Introduction
Postoperative fibrosis is a natural consequence of
surgical wound healing and epidural fibrosis is an expected healing
consequence after laminectomy. The source of fibrotic tissue after
spinal surgery is originally thought to arise from the disrupted
intervertebral disc and the disrupted epaxial muscles in the
surgical wound (1,2). This extradural fibrotic tissue may
extend to the vertebral canal and adhere to the dura mater and
nerve roots, causing recurrent symptoms including pain (3–5),
possibly leading to the failure of spinal surgery (6). Although there remains controversy
about the role of peridural fibrosis in failed back surgery
syndrome (7,8), it has been widely accepted that it is
a problematic clinical entity with no efficacious treatment options
(9–11). Furthermore, epidural adhesions make
re-exposure of the same operative area technically difficult and
dangerous because of the greatly increased risk in nerve root
injury and dural tears (12,13).
The control of scar formation has been one of the
main concerns in spine surgery. Numerous types of biological or
non-biological materials and methods for preventing epidural
fibrosis have been developed, including autologous fat grafts,
absorbable gelatin films and sponges, protein-based polymer,
high-molecular weight hyaluronan, collagen, gelatin foam (14,15),
which have been implanted to serve as a barrier (16,17)
to physically or chemically inhibit scar ingrowths.
Polylactic acid (PLA) and polyethylene glycol (PEG)
are water-soluble polymers without any electric charge and affinity
for any specific organ, and are non-immunogenic and non-toxic
(18,19). They exhibit good biological and
physicochemical properties. Due to their non-toxic, biodegradable
and hydrophilic characteristics, PLA and PEG have been widely used
in tissue engineering (18,19).
Although PLA and PEG significantly reduce post-laminectomy
proliferative scar without any impact on the integrity of
incisional wound healing, the process of re-absorption will result
in a fibrotic mass and form a gap between the sheet and the dura
(20,21). Moreover, PLA and PEG could not
prevent nerve root adhesion.
Because of its capacity to inhibit DNA-dependent RNA
synthesis (22), mitomycin C
(MMC), a well-known chemotherapeutic agent, has been proposed as a
potential adjuvant for the control of scar tissue in surgical
wounds and used successfully for a long time to prevent scar
formation and fibrosis (23,24).
In the present study, we used two control-release
delivery films, in which a small dose of MMC (0.01%) was absorbed
in the PLA and PEG films. In our preliminary studies, we
established a stable controlled-release delivery system (25). There are many studies on the
suppressing effect of MMC on the post-laminectomy scar invasion,
but there few studies on its repairing effect on the insignificant
cerebrospinal fluid leaks. The purpose of our research was to
investigate and compare the efficacy of MMC-PLA and MMC-PEG films
as a biophysical and chemical barrier to suppress post-laminectomy
scar invasion. Another purpose of our investigation was to assess
whether the barrier film contained MMC would influence the dural
spontaneous healing causing cerebrospinal fluid leakage.
We hypothesized that with the decomposition of PLA
and PEG films, MMC would be continuously released to inhibit scar
ingrowths without affecting the healing of the dura. If true, the
barrier film containing a low concentration of MMC is safe to be
used for spinal surgery without affecting the healing of the
cerebrospinal fluid leaks. Particularly, it is well-adapted for the
re-exposure of the same operative area.
Materials and methods
All the procedures were performed with the
permission of the Ethics Committee of Nanjing Medical University,
and all the rats were obtained from the Animal Experimental
Research Center of Jiangsu Province. A total of 72 male
Sprague-Dawley rats weighing 400+ g were used for the study, which
were divided into three groups. Group 1 (n=24, the control group):
only the standard surgical procedure was performed (L5 total
laminectomy, bilateral disk injury and central dura injury); group
2 (n=24): the mitomycin C-polylactic acid (MMC-PLA)
controlled-release film group, in which the barrier film served as
a roof tenting over the space between the ronguered edges of the
laminectomy area, and group 3 (n=24): the mitomycin C-polyethylene
glycol (MMC-PEG) controlled-release film group, in which the
application of barrier film was the same as group 2.
Surgical procedure
All the procedures were performed by the same
surgeon applying a standard surgical procedure on each rat. A
posterior midline incision was made from L4 to L7 vertebrae to
expose the bony posterior elements and carried sharply down to the
lumbosacral fascia. With blunt dissection, the paraspinal
musculature was subperiosteally dissected and the lumbar vertebral
segments were exposed. Keeping the spinal cord and cauda equina
intact, bilateral laminectomies were performed at L5-L6, and the
dura mater and nerve roots were exposed. The neural elements and
dura were retracted gently and sufficiently medially to expose the
posterolateral aspect of the L5-L6 disc. Then a 26-gauge needle was
inserted into the bilateral exposed L5-L6 disc, creating a disc
injury. Next, with the help of a 10 times microscope, an incision
was made into the dura and subarachnoid space (durotomy) by an
18-gauge needle, creating a dura mater injury. The durotomy was
considered complete when cerebrospinal fluid was observed leaking
outside the dura.
In the control group, the lumbosacral fascia and
other layers were sutured directly. In the experimental groups (the
MMC-PLA and MMC-PEG groups), after creating a disc injury and a
dura mater injury, the barrier film was cut into a suitable size
(~2×2 cm, 25–30 mg PLA or PEG film containing 0.01% MMC) and then
applied as a roof tenting over the space between the ronguered
edges of the laminectomy area. Then the lumbosacral fascia and
other layers were sutured.
