and evaluate the distribution of
11R-fused recombinant proteins in vivo. mCherry-11R was
constructed, however MafA-mCherry-11R was not used, as long
recombinant proteins are susceptible to fracture. A total of 0.1 mg
mCherry-11R purified protein was injected into the tale vein of
C57BL/6 mice and 11R-nonfused mCherry was applied as a negative
control in this experiment (n=6 each). mCherry fluorescent signal
is unable to be detected across the skin and muscle in
protein-infused mice, therefore mice were sacrificed for detection
of signal. It has previously been demonstrated that PTD alters the
in vivo distribution of recombinant proteins by transducing
them into cells and preventing rapid elimination into the urine.
The present study examined recombinant protein distribution 12 h
following injection. mCherry-11R was primarily present in the liver
and intestine post-protein administration, however no fluorescent
signal was detected by the small animal system in the 11R-nonfused
mCherry control protein injected mice (Fig. 3A), which suggested that 11R
facilitated the in vivo distribution of mCherry protein into
the liver and intestine cells, and prevented its rapid
elimination.
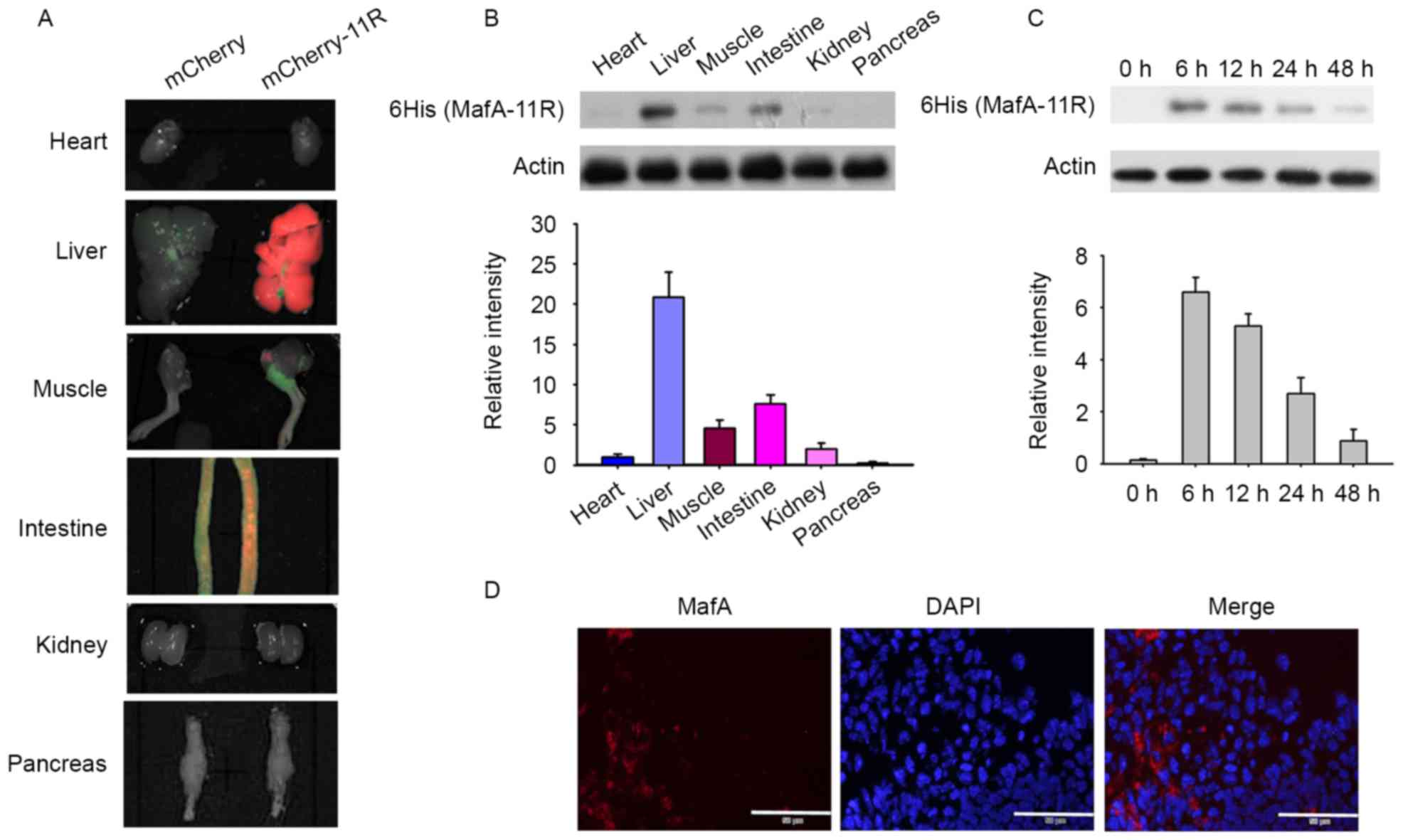 | Figure 3.Localization of the 11R-fused proteins
in vivo. (A) In vivo tissue distribution of
mCherry-11R was observed by an IVIS 200 series system. Heart,
liver, muscle, intestine, kidney and pancreas tissues were
harvested 12 h following mCherry-11R intravenous injection. (B) The
localization of MafA-11R was detected by western blotting. Major
tissues were harvested 12 h following MafA-11R administration.
Proteins were detected using anti-6-histidine or anti-actin
antibody. The relative amount of MafA-11R protein was quantified by
densitometry and the values normalized to actin. The heart reading
was defined as 1 and the remaining values were divided by the heart
reading. (C) Time course of penetration of MafA-11R proteins in
small intestine. Normal C57BL/6 mice were injected intravenously
