Introduction
Injuries and diseases that induce retinal neuronal
cell death may result in permanent blindness. Stimulating
endogenous neuronal regeneration is a potential strategy to treat
these retinal lesions. In non-mammalian vertebrates, endogenous
repair to restore neurons occurs very efficiently, even following
complete loss of the retina, either through retinal pigment
epithelium transdifferentiation or through stem cell proliferation
and differentiation (1–4). However, these endogenous repair
processes in mammalian retina are limited (5,6), as
the mammalian retina have no significant persistent neurogenic
source such as ciliary marginal zone; in mice, neurogenesis is
completed within the first 10 postnatal days (7). Nevertheless, great efforts have been
made in the past few decades to overcome the limits of these
regenerative processes in mammalian retina (1,8–11).
The field of retinal regeneration from Müller
glia-derived progenitor cells in mammals has recently become a
promising area of interest (1,2,12).
Müller glia are the sole glial cells generated by multipotential
retinal progenitors. In several vertebrate classes, in response to
acute retinal injury, Müller glial cells are able to
dedifferentiate, proliferate and acquire a progenitor-like state
(1,8,9). In
the retina of zebrafish, it has been reported that when retinal
neurons are destroyed, appropriate replacements for these missing
retinal cells are produced via progenitor cells derived from Müller
glia (10,11). A previous study demonstrated that
functional regeneration of the zebra fish retina is also achieved
through progenitors derived from Müller glia (11). These progenitor cells cycle
rapidly, progressing through the radial process of daughter Müller
glial cells to reach the outer nuclear layer (ONL), where they exit
the cell cycle and begin to differentiate into rod photoreceptors.
In N-methyl-D-aspartate-treated chicken retina, Müller glial cells
in the central retina re-enter the cell cycle, dedifferentiate,
acquire progenitor-like phenotypes and produce new neurons
(1). Our previous studies have
revealed that acute retinal degeneration induced by a
N-methyl-N-nitrosourea (MNU)-injection promoted the
transdifferentiation of Müller glial cells into photoreceptors
(13,14). The results from these previous
studies suggested that Müller glial cells may serve as a potential
source of neural regeneration in the mammalian retina; however, the
regenerative ability varies greatly across different species, with
warm-blooded species exhibiting the lowest regenerative ability
(1,3,8–10).
Therefore, a number of different methodologies have been evaluated
for the regulation of the endogenous repair capacity of retinal
Müller glial cells in warm-blooded vertebrates, amongst which the
sonic hedgehog (SHH) signaling pathway is considered one of the
most important (13,15–18).
A previous study involving zebrafish suggested that
SHH signaling is required for the proliferation and survival of
retinal progenitor cells (19).
Similarly, retinal marginal progenitors also depend on SHH as a
mitogen in chicks and in mice (7,20,21).
Our previous study also revealed that SHH was crucially involved in
stimulating the proliferation of Müller glial cells in
vitro, and exogenous SHH exposure exerted proliferative effects
on Müller glial cells in vivo following retinal injury
(14). In addition, SHH-treated
cells were shifted to neural lineage by expressing neuron-specific
class III β-tubulin (Tuj1), directing cell fate to rod cells
(14).
Although the activity of a commercially available
SHH was improved through a mutation at the amino (N)-terminus, as a
protein, the activity remains variable. Purmorphamine is a small
molecule that activates SHH signaling, potentially through
Smoothened (22). Therefore, the
present study investigated whether SHH may be replaced by
purmorphamine in the transdifferentiation of Müller glial cells to
retinal neurons, and thus, attempted to provide a more convenient,
effective and stabilized therapy.
Materials and methods
Ethical statement
The present study was approved by the Ethics
Committee of Fudan University (Shanghai, China). The protocol
involving the use of animals adhered to Statement for the Use of
Animals published by the Association for Research in Vision and
Ophthalmology (23), and the
experiments were conducted in accordance with Shanghai Experimental
Aanimal Management Method and Fudan University Guide for the Care
and Use of Laboratory Animals (24,25).
Müller glial cell culture
Primary cultures of retinal Müller glial cells were
prepared as previously described (14). Briefly, the eyes from postnatal day
7 Sprague-Dawley rats (5 rats each time, male, weighing ~20 g,
supplied by Department of Laboratory Animal Science of Fudan
University) were enucleated under sterile conditions. The retinal
tissues were then digested in 0.25% trypsin and 0.1% type I
collagenase at 37°C for 5 min. Dissociated retinal cells were
plated onto tissue culture dishes in monolayer-culture medium,
which was composed of Dulbecco's modified Eagle's medium/F12
supplemented with N-2 Supplement, 2 mM glutamine, 0.1%
penicillin-streptomycin and 10% fetal bovine serum (all purchased
from Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
and the plates were incubated at 37°C in humidified atmosphere
containing 5% CO2. The culture medium was changed every
2 days. A further purified flat cell population was obtained after
3 passages.
Cell transdifferentiation
methodology
To examine the regenerative potential of Müller
glial cells, 1×104 cells/ml were plated on poly-D-lysine
(500 µg/ml) and laminin (5 µg/ml) coated glass coverslips. To
measure the effects on proliferation, the 20 kDa N-terminal
signaling domain of SHH (SHH-N; 10 or 20 nM; R&D Systems, Inc.,
Minneapolis, MN, USA) and purmorphamine (0.1 or 0.5 µM;
Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) were added to the
culture medium, with or without cyclopamine (10 µg/ml;
Sigma-Aldrich, Merck KGaA) on the first day of culture and
maintained at the same concentration throughout the 2-day culture
period. A total of 7 treatment groups were established: i) 10 nM
SHH-N; ii) 20 nM SHH-N; iii) 0.1 µM purmorphamine; iv) 0.5 µM
purmorphamine; v) 20 nM SHH-N + 10 µg/ml cyclopamine; vi) 0.5 µM
purmorphamine + 10 µg/ml cyclopamine; and vii) the control group
(culture medium only). In addition, Dickkopf-related 1 (DKK1, 0.1
µg/ml; R&D Systems, Inc.) was added to purmorphamine-stimulated
Müller glial cells to determine whether the Wnt pathway was
involved. Following 2 days of culture, cells on the coverslips were
fixed in 4% paraformaldehyde at 4°C for 10 min and processed for
immunocytochemistry to detect proliferation-associated markers.
Progenitor cell markers were evaluated following 7 days of
treatment with purmorphamine or SHH-N. Cell proliferation was
examined by adding 5-bromo-2′-deoxyuridine (BrdU, 10 µM; R&D
Systems, Inc.) to the culture medium during the final 18 h of this
7-day treatment. Subsequently, the cells were transferred to fresh
culture medium, without purmorphamine or SHH-N, for a further 2
days to investigate Müller glia-derived cell differentiation.
Intravitreal injection
Photoreceptor apoptosis was induced in Sprague
Dawley® rats (male, aged 8–10 weeks, 300 g, 7 rats per
group, repeated 3 times, supplied by Department of Laboratory
Animal Science of Fudan University) by a single intraperitoneal
injections of 60 mg/kg MNU (Sigma-Aldrich, Merck KGaA). All the
animals were kept in an air-conditioned room at 22±2°C and 60±10%
relative humidity under a 12:12 h light/dark cycle (lights on at 7
am), food and water were available ad libitum. The animals
were treated according to the Fudan University Guide for the Care
and Use of Laboratory Animals.
