Introduction
Myelin is the spirally wrapped cell membrane that
surrounds and insulates axons in the peripheral and central nervous
systems (PNS and CNS, respectively). CNS myelin is synthesized by
oligodendrocyte (OL). OL myelination permits salutatory propagation
of nerve signals, and is critical for cognitive and motor functions
in the CNS (1). Myelination is a
multistep process involving the proliferation of OL precursor cells
(OPCs), timely differentiation into postmitotic OLs, ensheathment
of axons, initiation of myelin wrapping, and expansion of the
myelin sheath during myelination (2). Independent of underlying mechanisms
and causes, demyelinating diseases, such as multiple sclerosis
(MS), generally result in permanent damage, functional loss and
persisting disabilities. Such demyelination, however, often
triggers a spontaneous myelin repair process, also termed
remyelination (3). Remyelination
results in myelin reconstitution and functional recovery via
recruitment and activation of resident OPCs that differentiate and
replace lost OLs (4).
Remyelination is best investigated in models where demyelination
occurs at a predictable anatomical site, and follows a well-defined
and reproducible kinetic and time schedule.
A primary demyelinating disease is MS, which is a
recurrent progressive disease. It is estimated that ~2.5 million
people worldwide suffer from MS (5). The majority are young adults (age,
20–40 years) with females outnumbering males by 2:1 (5). The specific symptoms are determined
by the location of the demyelinated lesions. They may include
muscle weakness, loss of sensitivity, incontinency, visual
troubles, ataxia, fatigue and mood alterations (6). The etiology remains unclear, and
there is no effective treatment for this disease.
Studies of signaling pathways, including bone
morphogenetic protein/Id, Wnt/β-catenin and Notch/Hes signaling
have been shown to negatively regulate OL differentiation (7,8).
TCF7L2 is one of the four members of the TCF/lymphoid enhancer
binding factor 1 (LEF1) family (gene symbols, TCF7L1, TCF7L2, TCF7
and LEF1) that are essential for mediating Wnt/β-catenin signaling
in Wnt-activated cells (9).
Previously, many studies indicated that TCF7L2 expression in the
oligodendroglial lineage cells inhibits or delays OL
differentiation, and inhibits remyelination in demyelinating
diseases (10,11). However, recently, two studies
concluded that TCF7L2 positively regulates OL differentiation
(12,13). In addition, TCF7L2 affect OL
differentiation via different mechanisms (10,12,13).
The aim of the current study was to investigate the
role of Tcf7l2 in myelination and remyelination, which may have
clinical significance in the development novel therapeutic
targets.
Materials and methods
Transgenic mice
The transgenic Cnp-Cre and
Tcf7l2fl/fl (exon11 flanked by loxP sites) mice
were provided by Dr Hui Fu (Department of Anatomy, Basic Medical
School of Wuhan University, Wuhan, China). Male and female mice
were used in the current study. Tcf7l2fl/fl and
Cnp-cre. C57BL/6 served as the genetic background for the
Tcf7l2fl/fl mice. All animal experiments were
performed in accordance with the National Institutes of Health
guide for the care and use of Laboratory animals (NIH publications
no. 8023, revised 1978) and were approved by Institutional Animal
Care and Use Committee at Wuhan University (Wuhan, China).
mRNA in situ hybridization
Sections containing the spinal cord (25.4×76.2 mm)
were hybridized overnight with a labeled RNA probe (0.8–1.2 µg/ml)
at 65°C. The sections were washed in 2X saline-sodium citrate (SSC)
at 67°C, incubated with RNase (1 µg/ml, 2X SSC) at 37°C, washed in
0.2X SSC at 67°C, blocked in 1X phosphate-buffered saline (PBS)
with 10% lamb sera, and incubated in alkaline phosphatase-labeled
anti-digoxigenin antibody (catalog no. 11093274910; Roche
Diagnostics GmbH-Mannheim, Germany; 1:2,000, 10% lamb sera)
overnight at 4°C. Sections were washed and stained with nitro blue
tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate or BM purple
(Roche Diagnostics GmbH). Staining was terminated following visual
inspection using a light microscope. Finally, sections were washed
in 1X PBS three times, fixed in 4% paraformaldehyde and
coverslipped with glycerol.
Immunofluorescence and antibodies
Slides were incubated with antibodies (anti-CNP,
catalog no. NE1020, 1:200, Calbiochem; anti-APC, catalog no. OP80,
1:60, EMD Millipore, Billerica, MA, USA; anti-PDGFRA, catalog no.
APA5, 1:300, BD Bioscience, Franklin Lakes, NJ, USA) overnight at
4°C. Sections were then washed three times with 1X PBS, incubated
with Alexa-488- or Alexa-555-conjugated secondary antibodies (goat
anti-mouse IgG-Alexa Flour 555, catalog no. A21422, 1:500, Cell
Signaling Technology, Inc., Danvers, MA, USA; Goat anti-rabbit
IgG-Alexa Flour 488, catalog no. 4412s, 1:500, Cell Signaling
Technology, Inc.) and fluorescent images were obtained using a
Nikon epifluorescence microscope (Nikon Corporation, Tokyo, Japan).
Antibodies used in the study were as follows: Anti-APC, WNT
signaling pathway regulator (APC; 1:60; cat. no. OP80; EMD
Millipore), anti-2′,3′-cyclic nucleotide 3′ phosphodiesterase (CNP;
1:200; cat. no. NE1020; EMD Millipore), anti-myelin basic protein
(MBP; 1:200; cat. no. NE1018; EMD Millipore,) and
anti-platelet-derived growth factor receptor α (PDGFRA; 1:300; cat.
no. APA5; BD Biosciences).