No other medical treatment that could increase or
reduce the potential effects of the agents was used. The rats were
closely followed up during the first 24 h for the neurologic
status. The standard diet and the same conditions were provided for
each rat. All the rats were followed up for 4 weeks. We observed
the postoperative recovery of all rats and evaluated whether there
were neurologic deficits or cerebrospinal leak. The rats were
sacrificed on the 28 th day after operation by a lethal dose of
pentobarbital.
Magnetic resonance image (MRI)
examination
MRI examination was carried out at the 4 th week
after surgery. Six rats were selected from each group randomly.
After general anesthesia, the whole spine columns from L4 to L7
including the surrounding muscle tissues were dissected and removed
to perform the MRI examination. The MRIs were acquired by Bruker
7.0T micro-MR imaging system (Siemens Corp., Berlin, Germany) and
Multi-Slice Multi-Echo T2-weighted imaging (MSME T2WI) sequence.
Two sets of MR images were performed: they were spin echo
T1-weighted images in sagittal and transverse planes (TR_2710 ms,
TE_22 ms) and the gradiently recalled echo T2-weighted images in
the same locations with spin echo images for sagittal and
transverse images (TR_4391 ms, TE_33 ms). For each imaging slice
encompassing the operative level, the amount of epidural fibrosis
was graded on the scale of 0–4: grade 0, no scar/trace of a scar;
grade 1, >0 to ≤25% of the quadrant filled with scar; grade 2,
>25 to ≤50% of the quadrant filled with scar; grade 3, >50 to
≤75% of the quadrant filled with scar; and grade 4, 75–100% of the
quadrant being filled with scar (26). Briefly, the spinal canal in each
level was further subdivided into four quadrants by drawing
perpendicular lines from the central aspect of the dura sac. Three
radiologists analyzed the MR images of epidural fibrosis
independently with ImageJ software (version X; Media Cybernetics,
Silver Springs, MD, USA).
Macroscopic observation of the
epidural scar adhesion
Macroscopic evaluation was performed at the 4 th
week after operation. A total of 18 rats were randomly selected
from the remaining rats (6 from each group). Three pathologists
observed the epidural scar tissue and determined the degree of
epidural adhesion according to the Rydell standard independently.
We observed the rats simultaneously to assure whether there was a
leakage of cerebrospinal fluid from the durotomy site. All the
pathologists were blinded to the groups. The degree of epidural
adhesion was graded on the scale of 0–3: Grade 0, epidural scar
tissue was not adherent to the dura mater; Grade 1, epidural scar
tissue was adherent to the dura mater, but easily dissected; Grade
2, epidural scar tissue was adherent to the dura mater and
difficult to dissect without disrupting the dura mater; Grade 3,
epidural scar tissue was firmly adherent to the dura mater and
could not be dissected.
Histological analysis
Histological analysis was performed at the 4 th week
after surgery. A total of 18 rats were randomly selected from
remaining rats (6 from each group). Subsequently, the spinal
columns, including surrounding muscle tissue, were resected. All
specimens were harvested to assess the whole anatomy of fibrosis
formation on the space between the dura mater and the surrounding
soft tissues. The specimens were cut and ground into ~100-micron
axial sections. All the sections were stained with hematoxylin and
eosin and Massons trichrome, and then photographed by a microscope
(BX-42; Olympus, Tokyo, Japan). Two histopathologists with similar
qualifications, who were blinded and independent to the treatment,
evaluated and measured the peridural fibrosis and the fibroblast
cell density according to the classification (Table I and II) described by Sen et al
(26).
 | Table I.Pathological analysis scoring system
of the peridural fibrosis and adhesion. |
Table I.
Pathological analysis scoring system
of the peridural fibrosis and adhesion.
| Grade | Pathological
features |
|---|
| Grade 0 | The dura is free of
scar tissue |
| Grade 1 | Presence of only thin
fibrous bands between dura and scar tissue |
| Grade 2 | Continuous adherence
between dura and scar tissue involving <2/3 of laminectomy
defect |
| Grade 3 | Scar tissue adherence
≥2/3 of laminectomy defect and/or extend to nerve roots |
 | Table II.Fibroblast cell density scoring
system. |
Table II.
Fibroblast cell density scoring
system.
| Grade | Cell density |
|---|
| Grade 1 | <100 cells in
each area at ×400 |
| Grade 2 | 100–150 cells in
each area at ×400 |
| Grade 3 | >150 cells in
each area at ×400 |
Biochemical analysis
Another six rats were randomly selected from each
group at the 4 th week after operation. The scar tissue, about
0.2×0.2×0.5 cm, was harvested from the space between the dura mater
and surrounding soft tissues from each rat. The dissected tissues
were rinsed, homogenated, centrifuged, and hydrolyzed for the
measurement of hydroxyproline (Hyp). The Hyp developer was then
added into the digested solution. The absorbance was read by a
spectrophotometer (Hitachi, Tokyo, Japan) at 550 nm, and the
contents of Hyp per milligram tissue were calculated.
Statistical analysis
The statistical analysis was performed by the SPSS
19.0 (SPSS, Inc., Chicago, IL, USA) statistical package. Data were
presented as means ± standard deviations. The differences between
groups were evaluated with the non-parametrical test (Mann-Whitney
U test). P-values <0.05 were considered statistically
significant.
Results
In all rats, the postoperative recovery was
uneventful except for two with skin infection (one rat in the
control group, the other in the polylactic acid (PLA) film group.
The infections were superficial and controlled with
povidone-iodine, without using antibiotics. There was no case with
neurologic deficits or cerebrospinal leak in any of the rats. No
rats suffered accidental death.
