Introduction
Craniopharyngiomas (CPs) are benign epithelial
tumors of the sellar region that arise along the path of the
craniopharyngeal duct. CPs are histopathologically classified into
two subtypes: Adamantinomatous craniopharyngioma (ACP) and squamous
papillary craniopharyngioma (SPCP). ACP often forms finger-like
protrusions that invade the surrounding brain tissue, inducing a
local inflammatory state between the tumor cells and parenchyma.
Therefore, total resection of the tumor is complex and can lead to
postoperative recurrence and a poorer clinical prognosis when
compared to SPCP (1).
Inflammation is a critical component of tumor
progression. It is now evident that the tumor microenvironment,
which is largely composed of inflammatory cells, is an
indispensable participant in the neoplastic process, stimulating
proliferation, survival and migration (2). In some cases of ACP, infiltration of
neutrophils, lymphocytes and eosinophils has been detected in the
tumor mass or the surrounding parenchyma. In addition, spontaneous
rupture of ACP cysts and spillage of cyst fluid during surgery can
lead to aseptic meningitis (3).
Inflammatory cells and the cytokines secreted may modulate the
local immune response and affect tumor cell growth. High levels of
IL-6 have been detected in cyst fluid and CP tissues; a previous
study indicated that IL-6 served an important role in the
inflammatory reaction that occurred in the interface between the CP
and the brain parenchyma (4). In
addition, in some cancer cells, IL-6 acts as an autocrine or
paracrine factor that promotes tumor cell proliferation, migration
and invasion in vitro (5).
These findings indicate that IL-6 may exert a biological influence
on ACP cells.
It has previously been demonstrated that IL-6
induces an epithelial-mesenchymal transition (EMT) phenotype in
breast cancer cells, promoting migration and invasion (6). Our previous research revealed that
EMT may serve a role in the pathogenesis and development of CP,
particularly ACP (7). However, the
role of IL-6, particularly in association with EMT, in ACP
progression is unclear. The present study investigated the
biological role and significance of IL-6 in ACP cells using cell
culture assays of primary human ACP cells. Activation of the
classic and trans-signaling pathways of IL-6 was revealed to induce
an EMT phenotype to promote the migration of human ACP cells;
however, IL-6 did not affect the viability of ACP cells.
Materials and methods
Patients and tissue specimens
A prospective cohort of study patients were selected
for clinicopathological analysis according to the inclusion and
exclusion criteria as described previously (7). A total of 49 CP biopsy specimens (37
ACPs and 12 SPCPs) were obtained from the archives of the
Department of Neurosurgery at the Nanfang Hospital, Southern
Medical University (Guangzhou, China) for use in the present study.
The biopsy specimens were originally collected between January 2005
and January 2009. Patient age ranged between 4 and 60 years, with a
mean of 29.39±18.04 years. Cystic fluid samples were obtained at
surgery from 6 of the patients with cystic ACP, and stored at
−80°C.
A total of 13 solid ACP specimens were obtained at
surgery from the Department of Neurosurgery at the Nanfang
Hospital, Southern Medical University for primary ACP and tumor
associated fibroblast (TAF) cell culture, between July 2010 and
March 2012. Patient age ranged between 3 and 50 years, with a mean
of 21.77±16.60 years. The present study was approved by the
Affiliated Hospital of Southwest Medical University (Luzhou,
China). Patients provided written informed consent.
Inflammatory density score
Specimens removed at surgery were immediately fixed
in 10% formalin, and subsequently embedded in paraffin. Sections
were subsequently stained with hematoxylin and eosin (both from
ZSGB-Bio, Beijing, China) for diagnosis and assessment of the
inflammatory density score. The inflammatory density score was
determined by counting the number of inflammatory cells adjacent to
the interface between the CP and the surrounding normal tissue, to
a depth corresponding with 1 high-power field (HPF magnification,
×400) in 10 consecutive fields. Inflammatory density was graded on
a 4-grade scale: Grade 0, no inflammation; grade 1, <15
cells/field; grade 2, 15–50 cells/field and grade 3, >50
cells/field. Density was recorded separately for each HPF and the
inflammatory score for each case was calculated as the average of
all HPFs examined. Cases were divided into three groups according
to the inflammatory score: Grades 0–1, mild; grade 2, moderate and
grade 3, severe. Slides were analysed with an Olympus BX-51
microscope with a DP-71 CCD camera (both from Olympus Corporation,
Tokyo, Japan).
Evaluation of calcification
The degree of CP calcification in biopsy specimens
from the 49 patients was determined using the criteria described in
our previous study (8). Briefly,
tumor calcification was identified as areas of high attenuation on
computerized tomography scans or as low signal (usually) on
magnetic resonance images. Tumors were classified as follows:
Calcification absent (−); solid lumps/a little calcification or
eggshell pattern lining the cyst wall (+, mild to moderate
calcification); popcorn-like foci (++, severe calcification).
Follow-up
The postoperative hypothalamic status scale (HSS) of
biopsy specimens from the 49 patients was determined 6 months after
surgery using the criteria described by Fahlbusch et al
(9): I, good (without any new
permanent neurological, neuropsychological or endocrinal deficits);
II, moderate (with new endocrine deficits requiring permanent
replacement therapy); III, fair (with neurological or
neuropsychological deficits, with autonomy); IV, poor (severe
neurological and/or hypothalamic disturbances with total
dependency, or mortality).
Cytokine antibody array
An antibody-based cytokine array system was used to
detect the levels of inflammatory cytokines in frozen ACP tissues
obtained at surgery belonging to the mild and severe inflammatory
groups, which had been stored in liquid nitrogen. The experiment
was conducted using the Human Inflammation Antibody Array III kit
(AAH-INF-G3; RayBiotech, Inc., Norcross, GA, USA) according to the
manufacturer's protocol.