Protein extraction and western
blotting
For western blot analysis, whole cell lysates were
prepared from the corpus callosum at the thirtieth day after birth
(P30). using RIPA buffer (Applygen Technologies, Inc., Beijing,
China), phenylmethylsulfonyl fluoride (Biosharp, Shanghai, China)
and PhosSTOP (Roche Diagnostics GmbH). The tissue samples were
minced using an electric homogenizer. The protein concentrations in
the centrifugation-clarified cell lysates were measured using a
bicinchoninic acid Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and equal quantities of protein
were separated on 10% SDS-PAGE gel and transferred (100 V for 2 h)
to Hybond polyvinylidene fluoride membranes (GE Healthcare Life
Sciences, Shanghai, China). For Western blotting, primary
antibodies against CNP (1:100), MBP (1:1,000), GAPDH (1:1,000),
β-actin (1:10,000) were used. Signals were developed using
horseradish peroxidase-conjugated secondary antibodies (goat
anti-mouse antibody conjugated to horse radish peroxidase, 1:5,000,
catalog no. AS1106, Aspen Biotechnology Co., Ltd., Hubei, China);
goat anti-rabbit antibody conjugated to horse radish peroxidase,
1:5,000, catalog no. AS1107, Aspen Biotechnology Co., Ltd.) and an
ECL kit (GE Healthcare Life Science).
Eriochrome cyanine
Slides were washed in 1X PBS three times, and dyed
with eriochrome cyanine (catalog no. B100835-5G, Shanghai Aladdin
Bio-Chem Technology Co., Ltd., Shanghai, China) for 30 min, and
then washed three times with 1X PBS. Sections were differentiated
by 10% iron alum (catalog no. 10009218, Sinopharm Chemical Reagent
Co., Ltd., Shanghai, China) for 8 min, and washed three times with
1X PBS, prior to coverslipping with neutral gum (catalog no.
1000416, Sinopharm Chemical Reagent Co., Ltd.).
Rotarod performance test
The rotarod performance test is based on a rotating
rod with forced motor activity applied by a mouse. The test is used
to measure parameters, such as riding time (sec) or endurance. In
the rotarod apparatus (IITC Life Science, Woodland Hills, CA, USA),
mice were placed individually on a horizontally oriented, rotating
cylinder suspended above a cage floor, which is low enough not to
injure the animal, but high enough to induce avoidance of fall. The
fall off time of the mouse from the rotating rod was recorded. The
difference in fall off times from the rotating rod between the two
groups was taken as an index of muscle relaxation and motor
coordination activity.
Cuprizone test
The TCF7L2 cKO and control mice (age, 8–10 weeks)
were fed with a mixture of 0.2% cuprizone (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) in ground chow for up to 6 weeks.