MRI evaluation
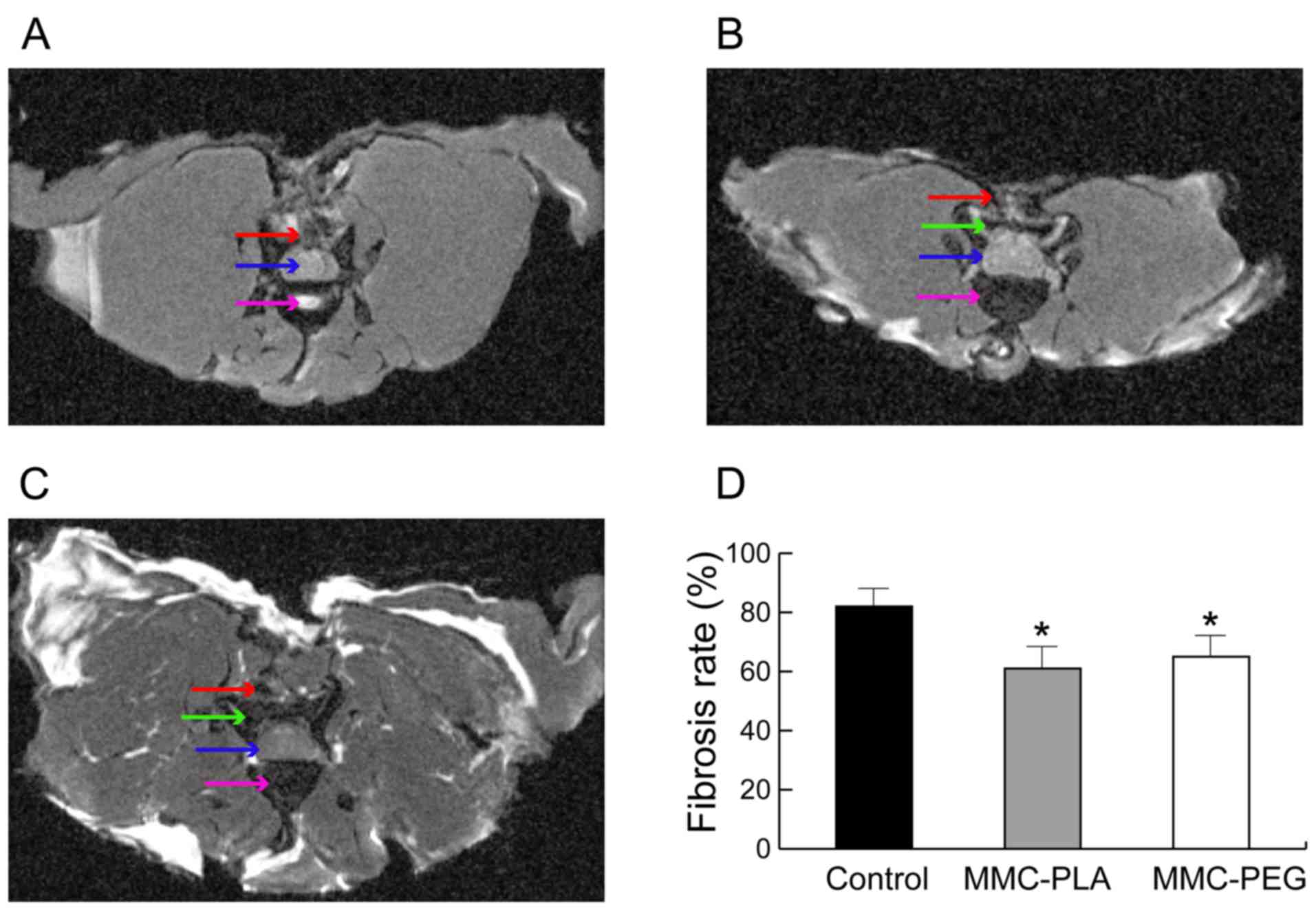
Transverse MRI obtained at the laminectomy level of
the control group rats exhibited noticeable fibrotic scarring
compression in the spine cord (Fig.
1A). However, the experimental groups showed a discrete
hypo-signal space between the dura mater and the surrounding
fibrosis tissue near the skin (Figs.
1B and C).
The MRI score demonstrated that it was higher in the
control group when compared with that in the experimental groups
(P<0.05). However, there was no significant difference between
experimental groups (P>0.05, Fig.
1D).