ACP cell culture
Primary ACP cell cultures were established using
solid tumor specimens obtained at surgery from the Department of
Neurosurgery at the Nanfang Hospital, Southern Medical University
(Guangzhou, China) in a similar manner as described by Hölsken
et al (10). Briefly, a
portion (1–10 cm3) of the tumor sample was placed in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing
penicillin/streptomycin (100 units/ml/100 µg/ml, Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) immediately following
surgical removal. Tumor tissue was subsequently separated into
small pieces, washed in phosphate-buffered saline (PBS) containing
penicillin, streptomycin (100 units/ml/100 µg/ml), trypsin and EDTA
(0.25 and 0.05%; Gibco; Thermo Fisher Scientific, Inc.) and
incubated for 20–25 min at 37°C and 5% CO2. A culture
solution was prepared using DMEM and DMEM/F12 (1:1; Gibco; Thermo
Fisher Scientific, Inc.) in a final ratio of 3:1, to which insulin
(5 µg/ml; Sigma-Aldrich; Merck KGaA), transferrin (5 µg/ml; EMD
Millipore, Billerica, MA, USA), T3 (2×10−9 mol/l;
Sigma-Aldrich; Merck KGaA), hydrocortisone (0.4 µg/ml;
Sigma-Aldrich; Merck KGaA), cholera toxin (2×10−10
mol/l; Sigma-Aldrich; Merck KGaA), antibiotics
(penicillin/streptomycin, 100 units/ml/100 µg/ml), fungizone (2.5
µg/ml; Gibco; Thermo Fisher Scientific, Inc.), L-glutamine (2 mM;
Gibco; Thermo Fisher Scientific, Inc.) and 10% FBS were added.
Following 2 days of culture, epidermal growth factor (EGF; 10
µg/ml; R&D Systems, Inc., Minneapolis, MN, USA) was added to
the culture medium and the medium was renewed every second day and
supplemented with EGF. Based on observations of cell morphology and
immunohistochemical staining for pan-keratin (CK; 1:500, #4545,
Cell Signaling Technology, Inc., Danvers, MA, USA), the cultures
that contained the most homogenous epithelial-like cells were
expanded. Cell morphology was analysed with a Leica DMIRE2 inverted
microscope (Leica Microsystems GmbH, Wetzlar, Germany).
TAF culture and analysis
The ACP tumor tissues obtained at surgery from
Nanfang Hospital were carefully dissected, minced with scalpels,
then transferred to new culture dishes with a little DMEM.
Following 4 h (37°C, 5% CO2), the tumor tissues were
cultured in DMEM supplemented with penicillin/streptomycin (100
units/ml/100 µg/ml) and 10% FBS. Following 1 week of culture, the
cells that had migrated from the tissue clumps were trypsinized,
transferred to new culture dishes and left for a short period
(10–20 min) to allow for surface attachment. Cells that had not
attached were then removed and the dishes with firmly attached
cells were washed with PBS to further eliminate loosely attached
cells. Based on observations of cell morphology and positive
staining for the fibroblast markers vimentin (1:500; 550513; BD
Biosciences, Inc., Franklin Lakes, NJ, USA) and α-SMA (1:100;
bs-10196R; BIOSS, Beijing, China) and negative staining for the
epithelial cell marker Pan-CK (dilution, 1:500, #4545, Cell
Signaling Technology, Inc.), the cultures that contained the most
homogenous fibroblastic-like cells were expanded. The culture
supernatant from the 3rd passage was then harvested for ELISA. Cell
morphology was analysed with a Leica DMIRE2 inverted
microscope.
Protein preparation and
immunoblotting
Total proteins from tumor tissue stored in liquid
nitrogen, obtained at surgery from the Department of Neurosurgery
at the Nanfang Hospital, Southern Medical University, were isolated
in lysis buffer (Beyotime Institute of Biotechnology, Shanghai,
China). The concentration of each fraction was evaluated by
photometric measurement (wavelength, 562 nm; Tecan Group Ltd.,
Männedorf, Switzerland) using a bicinchoninic acid assay kit
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). Following 10%
SDS-PAGE (50 µg/lane), separated proteins were transferred to
polyvinylidene difluoride membranes. The membranes were blocked in
5% skim milk in TBS buffer (50 mM Tris, pH 7.4, 150 mM NaCl) at
room temperature for 1 h. The membranes were then probed with
antibodies against IL-6 (1:400; ab9324; Abcam, Cambridge, UK),
IL-6R (1:400; BA0992; Wuhan Boster Biological Technology, Ltd.,
Wuhan, China), vimentin (1:500; 550513; BD Biosciences, Inc.),
E-cadherin (1:1,000; #4065; Cell Signaling Technology, Inc.) and
β-actin (1:10,000; SAB4200248; Sigma-Aldrich; Merck KGaA) overnight
at 4°C, and thereafter with horseradish peroxidase (HRP)-conjugated
rabbit anti-mouse/goat anti-rabbit secondary antibodies (1:4,000;
Southern Biotech, Birmingham, AL, USA), at room temperature for 1
h. The protein bands were detected by chemiluminescence using
Immobilon Western Chemiluminescence HRP substrate (EMD
Millipore).
Immunohistochemical staining
Immunohistochemical staining was performed using the
2-step plus poly-HRP method as described previously (7). Briefly, specimens removed at surgery
from the Department of Neurosurgery at Nanfang Hospital, Southern
Medical University, were fixed immediately in 10% formalin, and
subsequently embedded in paraffin wax. One representative section
of tissue from each patient was cut to 4 µm and placed on
poly-L-lysine-coated slides. The slides were deparaffinized,
rehydrated, immersed in 10 mM sodium citrate buffer (pH 6.0) and
pretreated in a microwave oven for 20 min, followed by a 15 min
rinse with PBS. Following blocking with 3% hydrogen peroxide for 10
min at room temperature, the slides were incubated at 4°C overnight
with primary antibodies: Anti-IL-6 (1:200; ab9324; Abcam),
anti-IL-6R (1:100; BA0992; Wuhan Boster Biological Technology,
Ltd.) and anti-gylcoprotein 130 (GP130) (1:100; bs-1459R; Beijing
Biosynthesis Biotechnology Co., Ltd., Beijing, China). The slides
were then stained with the 2-step plus Poly-HRP Anti-Rabbit
Immunoglobulin G (IgG) Detection system (PV-6001; ZSGB-Bio) for
IL-6R and GP130, or 2-step plus Poly-HRP Anti-Mouse IgG Detection
System (PV-6002; ZSGB-Bio) for IL-6. Following visualization of the
reaction with 3,3′-diaminobenzidine, the slides were counterstained
with hematoxylin at room temperature for 5 min and covered with a
glycerin gel. Negative controls consisted of tissue sections
incubated with PBS instead of the primary antibody. Slides were
analysed with an Olympus BX-51 microscope equipped with a DP-71 CCD
camera.