Cuprizone is a copper chelator that induces demyelination of the
corpus callosum if administered orally to adult mice. A normal diet
was provided for 2 weeks after cuprizone treatment. The
demyelination peaked in the fifth week. The remyelination occurred
thereafter and was allowed to develop for two weeks during the
current study.
Statistical analysis
Quantification was performed on data from at least
three independent experiments, and the data were presented as means
± standard error of the mean in the graphs. Student's t-test was
used for comparisons between two sets of data. ImageJ version 2.0
(National Institutes of Health, Bethesda, MD, USA) was used for
quantitative analysis of the mRNA and protein expression levels of
MBP, proteolipid protein 1 (PLP1), CNP, APC and PDGFRA. P<0.05
was considered to indicate statistically significant
differences.
Results
TCF7L2 deletion inhibits postnatal OL
differentiation during myelin formation
The Cre-loxP system was used to conditionally
knock out TCF7L2 in oligodendroglial lineage cells, and avoid the
lethality of TCF7L2-null newborns (14). Cnp-cre and
Tcf7l2fl/fl (exon11 is flanked by loxP sites)
mice were used to delete the TCF7L2 exon11 sequence in the OPCs,
which is the DNA binding domain (15).
Knockout of the TCF7L2 exon 11 sequence in the
oligodendroglial lineage cells (Fig.
1) demonstrated that the mRNA levels of MBP and PLP1 in the
spinal cords were significantly lower when compared with those of
the control group at P0 (P<0.001; Fig. 1A and C), and a similar reduction in
the spinal cords was observed at P7 (MBP, P<0.01; PLP,
P<0.05; Fig. 1B and D)
indicating that TCF7L2 positively regulates OL differentiation.
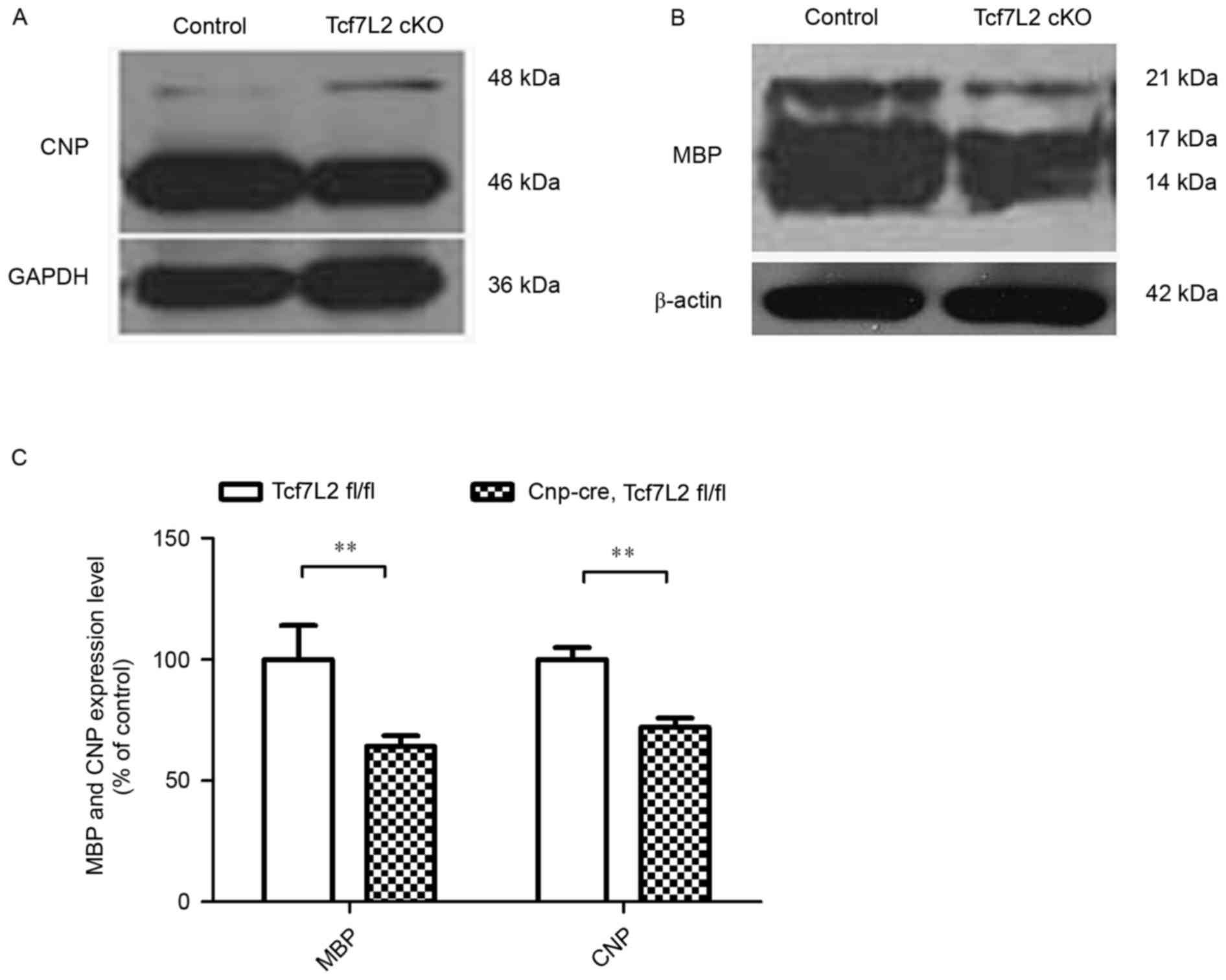
Furthermore, corpus callosum samples were obtained, and protein
samples from the Cnp-cre-mediated TCF7L2 KO mice and
littermate control mice were extracted at P30. Western blot
analysis revealed markedly decreased expression levels of CNP and
MBP in the TCF7L2 cKOs compared with the controls (Fig. 2A and B). Quantitative analysis
demonstrated that the expression levels of CNP and MBP were
significantly lower than those of the control mice (P<0.01;
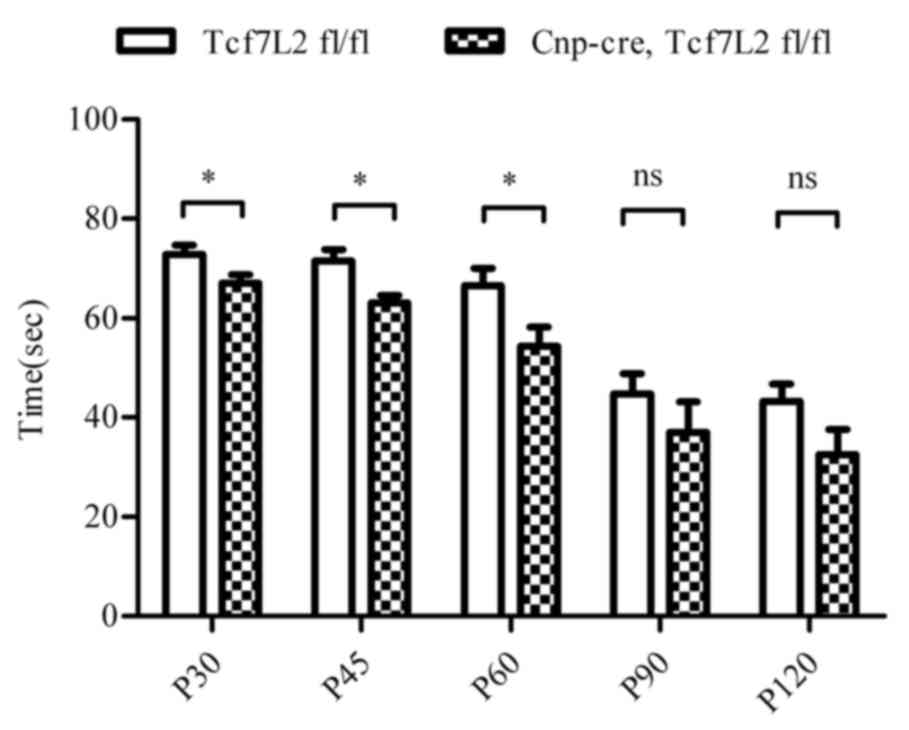
Fig. 2C). In order to exam motor
coordination activity, the rotarod performance test demonstrated
that the riding time on the rotating rod was significantly shorter
in the TCF7L2 cKO group when compared with the control mice at P30,
P45 and P60 (P<0.05; Fig. 3).
The riding time of the TCF7L2 cKO group mice was decreasing at P90
and P120 (P=0.131 and P=0.087, respectively). Together, these data
indicate that TCF7L2 promotes OL differentiation and myelination
during developmental myelination.