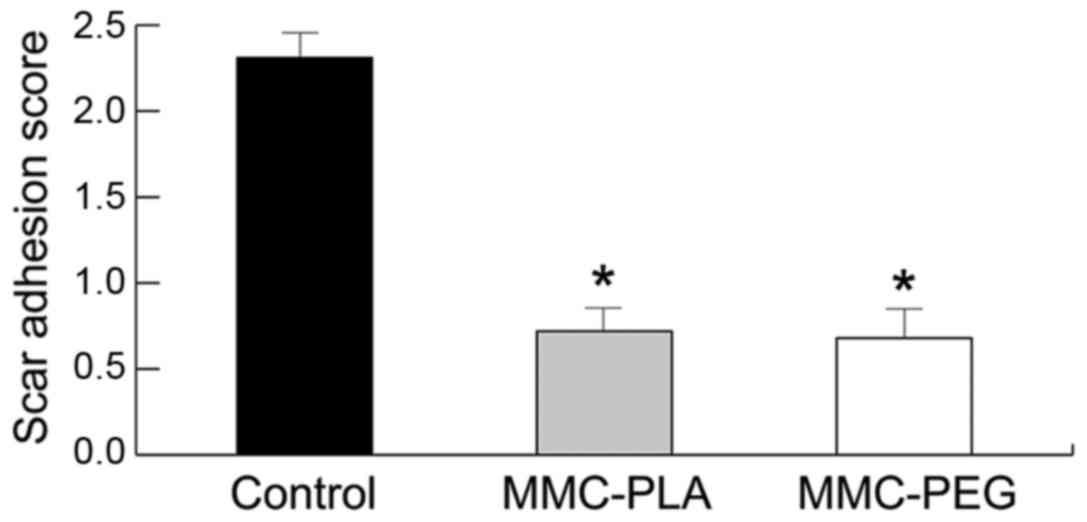
Macroscopic observation of the
epidural scar adhesion
Macroscopic observation by two independent
evaluators showed the soft or weak fibrosis adhesion around the
laminectomy areas in the experimental groups, which could be easily
dissected by manual traction and clean dura mater was exposed
without any evident adhesion or membrane. Leakage of cerebrospinal
fluid from the durotomy site was not observed in any of the rats in
the experimental groups. But in the laminectomy sites of rats in
the control group, there was a severe epidural scar tissue
disrupting dura mater around the laminectomy areas. Severe, thick,
and tenacious epidural scar adhesions were found between the dura
mater and surrounding tissue. Complete re-exposure of the dura
mater was impossible for the possibility to cause serious bleeding
and the risk of nerve root injury or dura tears. The grades of
epidural adhesion in rats according to the Rydell standard are
shown in Table III.
 | Table III.Grade of epidural scar adhesion
according to the Rydell standard. |
Table III.
Grade of epidural scar adhesion
according to the Rydell standard.
|
| Grade |
|---|
|
|
|
|---|
| Group | 0 | 1 | 2 | 3 |
|---|
| Control | 0 | 0 | 0 | 6 |
| aMMC-PLA | 4 | 2 | 0 | 0 |
| bMMC-PEG | 5 | 1 | 0 | 0 |
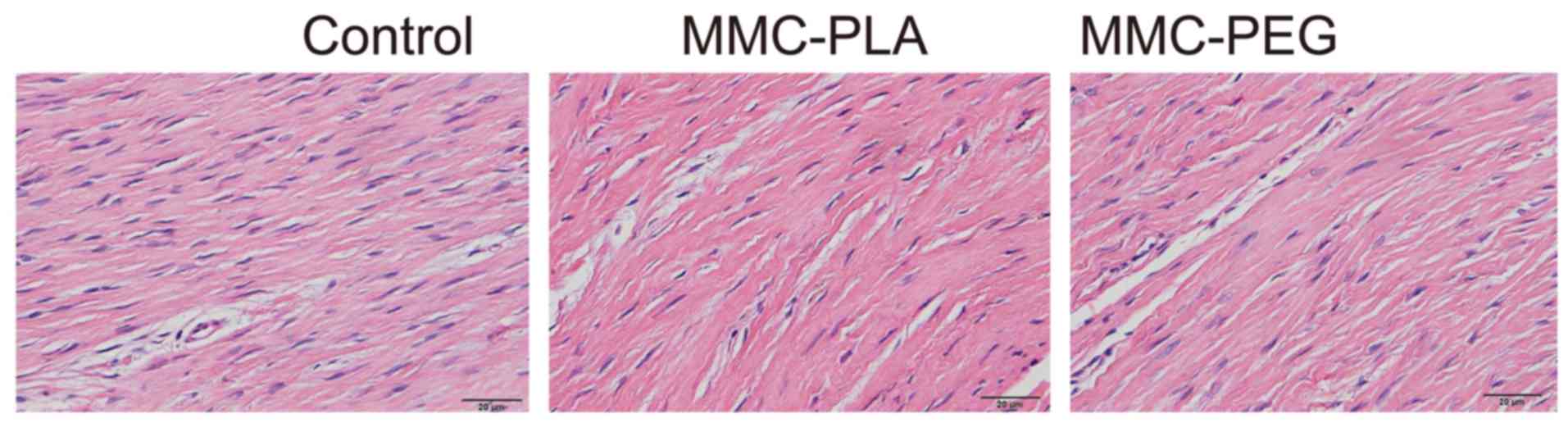
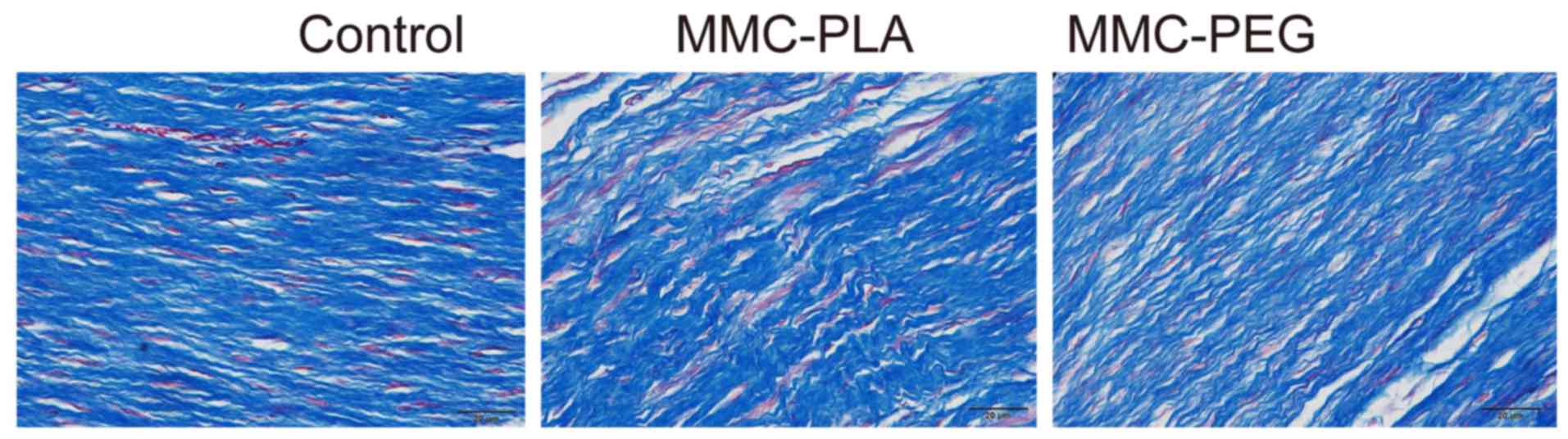
Histological analysis of epidural
adhesion
In the control group, marked epidural scar tissue
with widely spread adhesions to dura mater and dorsal aspect fascia
or muscle was noted primarily in the laminectomy sites. Dense scar
tissue mixed with extensive collagen tissue hyperplasia was
observed. There were also abundant fibroblasts and fibrocytes in
the laminectomy sites.