with MafA-11R protein (0.1 mg/mouse). Intestinal samples were
collected at the indicated times. The last reading was defined as 1
and the remaining values were divided by the last reading. (D)
MafA-11R immunostaining of the intestine tissue. Intestine tissues
were harvested 12 h following MafA-11R injection. Intestinal
sections were immunostained with anti-6-histidine antibodies. Scale
bars, 50 µm. 6 HIS, 6-histidine ladder; MafA, musculo aponeurotic
fibrosarcoma BZIP transcription factor A; 11R, 11 arginine;
mCherry-11R, 11R-fused mCherry; MafA-11R, 11R-fused MafA
protein. |
The MafA-11R protein may permeate into cells in
vitro, however the in vivo tissue distribution of
MafA-11R remains to be elucidated. To examine MafA-11R
distribution, the C57BL/6 mice were injected with 0.1 mg MafA-11R
protein, major organs were harvested 12 h following injection and
MafA-11R protein was then detected via anti-6-histidine
immunoblotting. As indicated in the representative images of heart,
liver, muscle, intestine, kidney and pancreas, the recombinant
MafA-11R was concentrated primarily in the hepatocytes and
intestine cells (n=6; Fig. 3B). A
low level of MafA-11R was additionally detected in the tissues of
the kidney, muscle, heart and pancreas, however became undetectable
at 24 h post injection (data not shown). Intestine samples were
collected at various times and probed with anti-6-histidine
antibody. The MafA-11R expression was evident in the intestine 6 h
post injection and then gradually began to decline (Fig. 3C). Immunofluorescence examination
confirmed the distribution of the MafA-11R protein in the intestine
12 h following injection (Fig.
3D).
MafA-11R protein decreases blood
glucose levels in diabetic mice
To test the function of the MafA-11R protein in
vivo, diabetic C57BL/6 mice received 0.1 mg MafA-11R or
nontherapeutic mCherry-11R protein (negative control; experimental
design in schematic Fig. 1A).
Non-fasting blood glucose levels were monitored as indicated.