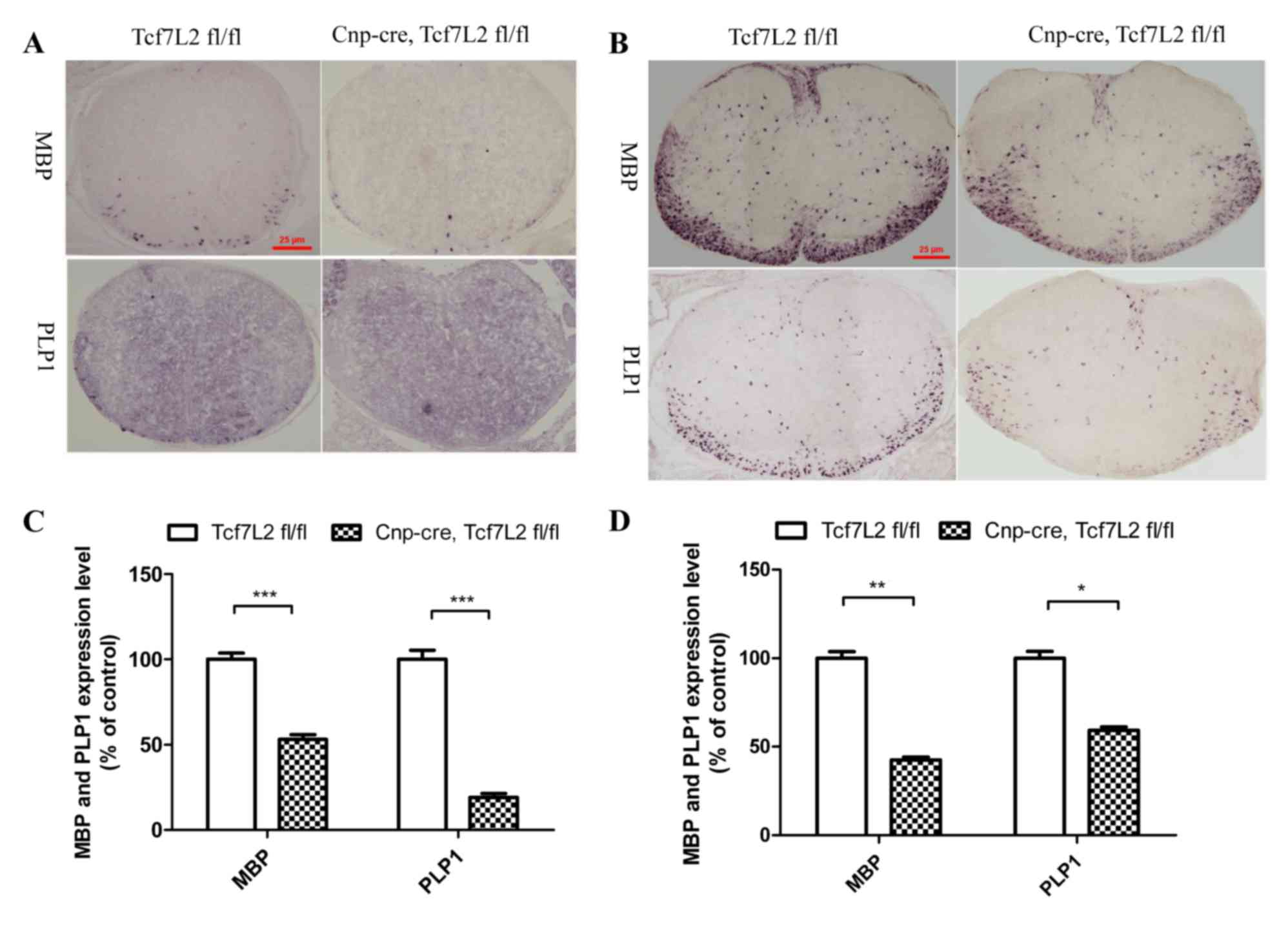 | Figure 1.TCF7L2 cKO inhibits OL
differentiation. (A) TCF7L2 cKO mice demonstrated that the mRNA
expression level of MBP and PLP1 decreased significantly when
compared with the control group in the spinal cord at P0 (n=5 per
group). Magnification, ×100. (B) The TCF7L2 cKO mice demonstrated
significantly decreased expression levels of MBP and PLP1 mRNA in
the spinal cord when compared with the control group at P7 (n=4 per
group). Magnification, ×100. (C) Quantitative analysis indicated
that the mRNA expression levels of MBP and PLP1 in the TCF7L2 cKO
group were significantly reduced compared with that of the control
group at P0 (P<0.001). (D) Quantitative analysis further
indicated that the MBP and PLP1 mRNA expression levels in the
TCF7L2 cKO group were significantly lower than those of the control
group at P7 (MBP, P<0.01; PLP, P<0.05). *P<0.05,
**P<0.01 and ***P<0.001. Error bars indicate the standard
error of the mean. TCF7L2, transcription factor 7 like 2; cKO,
conditional knockout; OL, oligodendrocyte; MBP, myelin basic
protein; PLP1, proteolipid protein 1. P0, the first day after
birth; P7, the seventh day after birth. |
TCF7L2 cKO inhibits OL differentiation
during remyelination
Various previous studies have proposed that TCF7L2
may inhibit OL differentiation and oligodendrogenesis during myelin
formation (16,17). There are few studies that have
revealed the role of TCF7L2 in remyelination. Based on the current
data, it was hypothesized that TCF7L2 promotes OL differentiation
during remyelination. Among the various animal and cell models
available to investigate remyelination, the cuprizone model stands
out due to its reproducibility, simplicity to induce demyelination
and low mortality rates (18). In
the present study, the 0.2% cuprizone demyelination/remyelination
model was used to determine the role of Tcf7l2 in
remyelination.
After feeding mice 0.2% cuprizone for 6 weeks,
myelin histochemistry with Eriochrome cyanine revealed that there
was almost no myelin in the corpus callosum samples between the two
groups (Fig. 4A). The magnified
view demonstrated further that the corpus callosum samples were
fully demyelinated in the two groups (Fig. 4A).