In the experimental groups, loosely arranged
epidural scar tissue with no or little dura adhesion to the dura
mater and dorsal aspect fascia or muscle was discovered. We could
also observe that the dura mater was slightly thickened and it was
separated from the epidural scar tissue through an empty
interspace. The collagen tissue hyperplasia was obviously decreased
(Figs. 2 and 3).
Inflammation was not observed in any of the
laminectomy sites in any of the rats. Peridural fibrosis and
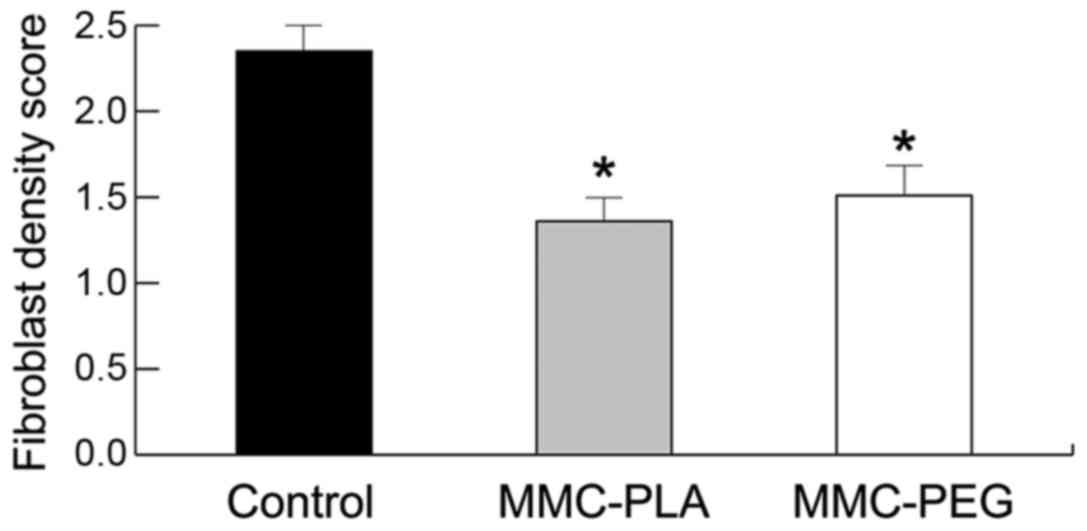
adhesions were evaluated and summarized in Fig. 4. Fibroblast cell density was
evaluated and summarized in Fig.
5.
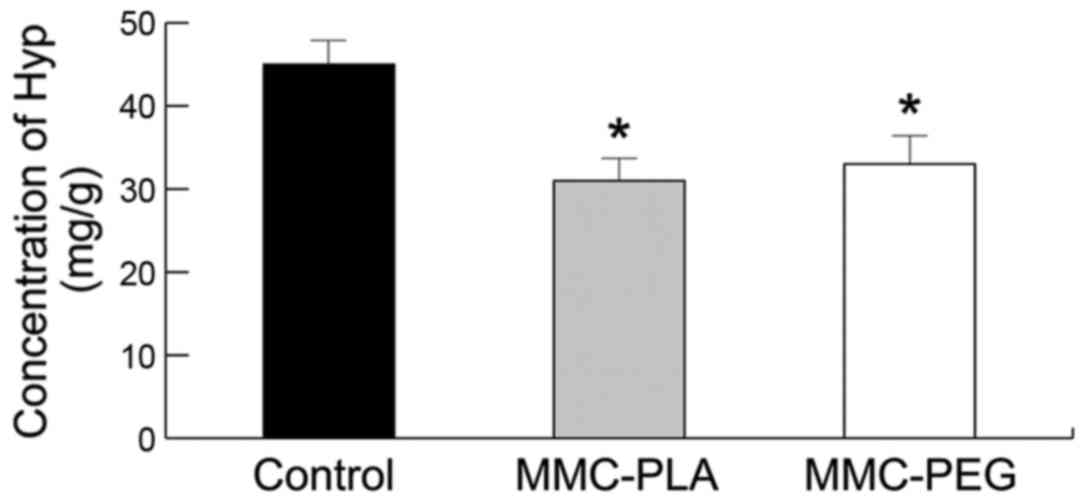
Biochemical analysis
The Hyp concentration in the epidural scar tissue in
the experiment groups (MMC-PLA and MMC-PEG) was significantly
reduced compared with that in the control group (P<0.05,
Fig. 6). The precise
concentrations of each group were as follows: the control group,
45.2132.53 mg/g; MMC-PLA, 31.4157.21 mg/g; MMC-PEG, 33.6736.91
mg/g.