Diabetic mice that received MafA-11R injections 4 times achieved
alleviation of hyperglycemia. Blood glucose levels were then kept
at a low level and increased within 15 days of the first injection
(Fig. 4B; n=10–12). A significant
decrease was not detected in the blood glucose levels of the
mCherry-11R injected control diabetic mice (Fig. 4B). The intraperitoneal glucose
tolerance test (IPGTT; Fig. 4C)
demonstrated that the MafA-11R injected mice exhibited a
significantly improved IPGTT curve 15 days following the first
injection (n=5, each), compared with the diabetic mice receiving
mCherry-11R.
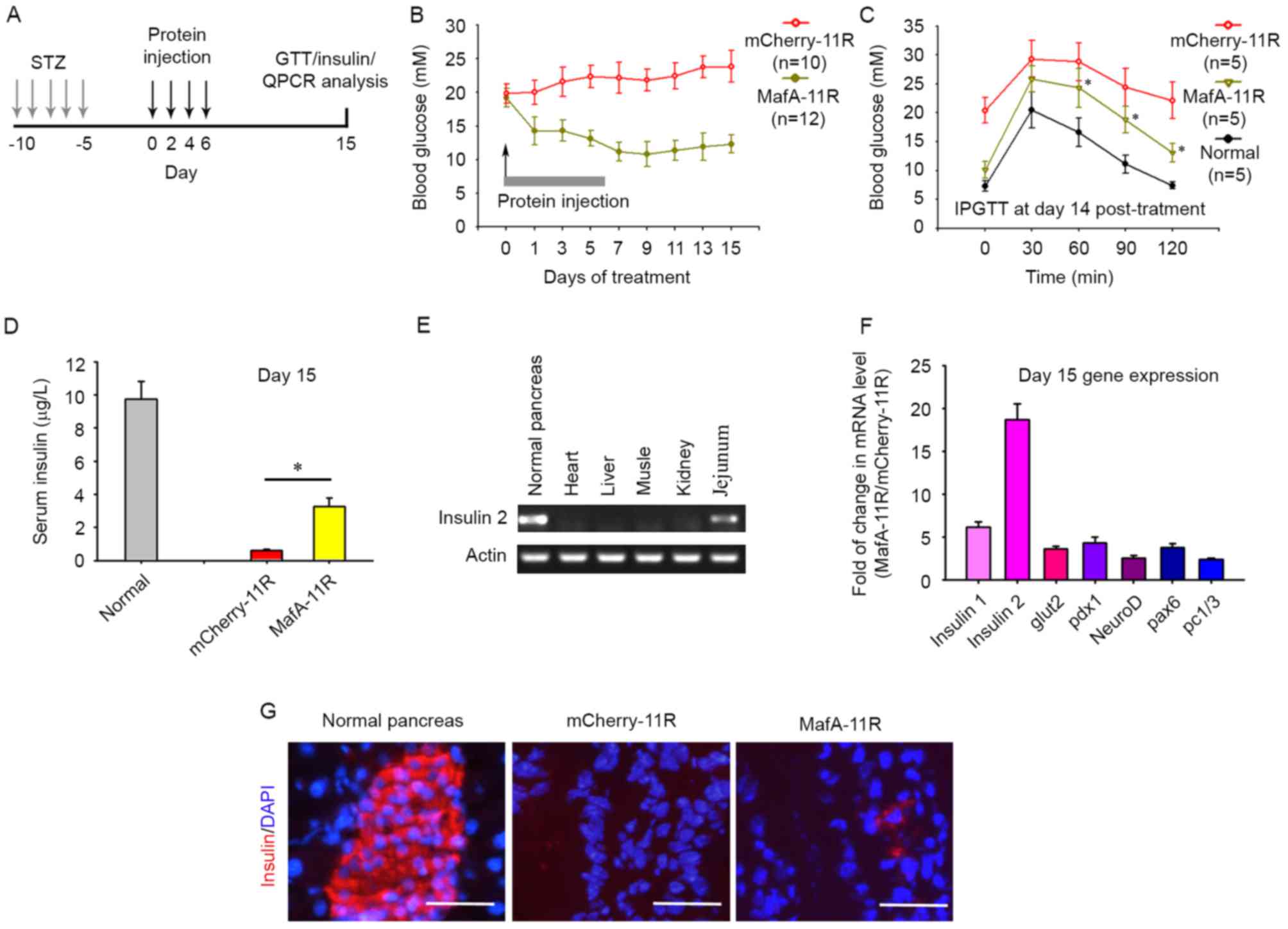 | Figure 4.MafA-11R protein promotes the
trans-differentiation of intestine cells into insulin-producing
cells. (A) Experimental timeline, timing of STZ treatment with
MafA-11R protein and the measurement of blood insulin levels, IPGTT
and the determination of gene expression by RT-qPCR analyses. (B)
Effects of MafA-11R protein on blood glucose levels. Diabetic
C57BL/6 mice were treated with intravenous injections of 0.1 mg
MafA-11R or mCherry-11R 4 times (arrow) and blood glucose levels
were determined by glucometer. (C) The IPGTT was performed as
described in materials and methods, and blood glucose was measured
at 0, 30, 60, 90 and 120 min in normal, MafA-11R or
mCherry-11R-treated mice. *P<0.05 compared with mCherry-11R. (D)
Serum insulin levels were measured in MafA-11R- and
mCherry-11R-treated mice 15 min following intraperitoneal glucose
stimulation 15 days post-treatment. *P<0.05 compared with
mCherry-11R. (E) Expression of insulin 2 in organs. Total RNA from
MafA-11R-treated diabetic mice at day 15 post-treatment and
expression of insulin 2 were examined by RT-qPCR. Data are from ≥6
mice/group and are representative of three independent experiments.
(F) RT-qPCR analyses of pancreatic gene expression in intestines.