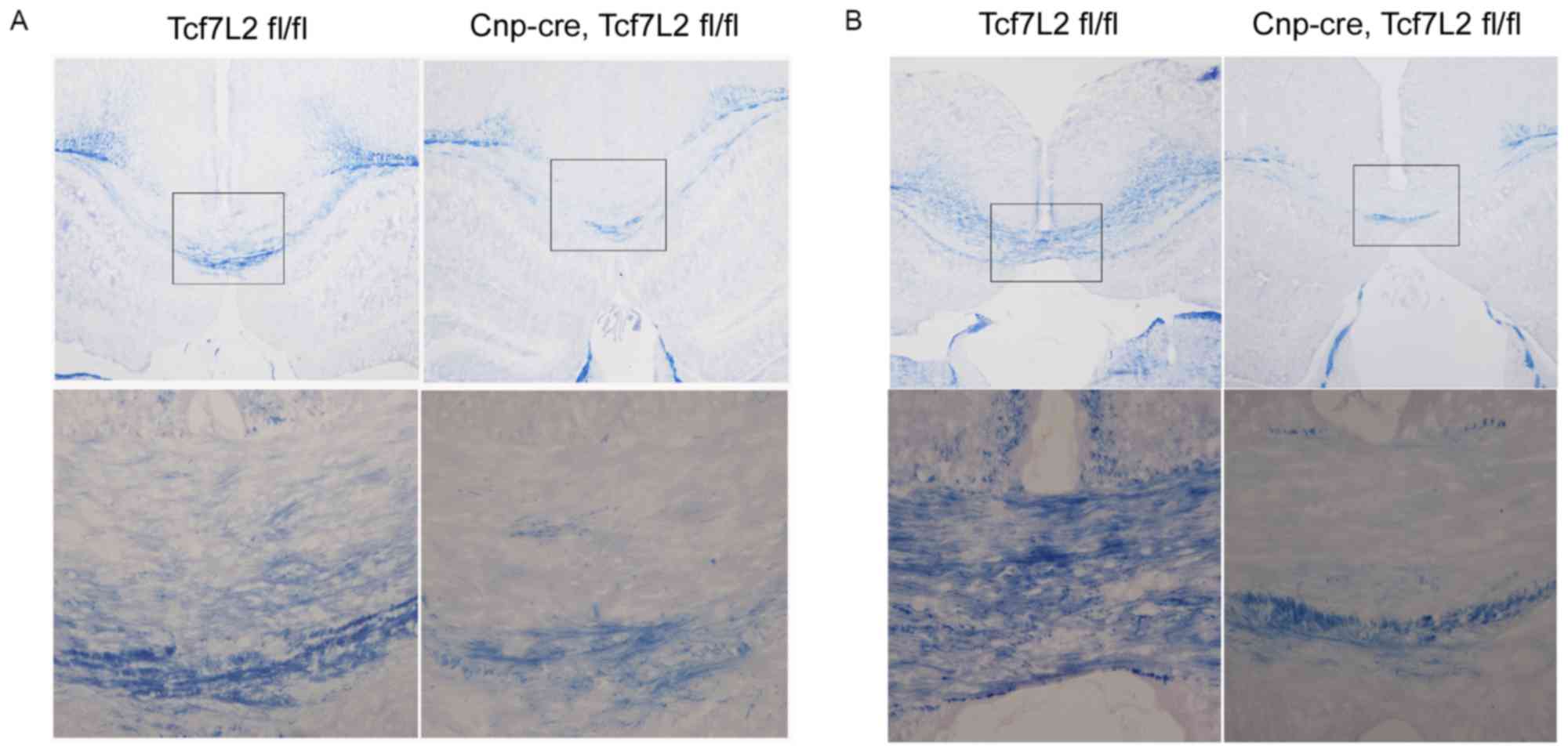 | Figure 4.TCF7L2 cKO inhibits remyelination. (A)
Subsequent to feeding the mice 0.2% cuprizone for 6 weeks, myelin
histochemistry with eriochrome cyanine demonstrated that there was
almost no myelin in the corpus callosum samples between the two
groups (n=4 per group; magnification, ×100). The bottom row
presents the magnified images of the boxed areas in the top row
(magnification, ×200). (B) Subsequent to returning to feeding with
normal chow for 2 weeks, the corpus callosum samples were almost
completely remyelinated in the control group (n=5 per group;
magnification, ×100). By contrast, remyelination did not occur in
the TCF7L2 cKO mice. The bottom row presents the magnified images
of the boxed areas in the top row (magnification, ×200). TCF7L2,
transcription factor 7 like 2; cKO, conditional knockout; CNP,
2′,3′-cyclic nucleotide 3′ phosphodiesterase. |
After returning to normal chow feed for 2 weeks, the
corpus callosum samples were almost completely remyelinated in the
control mice (Fig. 4B and C). By
contrast, remyelination did not occur in the TCF7L2 cKO mice
(Fig. 4B and C). Furthermore,
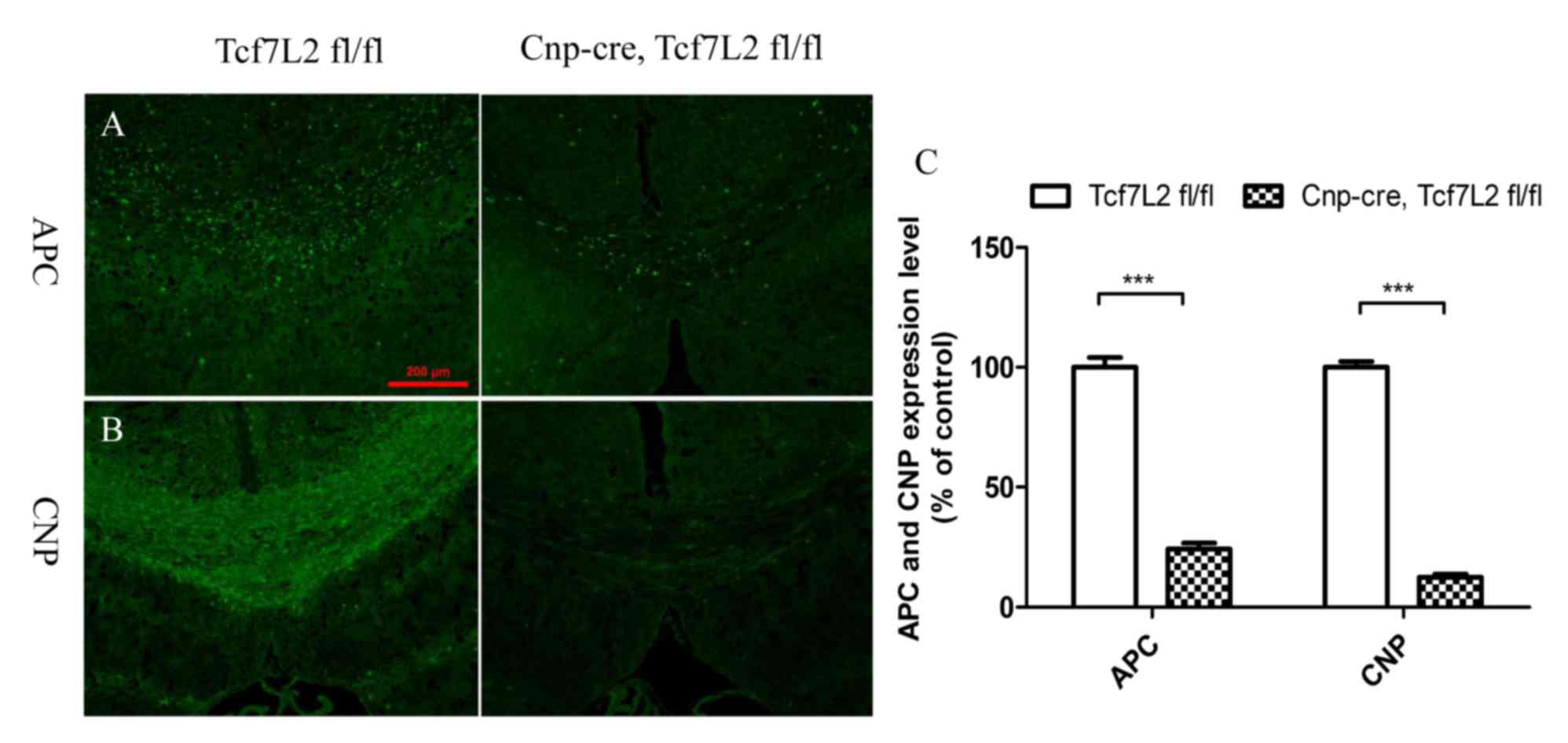
immunofluorescence demonstrated that the expression levels of APC