Discussion
The present study demonstrated that the MMC-PLA and
the MMC-PEG controlled-release delivery barrier films could be
implanted as a physical and chemistry barrier to effectively reduce
epidural fibrosis formation and scar adhesion without affecting the
cerebrospinal fluid leaks healing. The results provided sufficient
evidence that the MMC-PLA and the MMC-PEG controlled-release
delivery barrier films were a potential biomaterial to reduce
epidural fibrosis for future clinical application.
In MRI evaluation, the transverse MRI of the control
group exhibited noticeable fibrotic scarring compression in the
spine cord, and the experimental groups had a lower MRI score
(P<0.05). In macroscopic observation of the epidural scar
adhesion, the grade of the control group was 3 compared with the
experimental group grades from 0 to 1 (P<0.05). In histological
analysis of epidural adhesion, there was a dense scar tissue
adhered to dura mater in the control group, and loose scar tissues
without adherence to dura mater were present in the experimental
group. The density of collagen tissue in the section treated with
MMC-PLA or MMC-PEG was significantly less than that in the control
group. In biochemical analysis, the Hyp concentration in the
epidural scar tissue in the experiment groups (MMC-PLA and MMC-PEG)
was significantly reduced compared with that in the control group
(P<0.05). Also, there was no case of neurologic deficits or
cerebrospinal leakage in any rat. No rat suffered accidental
death.
The study demonstrated that with the decomposition
of PLA and PEG films, MMC would be continuously released and
inhibited the scar ingrowths without affecting the healing of the
dura.
Various reagents and materials have been used to
prevent epidural fibrosis after laminectomy. Several different
strategies have been extensively explored. Microsurgical technique
(27), drug use (28,29)
and low-dose radiation (30–32)
have been demonstrated to be effective in rat and human models. At
the same time, there are also studies on the biophysical barriers
which could be applied to avoid the contact between the epidural
scar and dura (33,34).
Bioresorbable PLA and PEG barrier films are
promising and currently used in surgical scenarios (35–37).
They are used for temporary wound support to reinforce soft tissue,
and minimize soft tissue attachments in the viscera by preventing
acute platelet deposition on damaged arteries and reducing adhesion
of platelets and leukocytes to the surface (35–37).
Both films could impede the migration of fibroblasts
from the raw surface of the erector spine musculature. They also
act as a surgical dissection plane, whereby the layer of organized
fibrous tissue enveloping the material forms a controlled,
distinguishable dissection plane allowing adjacent tissues to be
easily separated (35,38).
However, the ‘three-dimensional theory’, put forward
by Songer et al (39),
suggested that the PLA and PEG films could not function as a
three-dimensional barrier.
Compared with the PLA or PEG films, MMC could leak
into every space in the operation area and has been widely applied
as a potential adjuvant for the control of scar tissue in surgical
wounds (22,23,40).
However, the toxic characteristics of MMC are most likely
responsible for the greater tissue damage causing increased scar
tissue formation and even the failure of the wound healing
(41).
In most cases, insignificant cerebrospinal fluid
leaks during lumbar surgery are spontaneously repaired with the
help of fibroblasts or fibrocytes. Nevertheless, because the
barrier film prevents fibroblast migration and MMC prevents
fibroblast proliferation, these small leaks may do not heal
spontaneously and cerebrospinal fluid leaks develop (42).
In our study, compromised dura healing or
cerebrospinal fluid leaks were not identified with any of the test
products in the rats using MRI examination, macroscopic
observation, histological and biochemical analyses. The unique
feature of our study is that we used a controlled-release delivery
system in which a small dose of MMC was absorbed into the PLA and
PEG films to prepare the MMC-PLA and MMC-PEG films attempting to
prevent the epidural scar adhesions from developing cerebrospinal
fluid leaks. In our study, the two mitomycin C control-release
films of PEG and PLA barrier could function as a three-dimensional
barrier. At the same time, as only a small dose of MMC was applied,
the MMC controlled-release film should not interrupt the integrity
of the spontaneous repairing of the dura and give rise to some
other troublesome side effects. We, therefore, believe that the use
of MMC controlled-release film represents a better way of creating
a barrier to the expansion of the troublesome epidural
fibrosis.
In conclusion, the present study suggests that the
two mitomycin-C controlled-release barrier films of PEG and PLA is
an effective and safe material to decrease epidural scar adhesion
after spinal laminectomy in the rat model. Our findings indicate
that applying the mitomycin-C controlled-release barrier films in
clinical practice may potentially minimize post-operative
complications.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81301523 and 81271987) and
and Jiangsu High Level Medical Personnel ‘111111 Project Reasearch’
(grant no. LGY2016018).
References
|
1
|
Key JA and Ford LT: Experimental
intervertebral-disc lesions. J Bone Joint Surg Am. 30A:621–630.
1948. View Article : Google Scholar
|
|
2
|
Yang L, Tang J, Chen H, Ge D, Sui T, Que
J, Cao X and Ge Y: Taurine reduced epidural fibrosis in rat models
after laminectomy via downregulating EGR1. Cell Physiol Biochem.
38:2261–2271. 2016. View Article : Google Scholar
|
|
3
|
Bartynski WS and Petropoulou KA: The MR
imaging features and clinical correlates in low back pain-related
syndromes. Magn Reson Imaging Clin N Am. 15:137–154. 2007.