Immediately following MNU administration, the left
eyes (control group) were intravitreally injected with sterile
saline and the right eyes (treatment group) were injected with
purmorphamine (260 ng) or SHH-N (400 ng), with or without
cyclopamine (5,000 ng); all injection volumes were 5 µl and the
rats received daily injections for 7 consecutive days. Rats were
then sacrificed with 10% chloral hydrate on day 1, 3, 7 or 15.
Enucleated eyes were harvested and fixed in 4% paraformaldehyde at
4°C overnight. The cryostat section (10 µm) were produced as
previously described (13).
Immunofluorescence analysis
Immunofluorescence was performed as previously
described (13) using the
following primary antibodies: Mouse anti-proliferating cell nuclear
antigen (cat. no. 2586S; PCNA; 1:1,000; 4°C overnight; Cell
Signaling Technology, Inc., Danvers, MA, USA); rabbit
anti-glutamine synthetase (cat. no. G2781; GS; 1:2,000; 4°C
overnight; Sigma-Aldrich, Merck KGaA); mouse anti-vimentin (cat.
no. V6630; 1:20; 4°C for 48 h; Sigma-Aldrich, Merck KGaA); rabbit
anti-vimentin (cat. no. 2707-1; 1:200; 4°C overnight; Epitomics,
Inc.; Abcam, Cambridge, MA, USA); mouse anti-nestin (cat. no.
MAB353B; 1:500; 4°C overnight; EMD Millipore, Billerica, MA, USA);
mouse anti-paired box protein 6 (cat. no. AB 528427; Pax6; 1:500;
4°C for 24 h; Developmental Studies Hybridoma Bank, Iowa, IA, USA);
goat anti-sex determining region Y-box 2 (cat. no. AF2018; Sox2;
1:500; 4°C for 24 h; R&D Systems, Inc.); mouse anti-Tuj1 (cat.
no. T8328; 1:500; 4°C overnight; Sigma-Aldrich, Merck KGaA); mouse
anti-syntaxin1 (cat. no. S0664; 1:1,000, 4°C overnight;
Sigma-Aldrich, Merck KGaA); rabbit anti-calbindinD-28 K (cat. no.
AB1778; 1:500, 4°C overnight; EMD Millipore); mouse anti-protein
kinase Cα (cat. no. SAB4200739; PKCα, 1:500, 4°C overnight;
Sigma-Aldrich, Merck KGaA); mouse anti-thy1.1 (cat. no. M7898;
1:100, 4°C overnight; Sigma-Aldrich, Merck KGaA); rabbit anti-BrdU
(cat. no. ab152095; 1:200; 4°C overnight; Abcam); mouse
anti-rhodopsin (cat. no. R5403; 1:2,000; 4°C overnight;
Sigma-Aldrich, Merck KGaA); rabbit anti-phosphorylated (p)-histone
H3 (cat. no. 9701S; pHH3; 1:1,000; 4°C overnight; Cell Signaling
Technology, Inc.). Following extensive washing with PBS, cell
coverslips or cryosections were treated with a 1:1,000 dilution of
the following secondary antibodies from Invitrogen (Thermo Fisher
Scientific, Inc.) for 1 h at room temperature in dark: Alexa Fluor
488-conjugated donkey anti-mouse immunoglobulin (IgG) (cat. no.
A-21202); Alexa Fluor 488-conjugated donkey anti-rabbit IgG (cat.
no. A-21206); Alexa Fluor 594-conjugated donkey anti-mouse IgG
(cat. no. A-21203); Alexa Fluor 594-conjugated donkey anti-rabbit
IgG (cat. no. A-21207); Alexa Fluor 594-conjugated donkey anti-goat
IgG (cat. no. A-11058). DAPI was used for nuclear counterstaining
(room temperature, 10 min). The images of coverslips and sections
were captured using fluorescence microscopy and confocal
microscopy. Image J 1.50i software (National Institutes of Health,
Bethesda, MD, USA) was used to determine the number of stained
cells and the expression level of target proteins (measured by the
integrated optical density and the relative IOD ratios) in stained
sections. Cell counts were recorded from five coverslips in each
culture condition, and at least three random fields were selected
for cell counting. Five cryostat sections from each animal were
selected for analysis, and at least three different animals were
analyzed in each condition.
Semi-quantitative polymerase chain
reaction (PCR) analysis
Following treatment, total RNA was isolated from
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), which was followed by cDNA synthesis with AMV reverse
transcriptase and amplification with gene-specific forward and
reverse primers (Table I) using a
PCR kit (AMV) version 3.0 (Takara Biotechnology Co., Ltd., Dalian,
China), as previously described (13). 30 cycles consisted of 30 sec at
94°C, 30 sec at 55°C and 1 min at 72°C. PCR products were
visualized on a 2% agarose gel. The images were detected with
ethidium bromide staining using a Gel Doc™
XR+ system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). GAPDH mRNA has been chosen as the internal standard in this
study. Every experiment was repeated 3 times.
 | Table I.Polymerase chain reaction primer
sequences. |
Table I.
Polymerase chain reaction primer
sequences.
| Gene | Accession no. | Sequence
(5′→3′) |
|---|
| GAPDH | NM_017008 | F:
CTGCCCAGAACATCATCCCT |
|
|
| R:
TGAAGTCGCAGGAGACAACC |
| nrl | NM_001106036 | F:
GCCTGAGGTCCCTGGAATGAGTGT |
|
|
| R:
TAGTGTTTGGGGCGGGGAAGAT |
| crx | NM_021855 | F:
CCTCACTATTCGGTCAATGCC |
|
|
| R:
ATGTGCCTGCCTTCCTCTTC |
| smo | NM_012807 | F:
CACCTCCAGCGAGACCCTA |
|
|
| R:
AGCCTCCCACAATAAGCA |
| gli1 | NM_001191910 | F:
GTGGCAACAGGACGGAACTT |
|
|
| R:
CGACTGTGAGACCCTATACCC |
Western blot analysis
Following treatment, cell lysates were prepared for
western blot analysis as previously described (13). Briefly, the cells were lysed on ice
by sonication in radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Haimen, China). The lysate was
incubated on ice for 30 min, centrifuged at a speed of 12,000 × g
for 15 min. Then, the supernatant was collected, protein
concentrations were determined by the bicinchoninic acid method and
30 µg protein of each sample was separated by 15% SDS-PAGE and
transferred to 0.45 µm polyvinylidene difluoride membranes (EMD
Millipore). Membranes were blocked for 1 h at room temperature in
5% non-fat dry milk in TBS with 0.01% Tween. The primary antibodies
used for western blotting were as follows: Mouse anti-cyclinD1
(cat. no. 2926P; 1:1,000, 4°C overnight, Cell Signaling Technology,
Inc.); mouse anti-cyclinD3 (cat. no. 2936S; 1:1,000, 4°C overnight,
Cell Signaling Technology, Inc.); mouse anti-GAPDH (cat. no.
KC-5G4; 1:10,000, 4°C overnight, KangChen Bio-tech, Inc., Shanghai,
China). Horseradish peroxidase (HRP)-conjugated secondary
antibodies were also used (cat. no. PA1-28761; 1:3,000, room
temperature for 1 h, Thermo Fisher Scientific, Inc.).