and CNP in the control group were significantly higher than the
TCF7L2 cKO group (Fig. 5A and B)
and almost the same as normal expression levels of APC and CNP
(data not shown). Quantitative analysis further indicated that the
expression levels of APC and CNP were significantly lower than
those of the control mice (P<0.001; Fig. 5C). In addition, the mRNA expression
level of MBP observed by in situ hybridization was
significantly higher in the control group (data not shown). These
data indicate that TCF7L2 cKO inhibits remyelination, whereas it
promotes OL differentiation during remyelination.
TCF7L2 does not affect OPCs during
remyelination
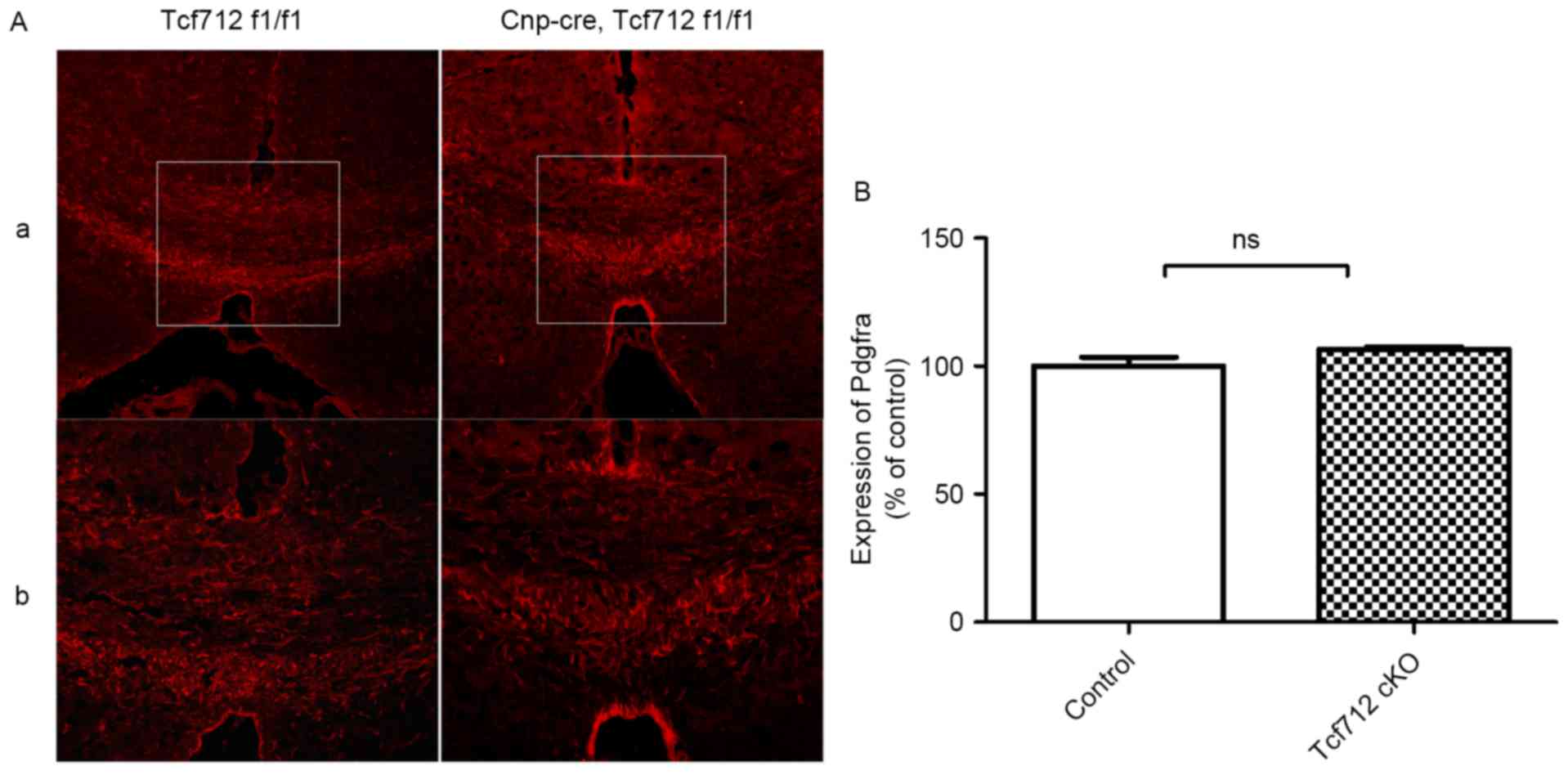
To determine whether OPCs were perturbed in TCF7L2
cKO mice, the expression level of PDGFRA, an OL precursor cell
marker, was examined during remyelination. After returning to
feeding with normal chow for 2 weeks, the expression level of
PDGFRA was almost the same in the two groups during remyelination
(Fig. 6Aa and Ab). Quantitative
analysis further indicated that there was no significant alteration
in terms of OPCs between the two groups during remyelination
(P>0.05; Fig. 6B). These data
indicate that TCF7L2 cKO does not affect OPCs during
remyelination.