View Article : Google Scholar
|
|
4
|
Sizer PS Jr, Phelps V, Dedrick G and
Matthijs O: Differential diagnosis and management of spinal nerve
root-related pain. Pain Pract. 2:98–121. 2002. View Article : Google Scholar
|
|
5
|
Lladó A, Guimerá J, Garcia F and Navarro
A: Expanded polytetrafluoroethylene membrane for the prevention of
peridural fibrosis after spinal surgery: An experimental study. Eur
Spine J. 8:138–143. 1999. View Article : Google Scholar :
|
|
6
|
Burton CV: Causes of failure of surgery on
the lumbar spine: Ten-year follow-up. Mt Sinai J Med. 58:183–187.
1991.
|
|
7
|
Ganzer D, Giese K, Völker L, Pietzner U,
Follak N and Merk H: Two-year results after lumbar microdiscectomy
with and without prophylaxis of a peridural fibrosis using Adcon-L.
Arch Orthop Trauma Surg. 123:17–21. 2003. View Article : Google Scholar
|
|
8
|
Vogelsang JP, Finkenstaedt M, Vogelsang M
and Markakis E: Recurrent pain after lumbar discectomy: The
diagnostic value of peridural scar on MRI. Eur Spine J. 8:475–479.
1999. View Article : Google Scholar :
|
|
9
|
Ozgen S, Naderi S, Ozek MM and Pamir MN:
Findings and outcome of revision lumbar disc surgery. J Spinal
Disord. 12:287–292. 1999. View Article : Google Scholar
|
|
10
|
Abdou M Samy and Hardy RW Jr: Epidural
fibrosis and the failed back surgery syndrome: History and physical
findings. Neurol Res. 21 Suppl 1:S5–S8. 1999. View Article : Google Scholar
|
|
11
|
BenDebba M, van Alphen H Augustus and Long
DM: Association between peridural scar and activity-related pain
after lumbar discectomy. Neurol Res. 21 Suppl 1:S37–S42. 1999.
View Article : Google Scholar
|
|
12
|
Chandler K and Cappello R: Laminectomy
membrane formation in dogs: Is the answer still elusive? Vet J.
172:1–2. 2006. View Article : Google Scholar
|
|
13
|
Ming J, Wei T, Lin C, Min T, Sheng W and
Chih Y: Spinal somatosensory evoked potential to evaluate
neurophysiologic changes associated with postlaminotomy fibrosis:
An experimental study. Spine. 32:2111–2118. 2007. View Article : Google Scholar
|
|
14
|
da Costa RC, Pippi NL, Graça DL, Fialho
SA, Alves A, Groff AC and Rezler U: The effects of free fat graft
or cellulose membrane implants on laminectomy membrane formation in
dogs. Vet J. 171:491–499. 2006. View Article : Google Scholar
|
|
15
|
Kato T, Haro H, Komori H and Shinomiya K:
Evaluation of hyaluronic acid sheet for the prevention of
postlaminectomy adhesions. Spine J. 5:479–488. 2005. View Article : Google Scholar
|
|
16
|
Temel SG, Ozturk C, Temiz A, Ersozlu S and
Aydinli U: A new material for prevention of epidural fibrosis after
laminectomy: Oxidized regenerated cellulose (interceed), an
absorbable barrier. J Spinal Disord Tech. 19:270–275. 2006.
View Article : Google Scholar
|
|
17
|
Akeson WH, Massie JB, Huang B, Giurea A,
Sah R, Garfin SR and Kim CW: Topical high-molecular-weight
hyaluronan and a roofing barrier sheet equally inhibit
postlaminectomy fibrosis. Spine J. 5:180–190. 2005. View Article : Google Scholar
|
|
18
|
Pasut G, Guiotto A and Veronese FM:
Protein, peptide and non-peptide drug PEGylation for therapeutical
application (Review). Expert Opin Ther Patents. 14:859–894. 2004.
View Article : Google Scholar
|
|
19
|
Schiavon O, Pasut G, Moro S, Orsolini P,
Guiotto A and Veronese FM: PEG-Ara-C conjugates for controlled
release. Eur J Med Chem. 39:123–133. 2004. View Article : Google Scholar
|
|
20
|
Tao H and Fan H: Implantation of amniotic
membrane to reduce postlaminectomy epidural adhesions. Eur Spine J.
18:1202–1212. 2009. View Article : Google Scholar :
|
|
21
|
Cao B, Yin J, Yan S, Cui L, Chen X and Xie
Y: Porous scaffolds based on cross-linking of poly(L-glutamic
acid). Macromol Biosci. 11:427–434. 2011. View Article : Google Scholar
|
|
22
|
Dogulu F, Kurt G, Emmez H, Erdem O, Memis
L, Baykaner K and Ceviker N: Topical mitomycin C-induced inhibition
of postlaminectomy peridural fibrosis in rabbits. J Neurosurg. 99
Suppl 1:76–79. 2003.
|
|
23
|
Sun Y, Wang LX, Wang L, Sun SX, Cao XJ,
Wang P and Feng L: A comparison of the effectiveness of mitomycin C
and 5-fluorouracil in the prevention of peridural adhesion after
laminectomy. J Neurosurg Spine. 7:423–428. 2007. View Article : Google Scholar
|
|
24
|
Lee JY, Stenzel W, Löhr M, Stützer H,
Ernestus RI and Klug N: The role of mitomycin C in reducing
recurrence of epidural fibrosis after repeated operation in a
laminectomy model in rats. J Neurosurg Spine. 4:329–333. 2006.