Chemiluminescent immunoreactivity was executed by incubating the
blot with the Immobilon Western HRP substrate (EMD Millipore) for 5
min at room temperature and detected using a ChemiDoc XRS+ System
(Bio-Rad Laboratories, Inc.). All the detections were repeated at
least in 3 samples. Bands were analyzed using Gel-Pro Analyzer
software (version 4.0, Media Cybernetics, Inc., MD, USA).
Statistical analysis
Differences between different groups were compared
by one-way analysis of variance and Least Significant Difference
post hoc analysis with Matlab R2012b (The Mathworks, Inc., MA,
USA). Data were expressed as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Purmorphamine promotes the
proliferation of Müller glial cells
Following 2 weeks of culture, immunocytochemistry
revealed that cultured cells co-expressed the specific markers of
Müller glia, GS and vimentin (Fig.
1A). The purity of cells achieved was 96% in
fluorescence-activated cell sorting analysis as previously reported
(14). The Müller glial cells were
cultured for 2 days in the presence of different concentrations of
SHH-N or purmorphamine, with or without cyclopamine. The
immunocytochemistry analysis of PCNA was used to assay the
proportion of cells in proliferation. Compared with the untreated
control cells, the percentage of PCNA-positive Müller glia
increased significantly following treatment with the two SHH-N
concentrations and the 0.5 mM purmorphamine treatment, whereas
cyclopamine inhibited the production of PCNA-positive Müller glial
cells compared with treatment with purmorphamine alone
(representative immunostaining image are presented in Fig. 1B). In our previous study (14), the mitogenic effects of SHH was
demonstrated to be concentration dependent, and no significant
increase was identified at concentrations >20 nM. In the present
study, the mitogenic effect of 0.5 µM purmorphamine was similar to
that of 20 nM SHH-N (Fig. 1B and
C). Therefore, 0.5 µM purmorphamine and 20 nm SHH-N were used
in subsequent experiments. These data indicated that the
proliferation of Müller glial cells was promoted by purmorphamine
treatment as well as SHH-N.
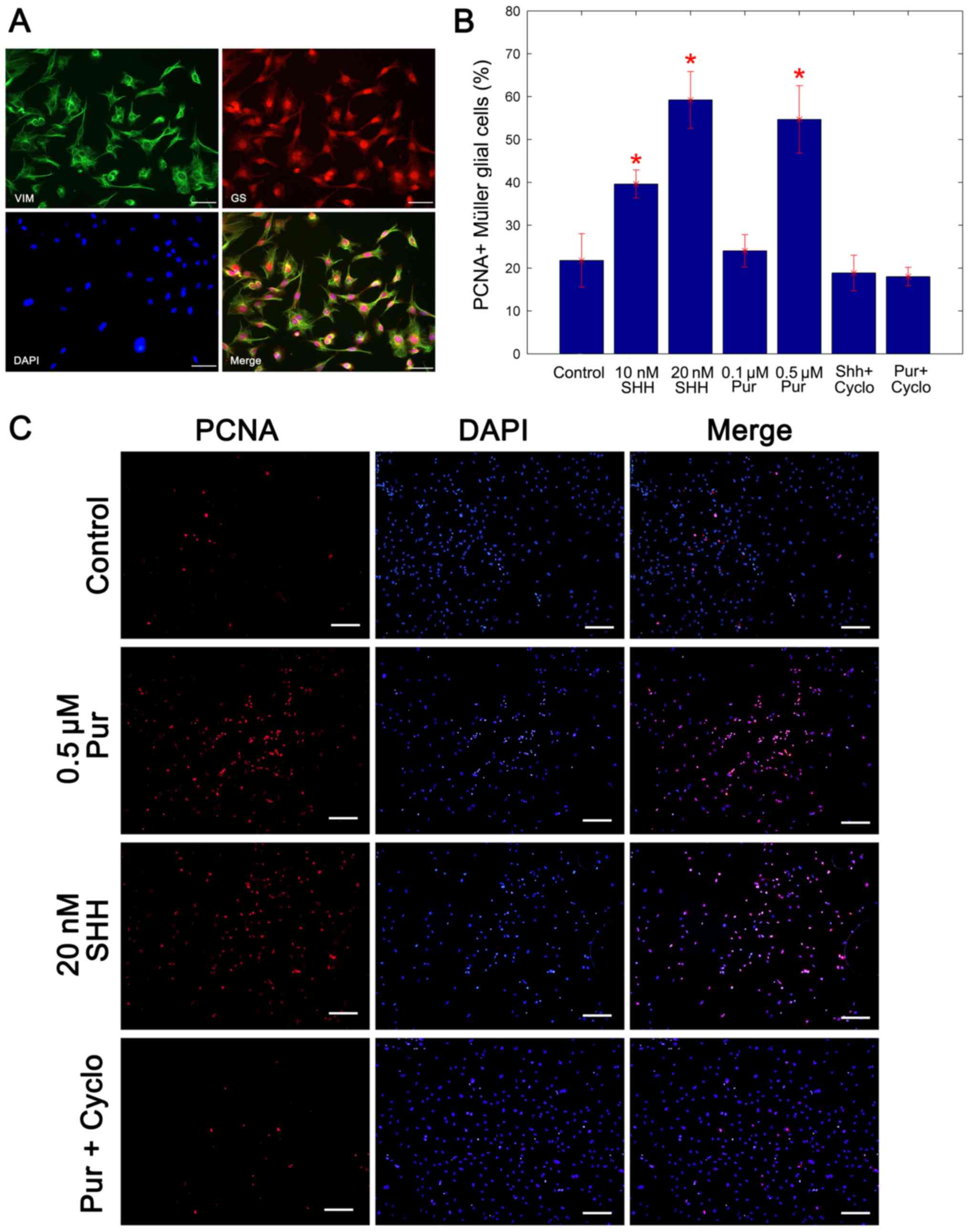 | Figure 1.Purmorphamine promotes the
proliferation of cultured Müller glial cells. (A) Double
immunocytochemistry showed that cells co-expressed specific markers
GS and Vimentin of Müller glial cells. Scale bar, 100 µm. (B)
Percentage of PCNA+ Müller glial cells was carried by a statistical
analysis. The mitogenic effect of 0.5 µM purmorphamine was
significantly greater than that the control and was similar to that
of the 20 nM SHH-N treatment. (C) Immunostaining was performed to
analyze the percentage of PCNA-positive cells. When compared with
the control, purmorphamine increased the percentage of
PCNA-positive cells, as did treatment with SHH-N. However,
co-treatment with purmorphamine and cyclopamine decreased the
percentage of PCNA-positive cells. Scale bar, 200 µm. *P<0.05
vs. control. Cyclo, cyclopamine; PCNA, proliferating cell nuclear
antigen; Pur, purmorphamine; SHH, sonic hedgehog; GS, glutamine
synthetase. |
Purmorphamine-treated Müller glial
cells express progenitor cell markers
To determine whether purmorphamine treatment
stimulated Müller glial cells to dedifferentiate into
progenitor-like cells, Müller glial cells were cultured in the
presence of 20 nM SHH-N or 0.5 mM purmorphamine for 7 days, with or
without cyclopamine. Purmorphamine treatment induced the expression
of the retinal progenitor cell marker Pax6 in 42.05±5.25% of cells
(Fig. 2A and B), the neurogenic
gene Sox2 in 36.08±4.8% of cells (Fig.