Discussion
There were various significant findings in the
present study. In contrast to previous reports, TCF7L2 promoted
postnatal OL differentiation during myelination. In addition,
TCF7L2 positively regulated OL differentiation during remyelination
and finally, TCF7L2 did not affect OPCs during remyelination.
It is well accepted that TCF7L2 is one of the four
members of the TCF/LEF1 family (TCF7, TCF7l1, TCF7l2 and LEF1),
which are essential for mediating canonical Wnt/β-catenin signaling
in Wnt-activated cells (9).
Previous studies indicated that TCF7L2 inhibits OL differentiation
(16,17), while a recent study reported that
TCF7L2 promotes OL differentiation (13). According to existing studies, it is
unclear whether TCF7L2 inhibits or promotes OL differentiation. The
results presented here do not support the hypothesis that TCF7L2
inhibits OL differentiation and myelination via Wnt/β-catenin
activation. The current study focused on the neonatal and postnatal
oligodendroglial lineage and demonstrated that, contrary to the
well accepted hypothesis that TCF7L2 inhibits OL differentiation
and myelination via Wnt/β-catenin activation, TCF7L2 functions as a
positive regulator of OL differentiation and myelination during
normal developmental myelination. This conclusion is consistent
with previous data derived from TCF7L2-null late embryos or
newborns (13,19).
In addition, the cuprizone
demyelination/remyelination model was used to determine the role of
TCF7L2 in remyelination. Various studies have provided compelling
evidence of the usefulness of this model to discover novel
therapeutic options to boost remyelination (20,21).
Remyelination, which resembles the physiological process of
myelination and has a high degree of complexity and regulation, is
classified into four consecutive steps: Proliferation of OPCs,
migration of progenitor cells towards the demyelinated axons, OPC
differentiation and interaction of premature OLs with the denuded
axon (3). These steps are
regulated by many intrinsic and extrinsic factors. For example,
transcription factors, Sonic hedgehog and oligodendrocyte
transcription factor 1 have been identified as important intrinsic
regulators for OL differentiation and myelination (22,23),
whereas astrocytes are regarded as important extrinsic regulators
for myelin repair. In the present study, immunohistochemistry was
used against the major myelin proteins, APC and CNP, and myelin
histochemistry with eriochrome cyanine was used to quantify
remyelination between the two groups. It was demonstrated that
TCF7L2 promotes remyelination, which is indispensable for clinical
demyelinating diseases. The conclusion concerning the role of
TCF7L2 in remyelination is compatible with a recent study (13).
The ablation of TCF7L2 directed by Cnp-cre
does not affect OPCs during remyelination. This demonstration most
likely reflects a specific requirement of TCF7L2 in OL
differentiation and remyelination rather than a general requirement
in OPCs.
While many regulators of OL differentiation and
myelination have been identified, the specific mechanisms remain
poorly understood. As TCF7L2 is an essential effector for
Wnt/β-catenin signaling, various previous studies indicated that
TCF7L2 inhibits oligodendrogenesis and OL differentiation through
Wnt/β-catenin activation (17,24).
One study concluded that TCF7L2 positively regulates OL
differentiation independent of Wnt/β-catenin signaling. Whereas a
recent study demonstrated that TCF7L2 interacts with a
transcriptional co-repressor, Kaiso to block β-catenin signaling at
the differentiation onset, and subsequently TCF7L2 recruits and
cooperates with SRY-box 10 to promote myelination during OL
maturation (12).
In conclusion, TCF7L2/TCF4 is required for OL
differentiation and remyelination; however, the mechanism by which
TCF7L2 controls myelination and remyelination remains unknown.
Additional studies are required to elucidate how TCF7L2 promotes OL
differentiation and remyelination, which is of particular clinical
importance in the diagnosis and treatment of demyelinating
diseases.
Acknowledgements
The authors would like to thank Dr Hui Fu
(Department of Anatomy, Basic Medical School of Wuhan University,
Wuhan, China) for providing the Cnp-cre and
Tcf7l2fl/fl mice. The study was supported by the
Fundamental Research Funds for the Central Universities of China
(2042017kf0129), grants from the Health and Family Planning
Commission of Hubei Province Scientific Research Project
(WJ2015MA007) and Wuhan Science and Technology Bureau Scientific
Research Project (2015060101010047).