View Article : Google Scholar
|
|
25
|
Liu J, Ni B, Zhu L, Yang J, Cao X and Zhou
W: Mitomycin C- polyethylene glycol controlled-release film
inhibits collagen secretion and induces apoptosis of fibroblasts in
the early wound of a postlaminectomy rat model. Spine J.
10:441–447. 2010. View Article : Google Scholar
|
|
26
|
Sen O, Kizilkilic O, Aydin MV, Yalcin O,
Erdogan B, Cekinmez M, Caner H and Altinors N: The role of
closed-suction drainage in preventing epidural fibrosis and its
correlation with a new grading system of epidural fibrosis on the
basis of MRI. Eur Spine J. 14:409–414. 2005. View Article : Google Scholar
|
|
27
|
Canis M, Botchorishvili R, Tamburro S,
Safi A, Wattiez A, Mage G, Pouly JL and Bruhat MA: Adhesion
prevention in the surgical treatment of pelvic endometriosis.
Gynaecol Endosc. 10:99–106. 2001. View Article : Google Scholar
|
|
28
|
Zhang Z, Tarone G and Turner DC:
Expression of integrin α1 β1 is regulated by nerve growth factor
and dexamethasone in PC12 cells. Functional consequences for
adhesion and neurite outgrowth. J Biol Chem. 268:5557–5565.
1993.
|
|
29
|
Dumont RJ, Verma S, Okonkwo DO, Hurlbert
RJ, Boulos PT, Ellegala DB and Dumont AS: Acute spinal cord injury,
part II: Contemporary pharmacotherapy. Clin Neuropharmacol.
24:265–279. 2001. View Article : Google Scholar
|
|
30
|
Gerszten PC, Moossy JJ, Flickinger JC,
Gerszten K, Kalend A and Martínez AJ: Inhibition of peridural
fibrosis after laminectomy using low-dose external beam radiation
in a dog model. Neurosurgery. 46:1478–1485. 2000. View Article : Google Scholar
|
|
31
|
Gerszten PC, Moossy JJ, Bahri S, Kalend A
and Martínez AJ: Inhibition of peridural fibrosis after laminectomy
using low-dose external beam radiation in a rat model.
Neurosurgery. 44:597–602; discussion 602–603. 1999. View Article : Google Scholar
|
|
32
|
Gibbs IC, Patil C, Gerszten PC, Adler JR
Jr and Burton SA: Delayed radiation-induced myelopathy after spinal
radiosurgery. Neurosurgery. 64(suppl_2): A67–A72. 2009. View Article : Google Scholar
|
|
33
|
Pospiech J, Pajonk F and Stolke D:
Epidural scar tissue formation after spinal surgery: An
experimental study. Eur Spine J. 4:213–219. 1995. View Article : Google Scholar
|
|
34
|
Silva SS, Motta A, Rodrigues MT, Pinheiro
AF, Gomes ME, Mano JF, Reis RL and Migliaresi C: Novel
genipin-cross-linked chitosan/silk fibroin sponges for cartilage
engineering strategies. Biomacromolecules. 9:2764–2774. 2008.
View Article : Google Scholar
|
|
35
|
Klopp LS, Welch WC, Tai JW, Toth JM,
Cornwall GB and Turner AS: Use of polylactide resorbable film as a
barrier to postoperative peridural adhesion in an ovine dorsal
laminectomy model. Neurosurg Focus. 16:E22004. View Article : Google Scholar
|
|
36
|
Bakaltcheva I, Ganong JP, Holtz BL, Peat
RA and Reid T: Effects of high-molecular-weight cryoprotectants on
platelets and the coagulation system. Cryobiology. 40:283–293.
2000. View Article : Google Scholar
|
|
37
|
Fujii H, Fujii S, Togashi H, Yoshioka M,
Nakai K, Satoh H, Sakuma I, Kenmotsu O and Kitabatake A:
Attenuation of hypothermia-induced platelet activation and platelet
adhesion to artificial surfaces in vitro by modification of
hemoglobin to carry S-nitric oxide and polyethylene glycol. Thromb
Res. 100:519–528. 2000. View Article : Google Scholar
|
|
38
|
Welch WC, Cornwall GB, Toth JM, Turner AS,
Thomas KA, Gerszten PC and Nemoto EM: Use of polylactide resorbable
film as an adhesion barrier. Orthopedics. 25(10 Suppl):
s1121–s1130. 2002.
|
|
39
|
Songer MN, Rauschning W, Carson EW and
Pandit SM: Analysis of peridural scar formation and its prevention
after lumbar laminotomy and discectomy in dogs. Spine. 20:571–580,
discussion 579–580. 1995. View Article : Google Scholar
|
|
40
|
Lee JY, Stenzel W, Ebel H, Wedekind C,
Ernestus RI and Klug N: Mitomycin C in preventing spinal epidural
fibrosis in a laminectomy model in rats. J Neurosurg. 100(1 Suppl
Spine): 52–55. 2004.
|
|
41
|
Fielding JW, Crocker J, Stockley RA and
Brookes VS: Interstitial fibrosis in a patient treated with
5-fluorouracil and mitomycin C. BMJ. 2:551–552. 1979. View Article : Google Scholar :
|
|
42
|
Kuhn J, Hofmann B, Knitelius HO, Coenen HH
and Bewermeyer H: Bilateral subdural haematomata and lumbar
pseudomeningocele due to a chronic leakage of liquor
cerebrospinalis after a lumbar discectomy with the application of
ADCON-L gel. J Neurol Neurosurg Psychiatry. 76:1031–1033. 2005.
View Article : Google Scholar :
|