Total RNA from diabetic mouse intestine (day 15 post-MafA-11R
treatment) was analyzed via RT-qPCR for the expression of 7 key
pancreatic genes (insulin 1, insulin 2, glut2, pdx1, neuroD, pax6
and pc1/3). (G) Insulin immunostaining. Paraffin sections from the
intestine were stained with anti-insulin antibody. Bars, 50 µm.
MafA, musculo aponeurotic fibrosarcoma BZIP transcription factor A;
11R, 11 arginine; MafA-11R, 11R-fused MafA protein; STZ,
streptozotocin; IPGTT, intraperitoneal glucose tolerance test;
RT-qPCR, reverse-transcription-quantitative polymerase chain
reaction; mCherry-11R, 11R-fused mCherry; Pdx, pancreatic and
duodenal homeobox 1; neuroD, neuronal differentiation; glut,
glucose transporter 2; pc, proprotein convertase 1/3; pax, paired
box 6. |
To further assess the ability of glucose-stimulated
insulin release in the MafA-11R-treated diabetic mice, healthy
normal and treated mice were challenged at day 15 post first
injection with an intraperitoneal bolus of glucose. Sera collected
from normal, MafA-11R-treated, or mCherry-11R-treated mice at 15
min post glucose injection were assayed for insulin. Serum insulin
levels in MafA-11R-treated diabetic mice were 5.36 times greater at
day 15 compared with those in the mCherry-11R-treated group
(Fig. 4D), indicating an
improvement in the ability of the MafA-11R-treated mice to react to
a glucose challenge. Blood glucose levels were decreased at day 15
in MafA-11R-treated mice, however the insulin released (3.27 µg/l)
following 15 min of glucose stimulation was decreased compared with
that in normal nondiabetic mice (9.74 µg/l), suggesting functional
immaturity of newly formed insulin producing cells (n=6 per group;
P<0.05).
β-cell regeneration in MafA-11R-treated mice was not
observed (data not shown), therefore the present study hypothesized
that MafA-11R promoted insulin expression in other tissues.