ELISA
ACP cells and TAFs were grown to subconfluence
(2.5×106 cells) and then incubated in serum-free DMEM
for 24 h (37°C, 5% CO2). The supernatants were then
harvested for ELISA. Cystic fluid samples were obtained at surgery
from the Department of Neurosurgery at Nanfang Hospital, from 6
patients affected by cystic ACP, and stored in −80°C. To detect
sIL-6R in the supernatant of ACP cells, TAFs or the cystic fluid,
an ELISA kit (KA0523; Abnova, Taipei, Taiwan) was conducted
according to the manufacturer's protocol.
Migration assays
Boyden chamber assay
A Boyden chamber migration assay was used to
quantify the number of migratory cells (Costar; Corning
Incorporated, NY, USA). ACP cells that had been serum starved for 6
h were resuspended in serum-free culture (1×105
cells/300 µl) medium containing IL-6 (0–100 ng/ml) or IL-6 (100
ng/ml)/anti-hIL-6- antibody (10 µg/ml; MAB206; R&D Systems,
Inc.) and placed in the top chamber of the inserts
(1×105 cells/well). Following incubation for 20 h (37°C,
5% CO2), the inserts were removed; cells were fixed and
stained with Giemsa solution (Solarbio Life Sciences, Beijing,
China) at room temperature for 20 min, and were viewed and counted
under a light microscope. Images of representative microscopic
fields were captured. Quantification of cell migration was
expressed as the mean count of stained cells in 5 random fields of
each filter. Each experiment was repeated three times (n=3).
Wound-healing assay
ACP cells (1×105 cells/well) were grown
to confluence in 24-well plates. A uniform wound was made in each
plate using a 200-µl pipette tip, and the plates were washed with
PBS and incubated in serum-free culture medium containing IL-6
(0–100 ng/ml) or IL-6 (100 ng/ml)/anti-hIL-6 -antibody (10 µg/ml).
The wound area was observed with a Leica DMIRE2 inverted
microscope, and images were immediately captured 48 h following
wound generation. Experiments were repeated three times (n=3).
Cell proliferation assay
ACP cells were resuspended in serum-free culture
(1×103 cells/100 µl/well) medium containing IL-6 (0–100
ng/ml) and were seeded in 96-well plates. The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT;
20 µl/well) assay was used to determine cell viability at 0, 12, 24
and 48 h after seeding. The absorbance at 570 nm was measured using
a Quant Universal Microplate Spectrophotometer (BioTek Instruments,
Inc., Winooski, VT, USA).
Statistical analysis
All numerical data are presented as the mean ±
standard deviation, all experiments were repeated three times.
Statistical differences between clinical variables and inflammatory
scores were evaluated using the χ2 test or Fisher's
exact test for categorical variables and the non-parametric
Spearman correlation test for continuous variables. One-way
analysis of variance was used to assess the significance of
differences among experimental groups and controls. Multiple
comparisons was performed using Fisher's least significant
difference test. The results were analyzed using SPSS statistical