2A and C) and the neural precursor marker nestin in 62.44±6.63%
of cells (Fig. 2A and D). In
addition, the effects of purmorphamine were similar to those of
SHH-N (Fig. 2A). The expression of
these progenitor cell markers was markedly reduced when cells were
co-treated with purmorphamine and cyclopamine (Fig. 2A) (SHH with cyclopamine, data not
shown). These results suggested that purmorphamine may stimulate
the multi-potency of Müller glia.
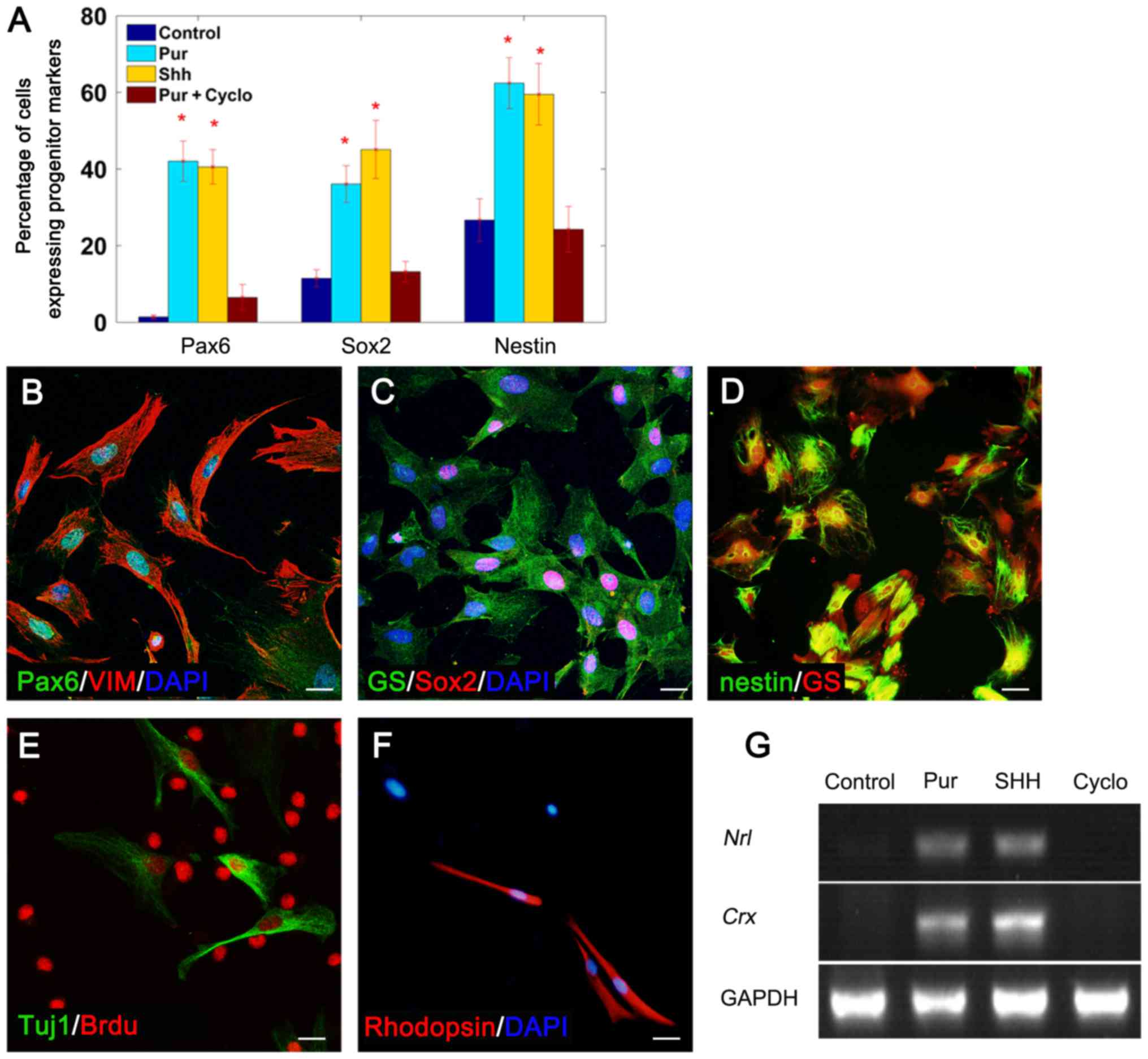 | Figure 2.Müller glial cells dedifferentiated
into progenitor cells and generated rod-like cells in the presence
of purmorphamine. (A) The percentage of cells expressing progenitor
cell markers under different treatment conditions. Double staining
revealed that purmorphamine treatment induced Müller glia (VIM- or
GS-positive) to express the progenitor cell markers (B) Pax6, (C)
Sox2 and (D) nestin. Following 2 days in fresh medium culture,
Müller glia-derived progenitor cells expressed (E) the neuron
marker Tuj1 and (F) the rod cell-specific marker rhodopsin. (G)
mRNA transcripts of rod development genes, nrl and
crx, were detected by semi-quantitative polymerase chain
reaction following treatment with purmorphamine or SHH-N; however,
no expression was detected in control cells or cells co-treated
with cyclopamine. *P<0.05 vs. control. Scale bars, 30 µm.
SHH+cyclo, data not shown. BrdU, 5-bromo-2′-deoxy uridine; cyclo,
cyclopamine; crx, cone-rod homeobox; GS, glutamine
synthetase; nrl, neural retina-specific leucine zipper;
Pax6, paired box protein-6; Pur, purmorphamine; SHH, sonic
hedgehog; Sox2, sex determining region Y-box 2; Tuj1, specific
class III β-tubulin; VIM, vimentin. |
Müller glia-derived cells express Tuj1
and rhodopsin following purmorphamine treatment
The regenerative potential of purmorphamine-treated
Müller glia was further investigated. Cells were cultured in
purmorphamine with or without cyclopamine for 7 days, and then
grown in fresh medium without purmorphamine for a further 2 days.
Following this, cells were collected for immunofluorescence
staining and PCR analysis. Immunofluorescence indicated that Müller
glia-derived cells expressed the neuronal marker Tuj-1 (Fig. 2E) and the rod cell-specific marker
rhodopsin (Fig. 2F), which
suggested that they have altered their neuronal lineage and have
obtained the ability to generate retinal neurons. The PCR results
demonstrated that purmorphamine induced Müller glial cells to
express neural retina-specific leucine zipper (nrl) and
cone-rod homeobox (crx; Fig.
2G), which have been reported to participate in the generation
of rod cells and the regulation of rhodopsin transcription
(26). To examine whether Müller
glia-derived cells differentiated into other retinal cell types,
specific antibodies for retinal neurons (PKCα for bipolar,
calbindin for horizontal, syntaxin1 for amacrine and Thy1.1 for
ganglion cells) were used; however, expression of these markers was
not detected. These data revealed that purmorphamine treatment
promoted Müller glial cells to transdifferentiate in the direction
of retinal neurons.