Adenovirus-mediated overexpression of MafA may induce insulin gene
expression in the rat small bowel, therefore the intestine was
harvested at day 15 post injection in addition to the kidney,
liver, heart and spleen. The transcription of insulin 2 in
MafA-11R-treated mice was detected via RT-PCR. Fig. 4E indicated the expression of
insulin 2 in the jejunum, however not in other tissues. The control
mCherry-11R-treated mice did not exhibit any signal in all the
tissues tested.
To determine the molecular mechanism underlying
MafA-11R-mediated insulin production in the intestine, RT-qPCR was
used to examine the expression of key genes associated with
pancreatic regeneration. MafA-11R treatment of diabetic mice at day
15 resulted in markedly upregulated levels of insulin 1
(6.17-fold), insulin 2 (18.64-fold), and slightly increased levels
of glut2 (3.62-fold), pdx1 (4.29-fold), neuroD (2.53-fold), pax6
(3.75-fold) and pc1/3 (2.43-fold), compared with their
corresponding control values (mCherry-11R treated intestine;
Fig. 4F). An immunofluorescence
study indicated that 15 days following MafA-11R treatment in
diabetic mice, insulin-positive cells were present in the jejunum
section (Fig. 4G). A total of 30
sections derived from 3 MafA-11R-treated mice were examined and the
density of insulin-positive cells was 2.38±0.41
cell/mm2. Conversely, sections derived from mCherry-11R
treated mice did not indicate insulin-positive cells. The
amelioration of hyperglycemia and the detection of insulin-positive
cells in the jejunum suggested that insulin was induced following
MafA-11R administration.
Discussion
It has been previously demonstrated that PTD-fusion
proteins are efficiently introduced into cultured cells and live
tissues when injected into mice. PTDs may be applicable for various
animal models that require repeated intracellular delivery of full
length proteins in vivo (18,19).
The present study demonstrated that 11R-fused proteins
(mCherry-11R, MafA-11R) directly transduced the small intestine and
the MafA-11R protein injection ameliorated hyperglycemia in mice
with streptozotocin-induced diabetes. It was additionally observed
that IPGTT and glucose-stimulated insulin release were improved in
MafA-11R-treated diabetic mice and the MafA-11R protein induced
insulin expression in the jejunum cells. The present study
therefore constituted a demonstration that protein therapy in the
form of in vivo MafA-11R delivery to animals may act as a
novel therapeutic strategy, which does not present the adverse
effects associated with viral vector-mediated gene therapies.
The results indicated that in vivo delivery
of MafA-11R promoted the development of various jejunum cells into
insulin producing cells. This effect was not observed in the liver
or other tissues. It has previously been demonstrated that there is
a developmental association between the gut and pancreas (20,21).
Various gut cells express the same molecules that have been
demonstrated to convey glucose responsiveness in pancreatic
β-cells. Commitment to the gut endocrine lineage requires the
initiation of Neurog3 expression (22). Expression of NeuroD is required for
the development of various gut endocrine cells (13). Pax4 and Pax6 are important in the
differentiation into endocrine cells in the pancreas and intestine
(13,14). Therefore, the gut cells may be
amenable to minimal engineering to secrete insulin, serving as
β-cell surrogates. Various groups have demonstrated the successful
induction of islet neogenesis in the liver using adenoviral vectors
to deliver pancreatic transcription factors (23–25).
MafA, in combination with Pdx-1 and NeuroD, resulted in the
induction of long-term expression of insulin in the liver (26). In the present study, delivery of
recombinant MafA did not induce insulin gene expression in the
liver, which led to the hypothesis that combination with further
transcription factors was necessary. Wang et al (27) demonstrated that a host response to
an adenovirus, in combination with expression of pro-endocrine
pancreatic transcription factors, is sufficient to induce insulin
production in the liver of diabetic mice.