software for Windows, version 13.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Correlation of inflammatory density
with clinicopathological characteristics
Inflammation was present in 77.6% of CP cases: Mild
in 24 (49%), moderate in 15 (30.6%) and severe in 10 (20.4%). A
total of 11 cases in the mild group (11/49, 22.4%) cases were free
of inflammation. The associations between clinicopathological
parameters and the inflammatory density score are presented in
Table I. There were no
statistically significant differences between the inflammatory
density scores and age, sex, tumor size and recurrence. However,
there was a statistically significant difference between the
inflammatory density score and pathological classification
(P=0.037), the extent of surgery (P=0.017), the degree of
calcification (r=0.326, P=0.022) and HSS (r=0.376, P=0.008).
 | Table I.Evaluation of the associations between
inflammatory density score and clinicopathological characteristics
in craniopharyngioma. |
Table I.
Evaluation of the associations between
inflammatory density score and clinicopathological characteristics
in craniopharyngioma.
|
|
| Inflammatory density
score |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | No. of cases | Mild | Moderate | Severe | χ2 | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
| ≤17 | 17 | 8 | 4 | 5 | 1.449 | 0.485 |
|
>17 | 32 | 16 | 11 | 5 |
|
|
| Sex |
|
|
|
|
|
|
| Male | 31 | 16 | 11 | 4 | 3.027 | 0.220 |
|
Female | 18 | 8 | 4 | 6 |
|
|
| Pathological
classification |
|
|
|
|
|
|
| ACP | 37 | 16 | 11 | 10 | 6.603 | 0.037 |
| SPCP | 12 | 8 | 4 | 0 |
|
|
| Tumor size (mm) |
|
|
|
|
|
|
| ≤40 | 29 | 17 | 8 | 4 | 5.637 | 0.228 |
|
>40<60 | 19 | 7 | 7 | 5 |
|
|
| ≥60 | 1 | 0 | 0 | 1 |
|
|
| Recurrence |
|
|
|
|
|
|
| Yes | 15 | 6 | 3 | 6 | 4.900 | 0.086 |
| No | 34 | 18 | 12 | 4 |
|
|
| Extent of
surgery |
|
|
|
|
|
|
| STR | 20 | 7 | 5 | 8 | 8.188 | 0.017 |
| GTR | 29 | 17 | 10 | 2 |
|
|
| Degree of
calcification |
|
|
|
| r | P-value |
| − | 16 | 9 | 7 | 0 | 0.326 | 0.022 |
| + | 28 | 15 | 6 | 7 |
|
|
| ++ | 5 | 0 | 2 | 3 |
|
|
| HSS |
|
|
|
|
|
|
| I | 7 | 7 | 0 | 0 | 0.376 | 0.008 |
| II | 9 | 2 | 4 | 2 |
|
|
|
III | 28 | 15 | 9 | 4 |
|
|
| IV | 6 | 0 | 2 | 4 |
|
|
| Total | 49 | 24 (49.0%) | 15 (30.6%) | 10 (20.4%) |
|
|
Protein expression levels of
inflammatory cytokines in response to different levels of
inflammation in ACP
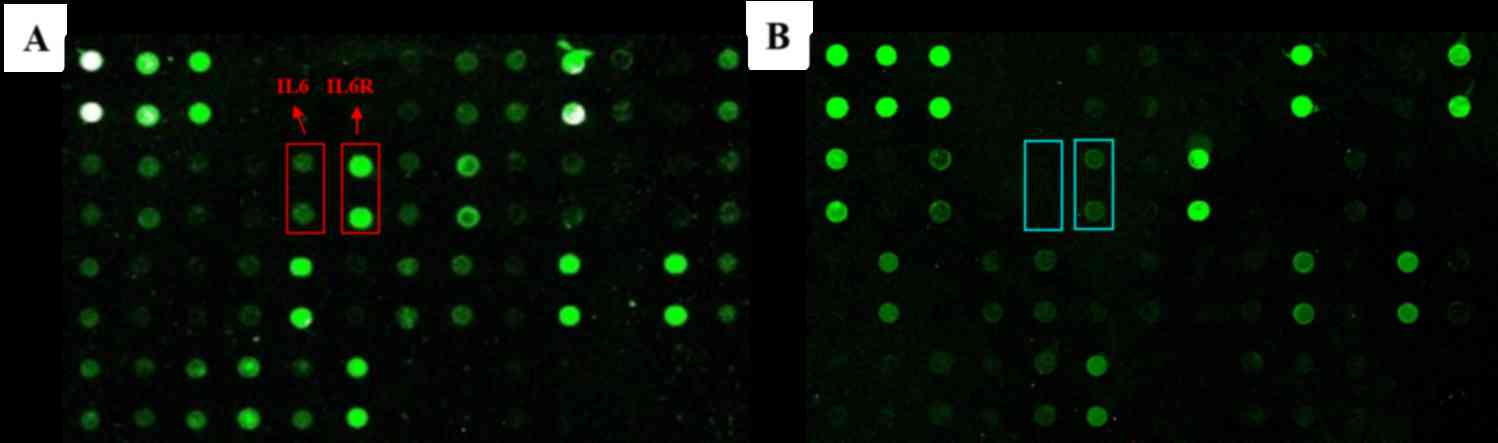
Antibody arrays were used to investigate the
expression profile of major inflammatory cytokines in ACPs in
response to various levels of inflammatory exposure. Representative
arrays analyzing tumor tissues in the mild and severe groups are
shown in Fig. 1. The mean optical
intensity of positive spots from tumor tissues was estimated. Among
these, spots corresponding to the IL-6 and IL-6R molecules were the
most strongly expressed in the severe group. The differential
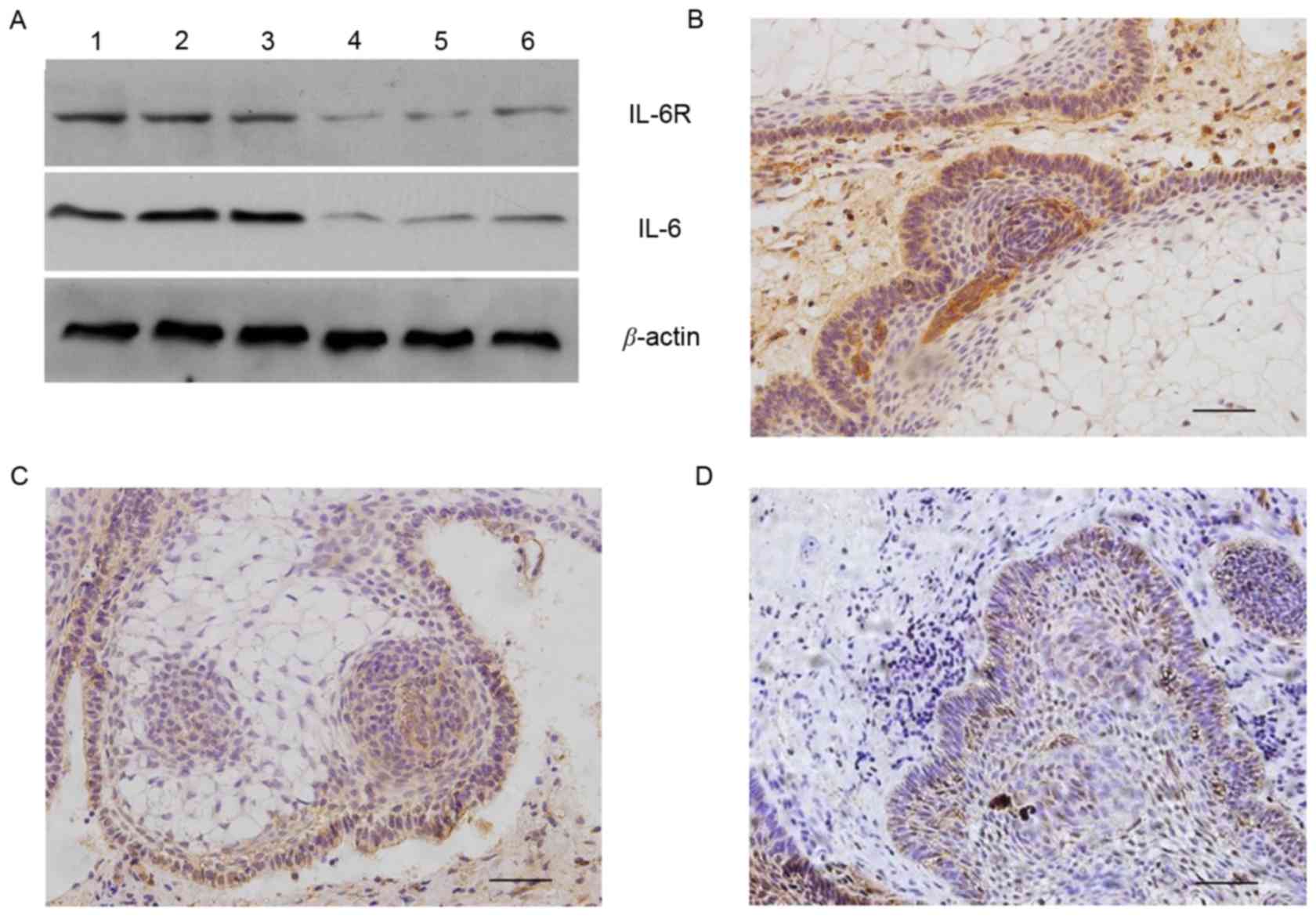
expression of IL-6 and IL-6R was further confirmed by western blot
analysis using tissue lysates that were different from those
employed in the antibody array analysis (Fig. 2A).