Purmorphamine promotes retinal
regeneration following photoreceptor degeneration
As aforementioned, purmorphamine stimulated the
proliferation and transdifferentiation of Müller glial cells in
vitro in a similar manner as SHH-N treatment. The present study
subsequently evaluated whether purmorphamine treatment was able to
control the proliferative and regenerative competence of these
cells in vivo. Intraocular injections of purmorphamine or
SHH-N with or without cyclopamine were administered to MNU-exposed
rats; the control retinas were treated with physiological saline.
The proliferative ability of cells was assessed by pHH3 and
vimentin double-immunofluorescence staining on day 3 (Fig. 3A-D). The results demonstrated that
MNU-induced degeneration of photoreceptors stimulated Müller glia
re-entry into the cell cycle in control retina, and there were
62.33±6.67 pHH3+ cells/section distributed in the inner
nuclear layer, 3 days following the injection of MNU (Fig. 3A and E). Treatment with
purmorphamine or SHH-N for 3 days, increased the number of
proliferating cells detected, with 106.22±7.22 pHH3+
cells/section (Fig. 3B and E) and
101.44±11.91 pHH3+cells/section (Fig. 3C and E), respectively. However,
proliferation decreased to only 28.78±7.63 pHH3+
cells/section in retinas co-treated with purmorphamine and
cyclopamine (Fig. 3D and E) (SHH +
cyclopamine, data not shown).
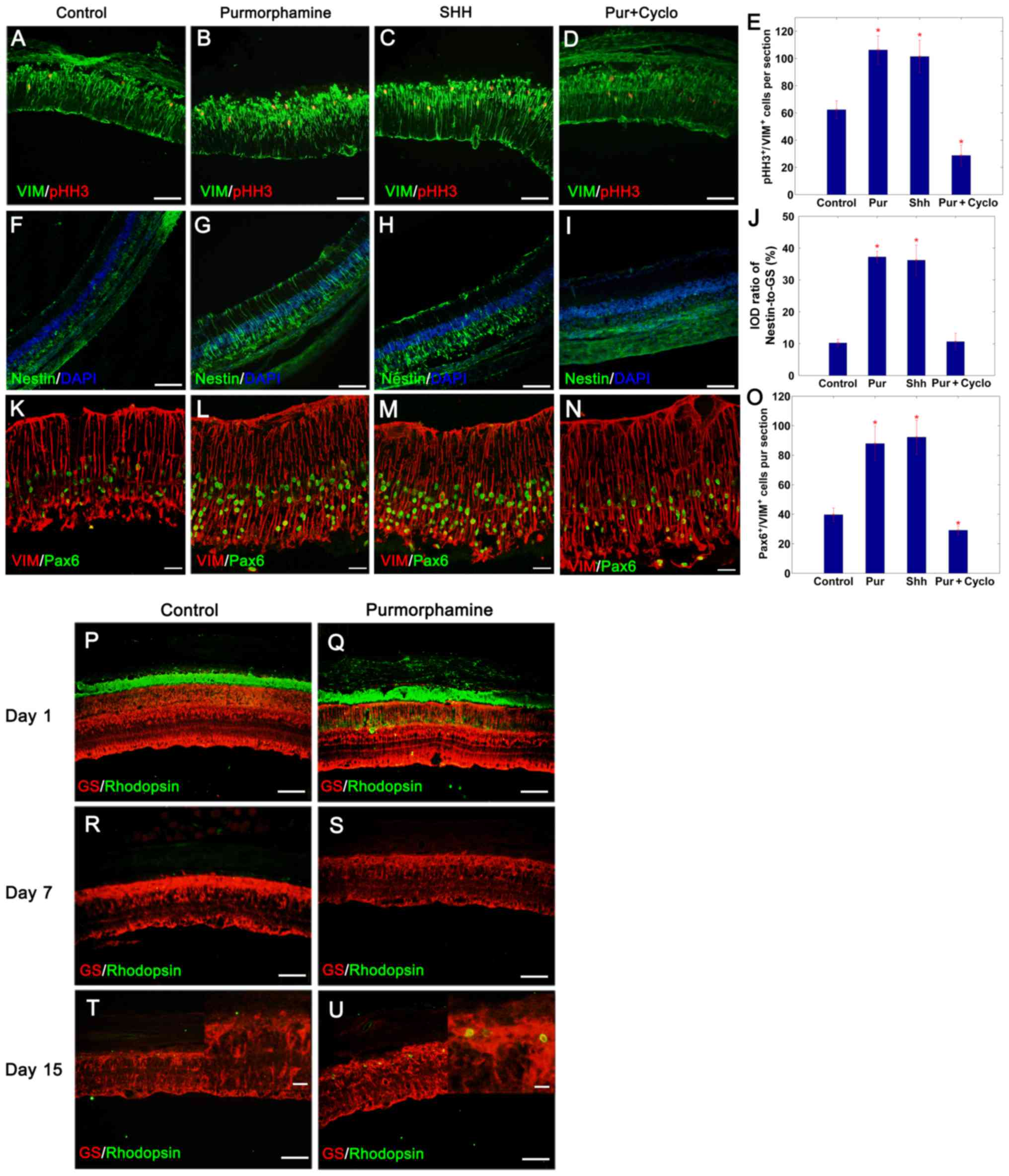 | Figure 3.Purmorphamine promotes retinal
regeneration in photoreceptor damaged retina. Compared with the (A)
control, the percentage of pHH3+/VIM+ Müller
glia increased significantly following an intravitreal injection of
(B) purmorphamine or (C) SHH-N on day 3. This mitogenic effect was
inhibited in purmorphamine + cyclopamine treated Müller glial cells
(D) SHH + cyclopamine, data not shown. (E) pHH3-labeling images
were analyzed to identify the number of pHH3/vimentin-positive
cells. (F-I) Following retinal damage, Müller glia dedifferentiated
into progenitor cells by expressing nestin. (J) The IOD ratio of
nestin/GS in purmorphamine-treated retina was significantly higher
than that exhibited by control retina (images not available, only
the graph is displayed). (K-N) Following retinal damage, Müller
glia dedifferentiated into progenitor cells by expressing Pax6. (O)
In addition, the number of Pax6+/VIM+ cells
was significantly greater compared with the control; the levels
were also similar to those produced by SHH treatment. (P and Q)
Photoreceptor degeneration was induced by MNU injection. With the
progression of photoreceptor degeneration, the expression of
rhodopsin completely disappeared on day 7 (R and S), with and
without purmorphamine. However, there were more rhodopsin-positive
cells produced with the purmorphamine injection, when compared with
the control retina on day 15 (T and U). *P<0.05 vs. control.
Scale bars: A-D, F-I and P-S, 100 µm; K-N, 30 µm; the magnified
images in (T) and (U), 10 µm. Cyclo, cyclopamine; GS, glutamine
synthetase; IOD, integrated optical density; Pax6, paired box
protein-6; pHH3, phosphorylated-histone H3; Pur, purmorphamine;
SHH, sonic hedgehog; VIM, vimentin. |
To verify the capacity of Müller glial cells to
generate neurons, the expression of progenitor cell markers nestin
and Pax6 were detected on day 3 (Fig.