Glossary
Abbreviations
Abbreviations:
|
TCF7L2
|
transcription factor 7 like 2
|
|
PNS
|
peripheral nervous system
|
|
CNS
|
central nervous system
|
|
OL
|
oligodendrocyte
|
|
OPC
|
oligodendrocyte precursor cells
|
|
MS
|
multiple sclerosis
|
|
LEF1
|
lymphoid enhancer binding factor 1
|
|
cKO
|
conditional knockout
|
|
PLP1
|
proteolipid protein 1
|
|
APC
|
adenomatous polyposis coli
|
|
CNP
|
2′,3′-cyclic nucleotide 3′
phosphodiesterase
|
|
MBP
|
myelin basic protein
|
|
PDGFRA
|
platelet-derived growth factor
receptor α
|
References
|
1
|
Bercury KK and Macklin WB: Dynamics and
mechanisms of CNS myelination. Dev Cell. 32:447–458. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ishii A, Fyffe-Maricich SL, Furusho M,
Miller RH and Bansal R: ERK1/ERK2 MAPK signaling is required to
increase myelin thickness independent of oligodendrocyte
differentiation and initiation of myelination. J Neurosci.
32:8855–8864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Franklin RJ and Ffrench-Constant C:
Remyelination in the CNS: From biology to therapy. Nat Rev
Neurosci. 9:839–855. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
El Waly B, Macchi M, Cayre M and Durbec P:
Oligodendrogenesis in the normal and pathological central nervous
system. Front Neurosci. 8:1452014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Milo R and Kahana E: Multiple sclerosis:
Geoepidemiology, genetics and the environment. Autoimmun Rev.
9:A387–A394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Compston A and Coles A: Multiple
sclerosis. Lancet. 372:1502–1517. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zuchero JB and Barres BA: Intrinsic and
extrinsic control of oligodendrocyte development. Curr Opin
Neurobiol. 23:914–920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He L and Lu QR: Coordinated control of
oligodendrocyte development by extrinsic and intrinsic signaling
cues. Neurosci Bull. 29:129–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fancy SP, Kotter MR, Harrington EP, Huang
JK, Zhao C, Rowitch DH and Franklin RJ: Overcoming remyelination
failure in multiple sclerosis and other myelin disorders. Exp
Neurol. 225:18–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lürbke A, Hagemeier K, Cui QL, Metz I,
Brück W, Antel J and Kuhlmann T: Limited TCF7L2 expression in MS
lesions. PLoS One. 8:e728222013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fancy SP, Harrington EP, Yuen TJ,
Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ,
Nusse R, et al: Axin2 as regulatory and therapeutic target in
newborn brain injury and remyelination. Nat Neurosci. 14:1009–1016.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao C, Deng Y, Liu L, Yu K, Zhang L, Wang
H, He X, Wang J, Lu C, Wu LN, et al: Dual regulatory switch through
interactions of Tcf7l2/Tcf4 with stage-specific partners propels
oligodendroglial maturation. Nat Commun. 7:108832016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hammond E, Lang J, Maeda Y, Pleasure D,
Angus-Hill M, Xu J, Horiuchi M, Deng W and Guo F: The Wnt effector
transcription factor 7-like 2 positively regulates oligodendrocyte
differentiation in a manner independent of Wnt/β-catenin signaling.
J Neurosci. 35:5007–5022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boj SF, van Es JH, Huch M, Li VS, José A,
Hatzis P, Mokry M, Haegebarth A, van den Born M, Chambon P, et al:
Diabetes risk gene and Wnt effector Tcf7l2/TCF4 controls hepatic
response to perinatal and adult metabolic demand. Cell.
151:1595–1607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Es JH, Haegebarth A, Kujala P,
Itzkovitz S, Koo BK, Boj SF, Korving J, van den Born M, van
Oudenaarden A, Robine S and Clevers H: A critical role for the Wnt
effector Tcf4 in adult intestinal homeostatic self-renewal. Mol
Cell Biol. 32:1918–1927. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye F, Chen Y, Hoang T, Montgomery RL, Zhao
XH, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, et al: HDAC1 and
HDAC2 regulate oligodendrocyte differentiation by disrupting the
beta-catenin-TCF interaction. Nat Neurosci. 12:829–838. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wood TL, Bercury KK, Cifelli SE, Mursch
LE, Min J, Dai J and Macklin WB: mTOR: A link from the
extracellular milieu to transcriptional regulation of
oligodendrocyte development. ASN Neuro. 5:e001082013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zendedel A, Beyer C and Kipp M:
Cuprizone-induced demyelination as a tool to study remyelination
and axonal protection. J Mol Neurosci. 51:567–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu H, Cai J, Clevers H, Fast E, Gray S,
Greenberg R, Jain MK, Ma Q, Qiu M, Rowitch DH, et al: A genome-wide
screen for spatially restricted expression patterns identifies
transcription factors that regulate glial development. J Neurosci.
29:11399–11408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
VonDran MW, Singh H, Honeywell JZ and
Dreyfus CF: Levels of BDNF impact oligodendrocyte lineage cells
following a cuprizone lesion. J Neurosci. 31:14182–14190. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hussain R, Ghoumari AM, Bielecki B,
Steibel J, Boehm N, Liere P, Macklin WB, Kumar N, Habert R,
Mhaouty-Kodja S, et al: The neural androgen receptor: A therapeutic
target for myelin repair in chronic demyelination. Brain.
136:132–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferent J, Zimmer C, Durbec P, Ruat M and
Traiffort E: Sonic Hedgehog signaling is a positive oligodendrocyte
regulator during demyelination. J Neurosci. 33:1759–1772. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niu J, Mei F, Wang L, Liu S, Tian Y, Mo W,
Li H, Lu QR and Xiao L: Phosphorylated olig1 localizes to the
cytosol of oligodendrocytes and promotes membrane expansion and
maturation. Glia. 60:1427–1436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sabo JK and Cate HS: Signalling pathways
that inhibit the capacity of precursor cells for myelin repair. Int
J Mol Sci. 14:1031–1049. 2013. View Article : Google Scholar : PubMed/NCBI
|