IL-6 and IL-6R are expressed in ACP
tissues
Of tumor tissues from 10 cases of ACP, 70 (7/10) and
60% (6/10) exhibited positive cytoplasmic immunoreactivity for IL-6
and IL-6R, respectively. Their immunostaining was predominantly
identified within the connective tissue mesenchymal cells, the
peripheral (basal palisaded) epithelial component and epithelial
whorls of ACP tissues. Conversely, the central stellate reticulum
components exhibited low or no immunoreactivity. Positive
cytoplasmic immunoreactivity for GP130 was observed in 90% (9/10)
of ACP tissues and was revealed to be equally distributed
throughout the tumor. These results demonstrated that tumor and
mesenchymal cells produce IL-6, indicating the presence of the IL-6
autocrine and/or paracrine systems in ACP (Fig. 2B-D).
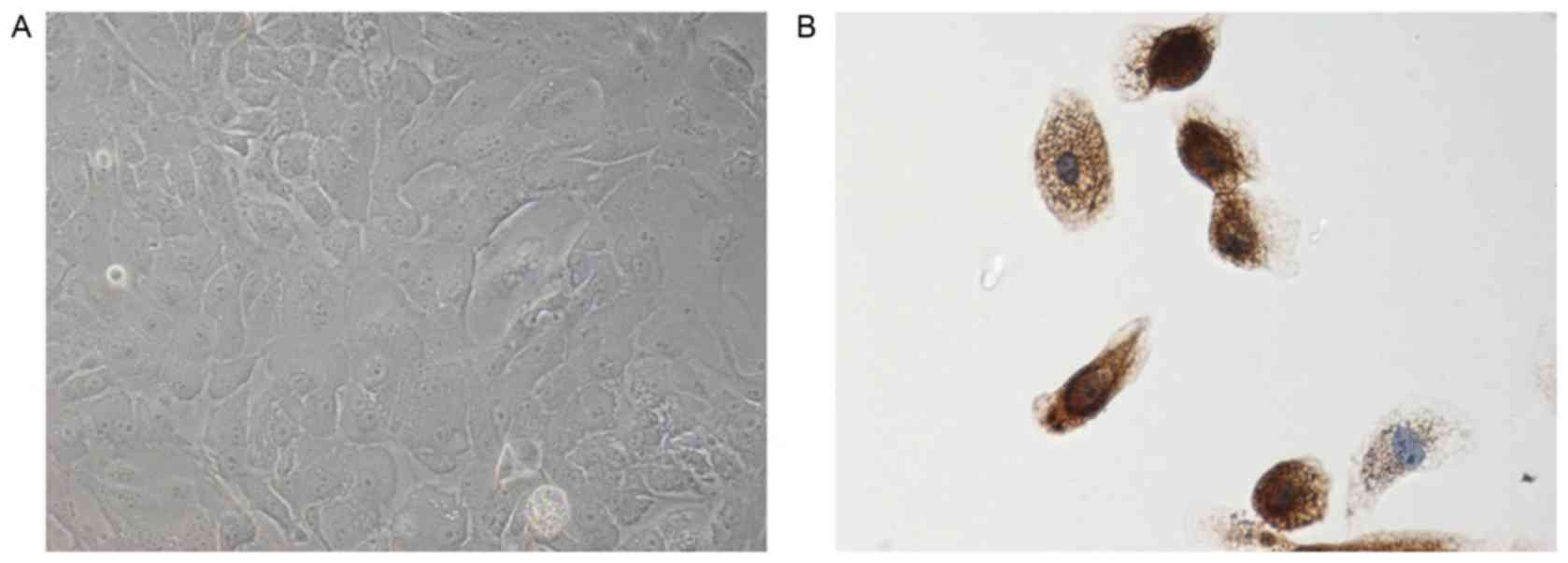
Establishment of primary ACP and TAF
cultures
ACP culture
To investigate the influence of IL-6 on the
biological characteristics of ACP cells, primary ACP cultures were
established directly from six different CP biopsy tissues. All
tumors were histologically classified as ACP, according to the 2007
World Health Organization classification system for tumors of the
nervous system (11). From each
tumor specimen, a primary ACP culture was prepared according to
established procedures to selectively raise epithelial cells. The
present study identified all of the ACP cells according to their
immunoreactivity for Pan-CK, a well-documented ACP cell marker
(10). Based on the
epithelial-like morphology and the Pan-CK phenotype, the epithelial
origin of ACP cell cultures was demonstrated (Fig. 3).
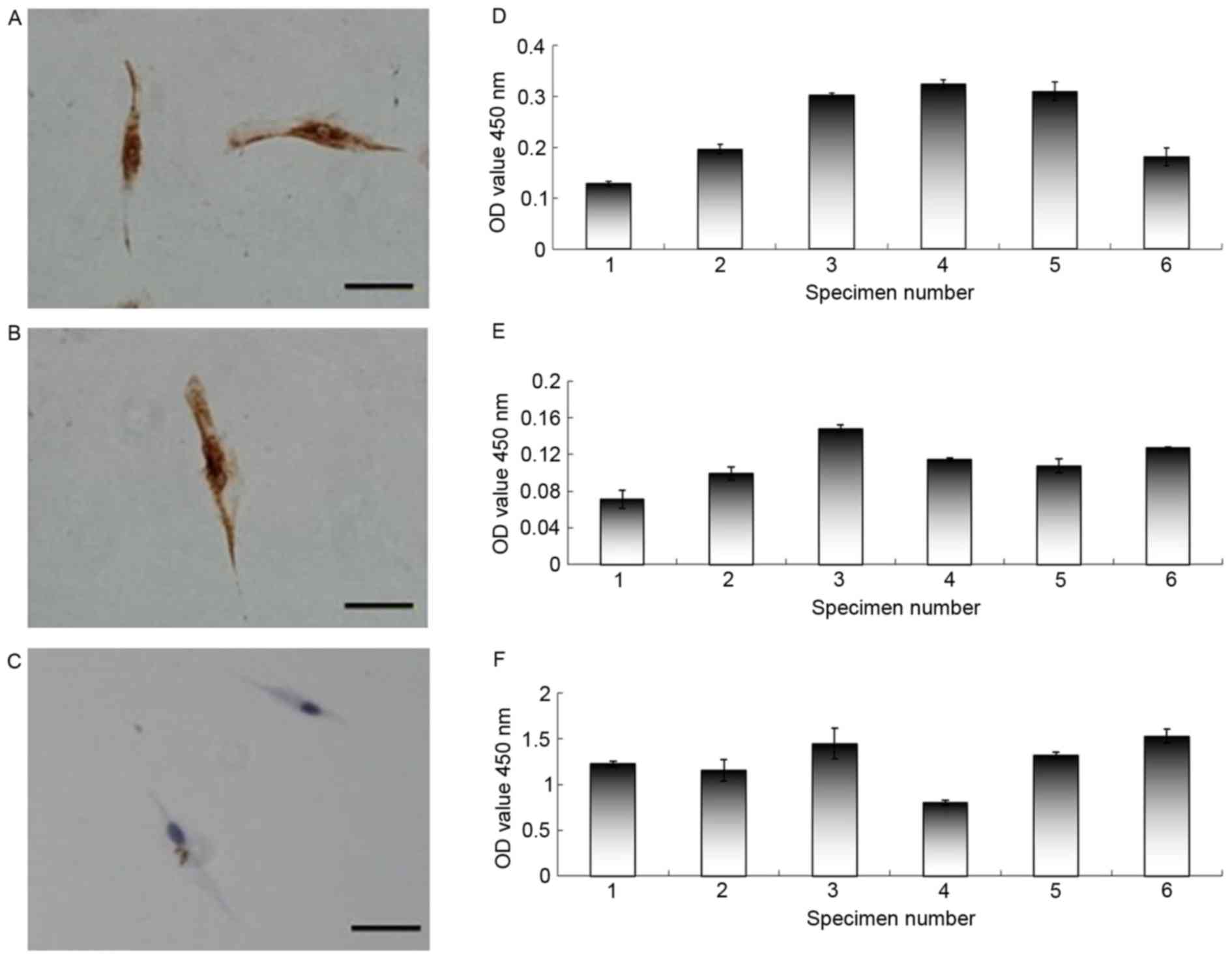