3F-J and K-O, respectively). The expression of nestin increased
following purmorphamine treatment (Fig. 3G and J) as observed via IOD
analysis. The IOD ratio of nestin/GS was 37.23±1.80% in
purmorphamine-treated retina (Fig. 3G
and J) and 10.22±1.15% in the control retina (Fig. 3F and J). Following retinal injury,
39.67±4.56 cells/section were double positive for Pax6 and vimentin
in the control group (Fig. 3K and
O). This population increased following the administration of
purmorphamine or SHH-N, with 87.89±11.82 cells/section (Fig. 3L and O) and 92.22±11.54
cells/section (Fig. 3M and O),
respectively. It was concluded that purmorphamine was able to
stimulate the proliferation of Müller glia-derived progenitors
following retinal injury similar to SHH.
To observe the effects of purmorphamine on the fate
of Müller glia-derived progenitors, rats were injected daily for 7
consecutive days immediately following the application of MNU, and
were sacrificed on day 1, 7 and 15. Compared with the retina on day
1 (Fig. 3P and Q),
rhodopsin-positive cells were not detected when the ONL disappeared
on day 7 following MNU-treatment (Fig.
3R and S). However, rhodopsin-positive rod-like cells
reappeared in purmorphamine-treated retina on day 15 compared to
control retina (Fig. 3T and
U).
Proliferation of Müller glial cells is
induced by purmorphamine through cyclin D1 and cyclin D3
It has been previously reported that the
proliferation of Müller glia is induced via the cyclin D1 and D3
associated pathway, and that SHH promoted this effect (13). To determine whether the effects of
purmorphamine are implemented through cyclin D1 and cyclin D3,
western blotting was performed 3 days following the addition of
purmorphamine or SHH-N, to evaluate the expression of cyclin D1 and
D3. The results revealed that the expression of these two proteins
was increased in purmorphamine and SHH-stimulated Müller glial
cells (Fig. 4A). Semi-quantitative
PCR analysis revealed that cultured Müller glial cells expressed
the SHH target genes smoothened homolog precursor (smo) and
GLI family zinc-finger 1 (gli1), and the levels of these
mRNA transcripts increased with the addition of purmorphamine and
decreased in cells co-treated with cyclopamine (Fig. 4B) (SHH + cyclopamine, data not
shown). It was concluded that purmorphamine promotes the
proliferation of Müller glial cells by activating the SHH-pathway.
Cyclin D1 and cyclin D3 expression levels have been demonstrated to
be increased by Wnt signaling pathway activation (16), and SHH activates the Wnt signaling
pathway by regulating the expression of E-cadherin (27,28).
Thus, the Wnt signaling pathway inhibitor, DKK1, was added to
purmorphamine-stimulated Müller glial cells. The proliferative
ability of Müller glial cells was not affected by the presence of
DKK1 (Fig. 4C and D). The data
indicated that purmorphamine preferentially induced the expression
of cyclin D1 and cyclin D3 through the activation of smo and
gli1, which in turn promoted the proliferation of Müller
glial cells.
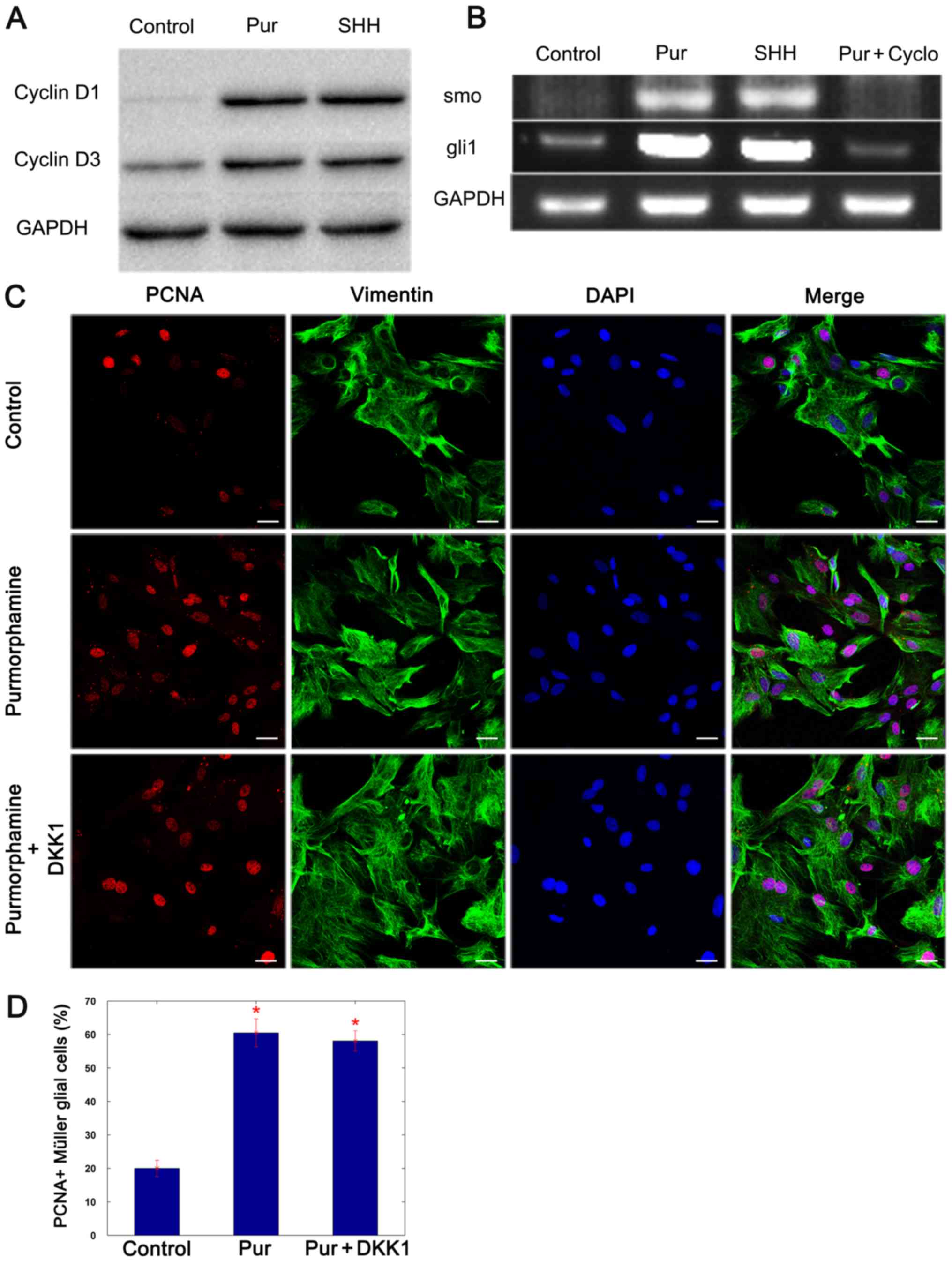 | Figure 4.Purmorphamine increases the
expression of cyclin D1 and cyclin D3 proteins by preferentially
activating the SHH signaling pathway. (A) Western blot analysis
revealed that the protein expression levels of cyclin D1 and cyclin
D3 were increased 3 days following treatment with purmorphamine or
SHH-N compared with control. (B) Semi-quantitative polymerase chain
reaction demonstrated that the expression of smo and
gli1 mRNA increased when cells were treated with
purmorphamine or SHH-N; however, the level of the transcripts
decreased in the presence of cyclopamine. (C and D) Double staining
of PCNA and vimentin indicated that the proliferation of Müller
glial cells was induced by purmorphamine; however, it was not
affected by treatment with the Wnt signaling pathway inhibitor
DKK1. *P<0.05 vs. control. Scale bars, 30 µm. Cyclo,
cyclopamine; DKK1, Dickkopf-related 1; gli1, GLI family
zinc-finger 1; PCNA, proliferating cell nuclear antigen; Pur,
purmorphamine; SHH, sonic hedgehog; smo, smoothened homolog
precursor. |
Discussion
In the past decade, there has been a paradigm shift
in our understanding of glial cells during the development and
regeneration of central nervous system (CNS). Glial cells provide
homeostatic support and serve as a source of stem cells in the
embryonic brain and the adult subventricular zone (SVZ) and
subgranular layer (SGL) (5,12);
however, active neurogenesis has not been detected in the adult
mammalian retina (7). By contrast,
neural regeneration has been observed in the injured retina, and
the source of injury-induced neurogenesis has been traced to Müller
glia (8,15,29).