The present study demonstrated that MafA-11R
effectively ameliorated hyperglycemia in diabetic mice, however
there are potential obstacles to overcome prior to the use of this
technique as a therapeutic strategy. One concern is the potential
toxicity of MafA-11R, as the 11R-fused protein exhibits potential
to enter any tissue or cell type. The present study excluded the
expression of insulin in tissues other than the intestine. The
diabetic mice treated with MafA-11R appeared normal, without
evidence of abnormal organ morphology. The animals gained body
weight and exhibited reduced blood glucose levels. However, a full
toxicity profile of MafA-11R is required in the future. Notably,
the MafA-11R-induced insulin producing cells were still premature
(the total amount of released insulin was decreased compared with
that in normal mice), therefore repeated administration of
MafA-11R, or administration in conjunction with transcription
factors may be necessary and requires further investigation.
Acknowledgements
The present study was supported in part by The
National Natural Science Foundation of China (grant nos. 81200560
and 81370948).
References
|
1
|
Docherty HM, Hay CW, Ferguson LA, Barrow
J, Durward E and Docherty K: Relative contribution of PDX-1, MafA
and E47/beta2 to the regulation of the human insulin promoter.
Biochem J. 389:813–820. 2005. View Article : Google Scholar :
|
|
2
|
Olbrot M, Rud J, Moss LG and Sharma A:
Identification of beta-cell-specific insulin gene transcription
factor RIPE3b1 as mammalian MafA. In: Proc Natl Acad Sci USA. 99.
pp. 6737–6742. 2002; View Article : Google Scholar :
|
|
3
|
Zhang C, Moriguchi T, Kajihara M, Esaki R,
Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, et
al: MafA is a key regulator of glucose-stimulated insulin
secretion. Mol Cell Biol. 25:4969–4976. 2005. View Article : Google Scholar :
|
|
4
|
Matsuoka TA, Kaneto H, Stein R, Miyatsuka
T, Kawamori D, Henderson E, Kojima I, Matsuhisa M, Hori M and
Yamasaki Y: MafA regulates expression of genes important to islet
beta-cell function. Mol Endocrinol. 21:2764–2774. 2007. View Article : Google Scholar
|
|
5
|
Schwarze SR and Dowdy SF: In vivo protein
transduction: Intracellular delivery of biologically active
proteins, compounds and DNA. Trends Pharmacol Sci. 21:45–48. 2000.
View Article : Google Scholar
|
|
6
|
Ho A, Schwarze SR, Mermelstein SJ, Waksman
G and Dowdy SF: Synthetic protein transduction domains: Enhanced
transduction potential in vitro and in vivo. Cancer Res.
61:474–477. 2001.
|
|
7
|
Matsushita M, Tomizawa K, Moriwaki A, Li
ST, Terada H and Matsui H: A high-efficiency protein transduction
system demonstrating the role of PKA in long-lasting long-term
potentiation. J Neurosci. 21:6000–6007. 2001.
|
|
8
|
Matsui H, Tomizawa K, Lu YF and Matsushita
M: Protein, Therapy: In vivo protein transduction by polyarginine
(11R) PTD and subcellular targeting delivery. Curr Protein Pept
Sci. 4:151–157. 2003. View Article : Google Scholar
|
|
9
|
Kim D, Kim CH, Moon JI, Chung YG, Chang
MY, Han BS, Ko S, Yang E, Cha KY, Lanza R and Kim KS: Generation of
human induced pluripotent stem cells by direct delivery of
reprogramming proteins. Cell Stem Cell. 4:472–476. 2009. View Article : Google Scholar :
|
|
10
|
Zhang H, Ma Y, Gu J, Liao B, Li J, Wong J
and Jin Y: Reprogramming of somatic cells via TAT-mediated protein
transduction of recombinant factors. Biomaterials. 33:5047–5055.
2012. View Article : Google Scholar
|
|
11
|
Kaneto H, Matsuoka TA, Nakatani Y,
Miyatsuka T, Matsuhisa M, Hori M and Yamasaki Y: A crucial role of
MafA as a novel therapeutic target for diabetes. J Biol Chem.