TAF culture
To determine the role of the tumor microenvironment
in the biological characteristics of ACP cells, primary fibroblast
cell cultures were established directly from three different ACP
tumor biopsy tissues. The adherent cells, which possessed a
fibroblast-like morphology, were successfully cultivated from ACP
tissues. To confirm that these cells were fibroblasts and not
contaminated with tumor cells, the adherent cells were assessed by
immunohistochemistry using vimentin, α-SMA and Pan-CK, which are
specific for human fibroblasts. The adherent cells were positively
stained for vimentin and α-SMA, and negatively stained for Pan-CK
(Fig. 4A-C).
sIL-6R expression in the supernatant of ACP
cells, ACP fibroblasts and ACP cystic fluid
Since some tumor tissues exhibited no IL-6R
expression, the expression of sIL-6R was also determined in the
supernatants of ACP cells, ACP fibroblasts and ACP cystic fluid by
ELISA. Various expression levels of sIL-6R were observed in the
supernatants of ACP cells, ACP fibroblasts, and ACP cystic fluid
(Fig. 4D-F). These results
indicated that ACP cells and tumor associated fibroblast cells in
the tumor microenvironment secrete the sIL-6R. The present study
hypothesized that after binding to its receptors, IL-6 may form a
complex to activate the IL-6 classic- and trans-signaling pathways,
promoting alterations in tumor cell characteristics, thus affecting
the prognosis of these patients.
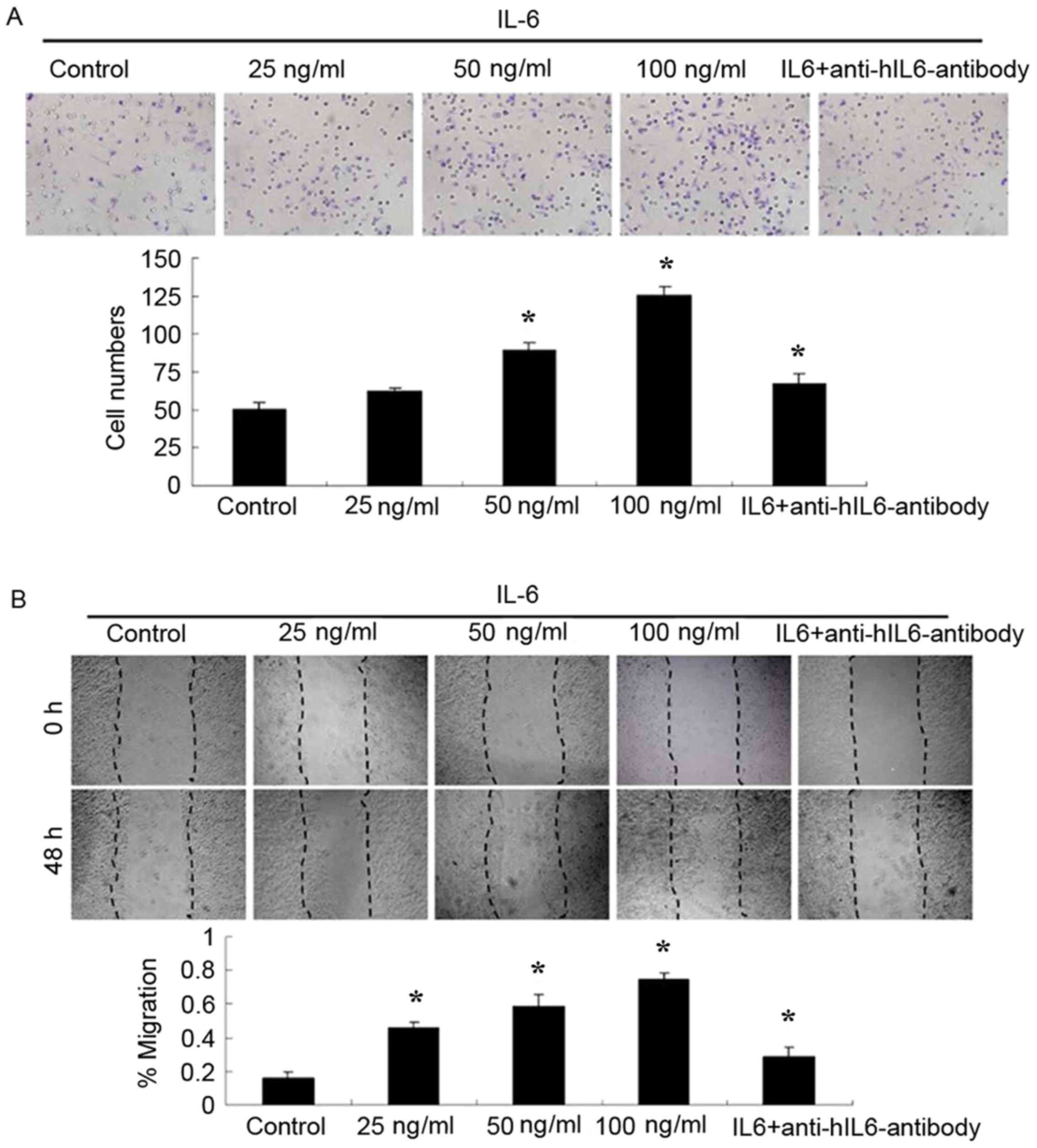
IL-6 promotes the migration of ACP cell
cultures
The effects of IL-6 on the migration of ACP cell
cultures was analyzed by Boyden chamber and wound-healing assays.