In addition, the application of SHH was able to facilitate the
process (13,14,30,31).
The results of the present study demonstrated that the application
of purmorphamine, an agonist of the SHH signaling pathway, promoted
Müller glia proliferation, dedifferentiation into progenitor cells
and the production of retinal neurons in vitro. The
proliferating cells co-expressed GS/vimentin and various progenitor
cell markers, including Pax6, Sox2 and nestin, alluding to their
progenitor nature. Intraocular injections of purmorphamine also
promoted retinal regeneration through Müller glia as demonstrated
by the increased number of proliferating Müller glial cells and the
re-expression of rhodopsin.
The response of Müller glia to disease and injury
includes the upregulated expression of glial fibrillary acidic
protein, an intermediate filament protein, cell hypertrophy and
proliferation, which is common to glia in the CNS, collectively
known as reactive gliosis (32).
Not all injuries and diseases lead to the proliferation of retinal
Müller glia. Rapid retinal degeneration typically leads to Müller
glia proliferation; however, in slow progressing retinal
degeneration, such as that exhibited by Borna disease
virus-infected and Royal College of Surgeons (RCS) rats, retinal
gliosis continues to be nonproliferative (32,33).
Given that the initial stages of reactive gliosis have been
observed to be neuroprotective and regenerative (32), extended and excessive proliferation
may serve as a barrier towards regeneration just as it does in the
CNS. The rapid downregulation of cyclin D1 and cyclin D3 expression
was previously reported to prevent uncontrolled proliferation of
Müller glia, and resulted in cells exiting mitosis and initiating
differentiation (34). In the
present study, there was ~2.5 times increase in mitotic Müller
glial cells which were induced by purmorphamine, when compared with
the control (Fig. 1B). However,
the period of proliferation was not prolonged, and the number of
dividing cells reached a peak on day 3, which then decreased on day
7 in the same manner as the controls (data not shown). These
results suggested that the application of purmorphamine may not
cause excessive proliferation, thus, the formation of glial scar
tissue may be avoided.
The importance of SHH has been demonstrated in the
maintenance of stem cells and progenitors in the CNS (35). SHH was reported to stimulate the
formation of proliferating Müller glia-derived progenitor cells in
chick and rat retina (13,14,36).
However, owing to the variable activity and the high cost of
megadose utilization, particularly for long-term treatment, the
application of recombinant SHH may be limited. Cell-permeable small
molecules may be a potential solution as they have similar
biological functions, stable activity and are available at a low
price (37). One such molecule is
purmorphamine, which has been demonstrated previously to activate
gli1 expression, a downstream target of the SHH signaling
pathway (22,38). The present study demonstrated that
purmorphamine promoted the proliferation and dedifferentiation of
Müller glia, and facilitated its fate shift to a neuronal lineage,
in the same manner as SHH treatment does. The use of purmorphamine
achieves efficient transdifferentiation, decreases the cost and
also makes in situ regeneration feasible as it has a stable
chemical nature and has an easy preparation procedure.
Extrinsic and intrinsic factors participate in the
activation process associated with Müller glial cells in retinal
regeneration, including SHH (14),
Notch (15), Wnt (16,17)
and fibroblast growth factor (18)
signaling pathways. Activation of these pathways, due to injury or
exogenous ligands, induces a subset of Müller glial cells into the
G1-S phase of the cell cycle (6,39).
Our previous study indicated that SHH may promote the proliferation
of Müller glial cells by increasing the expression of cyclin D1 and
cyclin D3 (14). It has been
reported that cyclin D1 expression may be increased by Wnt
signaling pathway activation (16); SHH is also a crucial signaling
pathway, which mostly functions with other morphogens, Wnts in
particular, rather than alone (40–42).
Therefore, in the present study, the Wnt signaling pathway
inhibitor DKK1 was applied to the culture medium. Purmorphamine
treatment activated the SHH signaling pathway, and increased the
levels of mRNA for gli1 and smo, and the protein
expression of cyclin D1 and cyclin D3, even in the presence of
DKK1. As DKK1 was an inhibitor of the Wnt signaling pathway, these
results suggested that purmorphamine may not function through the
Wnt pathway but induce the proliferation of Müller glial cells by
directly upregulating the expression of cyclin D1 and cyclin D3
which is similar to SHH. However, there is the possibility that
there may be other unknown pathways involved in this process, thus,
further research is required.
In conclusion, purmorphamine treatment efficiently
promoted the proliferation of Müller glial cells and the production
of photoreceptors, as an agonist of SHH. These results provide
evidence to support the possibility that small molecules may be
used as an alternative treatment strategy for the endogenous
regeneration of the retina via Müller glial cells. Further
investigations are required to focus on the efficient generation of
specific retinal neurons from Müller glia-derived cells to enhance
the potential of repair in the injured retina.
Acknowledgements
The present study was supported by The National
Natural Science Foundation of China (grant nos. 30971536 and
31571238).
References
|
1
|
Fischer AJ and Reh TA: Müller glia are a
potential source of neural regeneration in the postnatal chicken
retina. Nat Neurosci. 4:247–252. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hamon A, Roger JE, Yang XJ and Perron M:
Müller glial cell-dependent regeneration of the neural retina: An
overview across vertebrate model systems. Dev Dyn. 245:727–738.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brockerhoff SE and Fadool JM: Genetics of
photoreceptor degeneration and regeneration in zebrafish. Cell Mol
Life Sci. 68:651–659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang SZ and Yan RT: The retinal pigment
epithelium: A convenient source of new photoreceptor cells? J
Ophthalmic Vis Res. 9:83–93. 2014.PubMed/NCBI
|
|
5
|
Kriegstein A and Alvarez-Buylla A: The
glial nature of embryonic and adult neural stem cells. Annu Rev
Neurosci. 32:149–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilken MS and Reh TA: Retinal regeneration
in birds and mice. Curr Opin Genet Dev. 40:57–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moshiri A and Reh TA: Persistent
progenitors at the retinal margin of ptc+/- mice. J Neurosci.
24:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karl MO, Hayes S, Nelson BR, Tan K,
Buckingham B and Reh TA: Stimulation of neural regeneration in the
mouse retina. Proc Natl Acad Sci USA. 105:pp. 19508–19513. 2008;
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldman D: Müller glial cell reprogramming
and retina regeneration. Nat Rev Neurosci. 15:431–442. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lenkowski JR and Raymond PA: Muller glia:
Stem cells for generation and regeneration of retinal neurons in
teleost fish. Prog Retin Eye Res. 40:94–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raymond PA, Barthel LK, Bernardos RL and
Perkowski JJ: Molecular characterization of retinal stem cells and
their niches in adult zebrafish. BMC Dev Biol. 6:362006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahmad I, Del Debbio CB, Das AV and
Parameswaran S: Müller glia: A promising target for therapeutic
regeneration. Invest Ophthalmol Vis Sci. 52:5758–5764. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wan J, Zheng H, Chen ZL, Xiao HL, Shen ZJ
and Zhou GM: Preferential regeneration of photoreceptor from Müller
glia after retinal degeneration in adult rat. Vision Res.
48:223–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wan J, Zheng H, Xiao HL, She ZJ and Zhou
GM: Sonic hedgehog promotes stem-cell potential of Müller glia in
the mammalian retina. Biochem Biophys Res Commun. 363:347–354.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Del Debbio CB, Balasubramanian S,
Parameswaran S, Chaudhuri A, Qiu F and Ahmad I: Notch and Wnt
signaling mediated rod photoreceptor regeneration by Müller cells
in adult mammalian retina. PLoS One. 5:e124252010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Osakada F, Ooto S, Akagi T, Mandai M,
Akaike A and Takahashi M: Wnt signaling promotes regeneration in
the retina of adult mammals. J Neurosci. 27:4210–4219. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu B, Hunter DJ, Rooker S, Chan A, Paulus
YM, Leucht P, Nusse Y, Nomoto H and Helms JA: Wnt signaling
promotes Müller cell proliferation and survival after injury.
Invest Ophthalmol Vis Sci. 54:444–453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fischer AJ and Bongini R: Turning Müller
glia into neural progenitors in the retina. Mol Neurobiol.
42:199–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stenkamp DL, Frey RA, Prabhudesai SN and
Raymond PA: Function for hedgehog genes in zebrafish retinal
development. Dev Biol. 220:238–252. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moshiri A, McGuire CR and Reh TA: Sonic
hedgehog regulates proliferation of the retinal ciliary marginal
zone in posthatch chicks. Dev Dyn. 233:66–75. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jensen AM and Wallace VA: Expression of
sonic hedgehog and its putative role as a precursor cell mitogen in
the developing mouse retina. Development. 124:363–371.
1997.PubMed/NCBI
|
|
22
|
Sinha S and Chen JK: Purmorphamine
activates the Hedgehog pathway by targeting Smoothened. Nat Chem
Biol. 2:29–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
ARVO: Toolkit for Biomedical Researchers
Using Laboratory Animals. http://www.arvo.org/Journals_and_Publications/Toolkit_for_Biomedical_Researchers_Using_Laboratory_AnimalsNov
17–2014
|
|
24
|
Shanghai Experimental Animal Management
Method. http://www.shanghai.gov.cn/nw2/nw2314/nw2319/nw2407/nw26170/u26aw27198.html
|
|
25
|
Fudan University Guide for the Care and
Use of Laboratory Animals. http://lsem.fudan.edu.cn/wz/websit/article.jsp
|
|
26
|
Ohsawa R and Kageyama R: Regulation of
retinal cell fate specification by multiple transcription factors.
Brain Res. 1192:90–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao C, Ogle SA, Schumacher MA, Orr-Asman
MA, Miller ML, Lertkowit N, Varro A, Hollande F and Zavros Y: Loss
of parietal cell expression of Sonic hedgehog induces
hyperproliferation of surface mucous cells. Gastroenterology.
138:550–561, 561.e1-e8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao C, Ogle SA, Schumacher MA, Schilling
N, Tokhunts RA, Orr-Asman MA, Miller ML, Robbins DJ, Hollande F and
Zavros Y: Hedgehog signaling regulates E-cadherin expression for
the maintenance of the actin cytoskeleton and tight junctions. Am J
Physiol Gastrointest Liver Physiol. 299:G1252–G1265. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ooto S, Akagi T, Kageyama R, Akita J,
Mandai M, Honda Y and Takahashi M: Potential for neural
regeneration after neurotoxic injury in the adult mammalian retina.
Proc Natl Acad Sci USA. 101:pp. 13654–13659. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Todd L and Fischer AJ: Hedgehog signaling
stimulates the formation of proliferating Muller glia-derived
progenitor cells in the chick retina. Development. 142:2610–2622.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferraro S, Gomez-Montalvo AI, Olmos R,
Ramirez M and Lamas M: Primary cilia in rat mature Muller glia:
Downregulation of IFT20 expression reduces sonic hedgehog-mediated
proliferation and dedifferentiation potential of Muller glia
primary cultures. Cell Mol Neurobiol. 35:533–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bringmann A, Iandiev I, Pannicke T, Wurm
A, Hollborn M, Wiedemann P, Osborne NN and Reichenbach A: Cellular
signaling and factors involved in Müller cell gliosis:
Neuroprotective and detrimental effects. Prog Retin Eye Res.
28:423–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iandiev I, Biedermann B, Bringmann A,
Reichel MB, Reichenbach A and Pannicke T: Atypical gliosis in
Müller cells of the slowly degenerating rds mutant mouse retina.
Exp Eye Res. 82:449–457. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dyer MA and Cepko CL: Control of Muller
glial cell proliferation and activation following retinal injury.
Nat Neurosci. 3:873–880. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fuccillo M, Joyner AL and Fishell G:
Morphogen to mitogen: The multiple roles of hedgehog signalling in
vertebrate neural development. Nat Rev Neurosci. 7:772–783. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Todd L and Fischer AJ: Hedgehog signaling
stimulates the formation of proliferating Müller glia-derived
progenitor cells in the chick retina. Development. 142:2610–2622.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding S and Schultz PG: A role for
chemistry in stem cell biology. Nat Biotechnol. 22:833–840. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chechneva OV, Mayrhofer F, Daugherty DJ,
Krishnamurty RG, Bannerman P, Pleasure DE and Deng W: A Smoothened
receptor agonist is neuroprotective and promotes regeneration after
ischemic brain injury. Cell Death Dis. 5:e14812014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xia X and Ahmad I: Unlocking the
neurogenic potential of mammalian Muller glia. Int J Stem Cells.
9:169–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aviles EC, Wilson NH and Stoeckli ET:
Sonic hedgehog and Wnt: Antagonists in morphogenesis but
collaborators in axon guidance. Front Cell Neurosci. 7:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wilson NH and Stoeckli ET: Sonic Hedgehog
regulates Wnt activity during neural circuit formation. Vitam Horm.
88:173–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang M, Villaescusa JC, Luo SX, Guitarte
C, Lei S, Miyamoto Y, Taketo MM, Arenas E and Huang EJ:
Interactions of Wnt/beta-catenin signaling and sonic hedgehog
regulate the neurogenesis of ventral midbrain dopamine neurons. J
Neurosci. 30:9280–9291. 2010. View Article : Google Scholar : PubMed/NCBI
|


