280:15047–15052. 2005. View Article : Google Scholar
|
|
12
|
Nomura S, Nakamura T, Hashimoto T, Nishio
Y, Maegawa H, Kudo M and Kashiwagi A: MafA differentiates rat
intestinal cells into insulin-producing cells. Biochem Biophys Res
Commun. 349:136–143. 2006. View Article : Google Scholar
|
|
13
|
Schonhoff SE, Giel-Moloney M and Leiter
AB: Minireview: Development and differentiation of gut endocrine
cells. Endocrinology. 145:2639–2644. 2004. View Article : Google Scholar
|
|
14
|
van der Flier LG and Clevers H: Stem
cells, self-renewal, and differentiation in the intestinal
epithelium. Annu Rev Physiol. 71:241–260. 2009. View Article : Google Scholar
|
|
15
|
Wu KK and Huan Y: Streptozotocin-induced
diabetic models in mice and rats. Curr Protoc Pharmacol Chapter.
5:Unit 5.47. 2008. View Article : Google Scholar
|
|
16
|
Lu J, Li G, Lan MS, Zhang S, Fan W, Wang H
and Lu D: Pax4 paired domain mediates direct protein transduction
into mammalian cells. Endocrinology. 148:5558–5565. 2007.
View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Zacharakis G, Kambara H, Shih H, Ripoll J,
Grimm J, Saeki Y, Weissleder R and Ntziachristos V: Volumetric
tomography of fluorescent proteins through small animals in vivo.
In: Proc Natl Acad Sci USA. 102. pp. 18252–18257. 2005; View Article : Google Scholar :
|
|
19
|
Toro A and Grunebaum E: TAT-mediated
intracellular delivery of purine nucleoside phosphorylase corrects
its deficiency in mice. J Clin Invest. 116:2717–2726. 2006.
View Article : Google Scholar :
|
|
20
|
Yamada S, Kanno H and Kawahara N:
Trans-membrane peptide therapy for malignant glioma by use of a
peptide derived from the MDM2 binding site of p53. J Neurooncol.
109:7–14. 2012. View Article : Google Scholar
|
|
21
|
Slack JM: Developmental biology of the
pancreas. Development. 121:1569–1580. 1995.
|
|
22
|
Grapin-Botton A, Majithia AR and Melton
DA: Key events of pancreas formation are triggered in gut endoderm
by ectopic expression of pancreatic regulatory genes. Genes Dev.
15:444–454. 2001. View Article : Google Scholar :
|
|
23
|
Jenny M, Uhl C, Roche C, Duluc I,
Guillermin V, Guillemot F, Jensen J, Kedinger M and Gradwohl G:
Neurogenin3 is differentially required for endocrine cell fate
specification in the intestinal and gastric epithelium. EMBO J.
21:6338–6347. 2002. View Article : Google Scholar :
|
|
24
|
Kojima H, Fujimiya M, Matsumura K, Younan
P, Imaeda H, Maeda M and Chan L: NeuroD-betacellulin gene therapy
induces islet neogenesis in the liver and reverses diabetes in
mice. Nat Med. 9:596–603. 2003. View
Article : Google Scholar
|
|
25
|
Taniguchi H, Yamato E, Tashiro F, Ikegami
H, Ogihara T and Miyazaki J: beta-cell neogenesis induced by
adenovirus-mediated gene delivery of transcription factor pdx-1
into mouse pancreas. Gene Ther. 10:15–23. 2003. View Article : Google Scholar
|
|
26
|
Kaneto H, Matsuoka TA, Katakami N and
Matsuhisa M: Combination of MafA, PDX-1 and NeuroD is a useful tool
to efficiently induce insulin-producing surrogate beta-cells. Curr
Med Chem. 16:3144–3151. 2009. View Article : Google Scholar
|
|
27
|
Wang AY, Ehrhardt A, Xu H and Kay MA:
Adenovirus transduction is required for the correction of diabetes
using Pdx-1 or Neurogenin-3 in the liver. Mol Ther. 15:255–263.
2007. View Article : Google Scholar
|