As presented in Fig. 5, migration
of ACP cells was enhanced by IL-6 treatment for 24 h in a
dose-dependent manner. Since IL-6 was observed to promote the
growth of some tumor cells (12),
an MTT assay was performed to assess whether the
viability-associated effects of IL-6 influenced the number of
migratory cells of ACP. The results demonstrated that with an IL-6
dose range between 0 and 100 ng/ml, there were no significant
differences between the viability rates of cells treated for 12 or
24 h (data not shown). These results indicated that IL-6 may
promote tumor cell migration. Furthermore, antibody-based blockade
of IL-6 decreased the migration of ACP cell cultures significantly
(Fig. 5A and B).
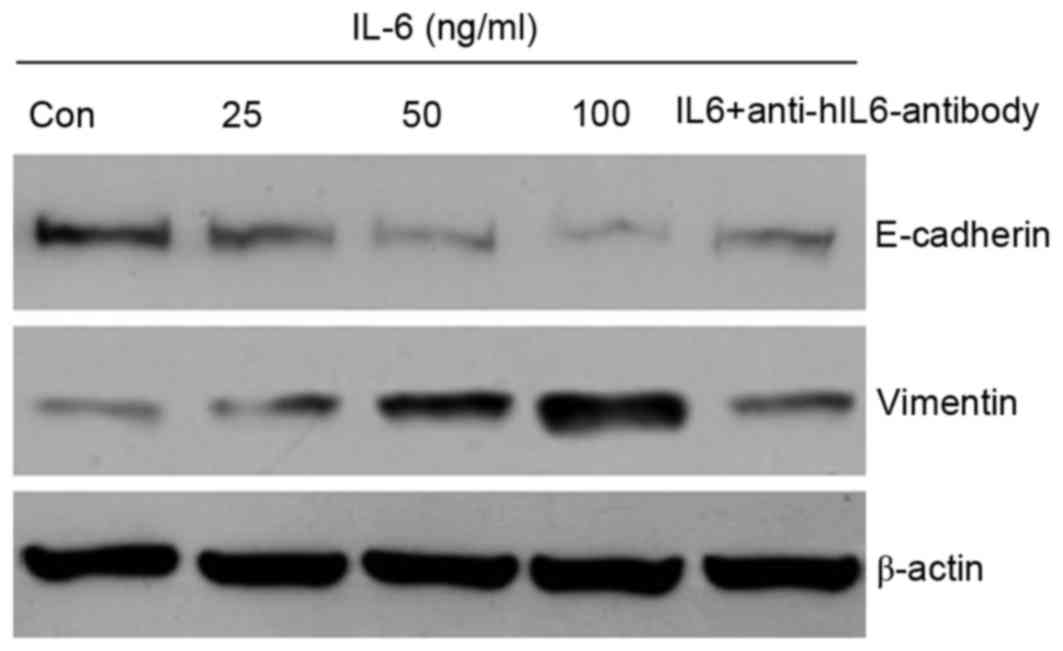
IL-6 promotes an EMT phenotype of ACP cells
Our previous study demonstrated that EMT serves a
potential role in the pathogenesis and development of CP,
particularly ACP (7). The present
study hypothesized that tumor cells undergoing EMT may exhibit
increased motility contributing to the invasiveness of ACP, which
usually leads to a worse prognosis than that for SPCP, in which
this feature is absent. Some cytokines have been revealed to induce
phenotypes consistent with EMT in transformed epithelial cells, as
well as carcinoma cell lines, such as breast cancer (6). The present study analyzed whether the
effects of IL-6 on the migration of ACP cell cultures were
associated with markers of EMT. Following treatment with IL-6 for
48 h, western blotting was performed to investigate the alterations
in vimentin and E-cadherin expression in whole cell extracts. As
presented in Fig. 6, the protein
expression of vimentin was upregulated, whereas E-cadherin
expression was downregulated in an IL-6 dose-dependent manner.
Discussion
CPs are benign epithelial tumors of the sellar
region; their aggressive behavior and potential for adhesion to
adjacent brain structures are conducive to significant regrowth or
recurrence even following total tumor removal. Histologically,
intense fibrillary gliosis rich in Rosenthal fibers and
infiltration of inflammatory cells are frequently present in the
surrounding parenchyma in CPs, particularly in ACP (13).
Chronic inflammation, which is implicated in all
stages of carcinogenesis, including initiation, promotion and
progression, represents a major pathological basis for the majority
of human malignancies. In the present study, CP inflammation was
associated with pathological classification, the rate of total
resection, calcification and postoperative HSS. Within the tumor
microenvironment, a number of proinflammatory mediators, such as
cytokines, participate in complex inflammatory signaling pathways
that facilitate the extravasation of tumor cells through the
stroma, thereby stimulating tumor progression (14). Results from the present study, as
well as a study by Mori et al (4), strongly implicated IL-6 as the key
molecule for ACP inflammation.
IL-6 is a multifunctional cytokine that exerts
pleiotropic activities on various cell types. It is usually
produced at local tissue sites during infection, trauma or in
tumors. IL-6 can bind to IL-6R (classic-signaling) and/or sIL-6R
(trans-signaling) to form a complex that activates aberrant
signaling pathways, which contribute to the promotion of
tumorigenesis and tumor angiogenesis, migration and invasion
(15). Numerous biological
activities are mediated by the IL-6 cytokine. Mori et al
(4) proposed that IL-6 may serve
an important role in the inflammatory reaction that occurs in the
interface between the CP and brain parenchyma. However, the
biological activities and mechanisms involved in CP cells remain
unclear.
The present study demonstrated that the IL-6, IL-6R
and GP130 proteins are present in some ACP tissues. In addition,
sIL-6R was observed to accumulate in the supernatant of ACP cells,
ACP fibroblasts and ACP cystic fluid. Therefore, autocrine and/or
paracrine sources of IL-6 may serve an important role in regulation
of the biological characteristics of ACP. IL-6 did not affect the
viability of ACP cells, however, IL-6 promoted the migration of ACP
cells following a primary ACP culture assay. Depletion of IL-6 from
the ACP medium abolished the IL-6 mediated stimulatory effect on
ACP cell migration.
In addition, the present study revealed that EMT may
serve a role in the pathogenesis and development of CPs,
particularly ACP. Overexpression of vimentin and decreased
expression of E-cadherin are closely associated with tumor
recurrence and poor postoperative hypothalamic function in patients
with CP. It has been well documented that an IL-6-mediated EMT
phenotype in some cancer cells, such as breast cancer and biliary
tract cancer cells, is clinically associated with unfavorable
outcomes in numerous human carcinoma types (6). In the present study, a rapid
dose-dependent increase in vimentin and decrease in E-cadherin was
observed following ACP cell stimulation with IL-6, and the
expression of these two proteins was reversed following IL-6
blocking with a neutralizing antibody. Therefore, EMT may be
involved in IL-6-promoted migration.
The IL-6 signaling network has been targeted with
several therapeutic antagonists in numerous human cancer
preclinical and clinical trials; however, this strategy has yet to
be applied for CP treatments. The present study observed an
IL-6-mediated EMT phenotype in ACP cells, which is clinically
associated with unfavorable outcomes in several human carcinoma
types. Further studies are required to fully elucidate the
mechanism of action of IL-6 and the specific signal transduction
pathways involved in ACP progression. However, the results may
support further evaluation of anti-IL-6 therapies in CPs,
particularly ACP.
In conclusion, a local inflammatory state between
tumor cells and parenchyma is generated by the enhanced
infiltration of leukocytes and tumor cell-derived cytokines,
particularly IL-6, at the adjacent tissue in CPs. To the best of
our knowledge, the present study is the first to demonstrate that
IL-6 may promote migration in vitro via classic- and
trans-signaling pathways by inducing EMT in ACP. Therefore,
anti-IL-6 strategies targeting its downstream pathways and proteins
may be promising for the treatment of ACP.
Acknowledgements
The present study was funded by the Natural Science
Foundation of Guangdong province (grant no. S2012020010939) and the
Natural Science Foundation of Luzhou Medical College (grant no.
2013ZRZD005).
Glossary
Abbreviations
Abbreviations:
|
ACP
|
adamantinomatous craniopharyngioma
|
|
CPs
|
craniopharyngiomas
|
|
EMT
|
epithelial-mesenchymal transition
|
|
HPF
|
high-power field
|
|
HSS
|
hypothalamic status scale
|
|
IL-6
|
interleukin-6
|
|
IL-6R
|
IL-6 receptor
|
|
sIL-6R
|
soluble IL-6R
|
|
SPCP
|
squamous papillary
craniopharyngioma
|
|
TAFs
|
tumor-associated fibroblasts
|
References
|
1
|
Karavitaki N, Cudlip S, Adams CB and Wass
JA: Craniopharyngiomas. Endocr Rev. 27:371–397. 2006. View Article : Google Scholar
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar
|
|
3
|
Satoh H, Uozumi T, Arita K, Kurisu K,
Hotta T, Kiya K, Ikawa F, Goishi J and Sogabe T: Spontaneous
rupture of craniopharyngioma cysts: A report of five cases and
review of the literature. Surg Neurol. 40:414–419. 1993. View Article : Google Scholar
|
|
4
|
Mori M, Takeshima H and Kuratsu J:
Expression of interleukin-6 in human craniopharyngiomas: A possible
inducer of tumor-associated inflammation. Int J Mol Med.
14:505–509. 2004.
|
|
5
|
Walter M, Liang S, Ghosh S, Hornsby PJ and
Li R: Interleukin 6 secreted from adipose stromal cells promotes
migration and invasion of breast cancer cells. Oncogene.
28:2745–2755. 2009. View Article : Google Scholar :
|
|
6
|
Sullivan NJ, Sasser AK, Axel AE, Vesuna F,
Raman V, Ramirez N, Oberyszyn TM and Hall BM: Interleukin-6 induces
an epithelial-mesenchymal transition phenotype in human breast
cancer cells. Oncogene. 28:2940–2947. 2009. View Article : Google Scholar
|
|
7
|
Qi ST, Zhou J, Pan J, Zhang C, Silky C and
Yan XR: Epithelial-mesenchymal transition and clinicopathological
correlation in craniopharyngioma. Histopathology. 61:711–725. 2012.
View Article : Google Scholar
|
|
8
|
Qi S, Huang G, Pan J, Li J, Zhang X, Fang
L, Liu B, Meng W, Zhang Y and Liu X: Involvement of osteopontin as
a core protein in craniopharyngioma calcification formation. J
Neurooncol. 98:21–30. 2010. View Article : Google Scholar
|
|
9
|
Fahlbusch R, Honegger J, Paulus W, Huk W
and Buchfelder M: Surgical treatment of craniopharyngiomas:
Experience with 168 patients. J Neurooncol. 90:237–250. 1999.
|
|
10
|
Hölsken A, Buchfelder M, Fahlbusch R,
Blümcke I and Buslei R: Tumour cell migration in adamantinomatous
craniopharyngiomas is promoted by activated Wnt-signalling. Acta
Neuropathol. 119:631–639. 2010. View Article : Google Scholar
|
|
11
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 11:97–109. 2007. View Article : Google Scholar
|
|
12
|
Smith PC, Hobisch A, Lin DL, Culig Z and
Keller ET: Interleukin-6 and prostate cancer progression. Cytokine
Growth Factor Rev. 12:33–40. 2001. View Article : Google Scholar
|
|
13
|
Karavitaki N and Wass JA:
Craniopharyngiomas. Endocrinol Metab Clin North Am. 37:173–193,
ix-x. 2008. View Article : Google Scholar
|
|
14
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar
|
|
15
|
Rose-John S, Scheller J, Elson G and Jones
SA: Interleukin-6 biology is coordinated by membrane-bound and
soluble receptors: Role in inflammation and cancer. J Leukoc Biol.
80:227–236. 2006. View Article : Google Scholar
|