Introduction
Hepatocellular carcinoma (HCC) is one of the major
leading causes of tumor-associated deaths, with high rates of
incidence and disease-related mortality and morbidity in the world
(1). As it is still difficult to
make an early diagnosis for HCC, most of the patients are diagnosed
at advanced stages. Despite the improvement of conventional
therapies for HCC, including surgery, chemotherapy and
radiotherapy, the length or quality of life of patients with HCC is
still poor. Therefore, it is urgent to develop a new preventive
strategy for liver cancer.
Resveratrol (RES, trans-3,5,4′-trihydroxystilbene)
is a polyphenol compound derived from grapes, berries, peanuts and
other sources, and it has inhibitory effects on several types of
cancer cell lines such as colon, lung and prostate and affects
diverse molecular targets (2).
Sirtuin 1 (SIRT1) has been reported to be a key target of
resveratrol in several tumor models (3,4).
Whether SIRT1 as a tumor promoter or tumor suppressor remains
controversial, it might depend on tumor type (5). Resveratrol suppresses tumor cell
growth and metastasis in colorectal cancer cells by targeting SIRT1
protein and regulating NF-κB signaling pathway (6). The forkhead box O transcription
factors (FoxOs) have emerged as critical transcriptional factors in
regulating metabolism and stress responses and been considered as
downstream targets of SIRT1. FoxO1 translocated into nucleus
increases FoxO1-DNA binding and expression of proapoptotic gene Bim
(7). SIRT1 might regulate cell
apoptosis by deacetylating FoxOs protein.
The phosphatidylinositol 3′-kinase (PI3K)/AKT
pathway plays an important role in cell survival and PI3K activity
has been linked to a variety of human cancers (8). AKT, a downstream kinase of PI3K,
regulates many cellular proteins including metabolism, apoptosis
and proliferation (9). PI3K
pathway phosphorylates FoxOs via activation of its downstream
kinase AKT (10). Inhibition of
PI3K pathway leads to dephosphorylation and nuclear translocation
of active FoxOs, which induce cell cycle arrest and apoptosis
(11). These indicate that FoxOs
are important downstream effectors of PI3K/AKT pathway. Resveratrol
has been shown to inhibit activation of multiple survival pathways
including PI3K/AKT pathway to induce apoptosis in various cancer
cells (9,12).
Deleted in liver cancer 1 (DLC1), a focal adhesion
protein, is identified as a putative tumor suppressor in HCC in
1998 (13). It functions as a
RhoGTPase activating protein (RhoGAP) (14). Activated protein kinase C (PKC) and
protein kinase D (PKD) stimulate the association between DLC1 and
14-3-3 protein, which blocks DLC1 nucleocytoplasmic shuttling and
inhibits RhoGAP activity of DLC1 (15). DLC1 activity could be regulated by
post-translational modification and it might be a substrate of AKT.
Expression of DLC1 suppresses cell proliferation,
anchorage-independent growth, tumorigenicity and invasiveness in
HCC cells (16). DLC1 inhibits
Rho-dependent stress fiber formation in fibroblasts and serves as a
tumor suppressor gene in human non-small cell lung carcinomas
(17). Thus, we hypothesized that
AKT involved in regulation of DLC1 mediated cell motility
inhibition in HCC.
The purposes of the present study were to determine
the molecular mechanism of resveratrol affected proliferation and
migration through SIRT1 mediated post-translational modification of
PI3K/AKT signaling pathway in HCC cells.
Materials and methods
Cell culture
The human hepatocellular carcinoma (HCC) cell lines
Bel-7402, SMMC-7721, hepatoblastoma cells HepG2 (The HepG2 cell
line was originally thought to be a hepatocellular carcinoma cell
line but was later shown to be from an hepatoblastoma,
PubMed=19751877), and human liver normal cell line HL-7702 were
obtained from the Cell Bank of Type Culture Collection of Chinese
Academy of Science (Shanghai, China). HepG2 cells were cultured in
DMEM, other cells in RPMI 1640 medium. All the experiments were
performed in medium containing 10% fetal bovine serum, 100 U/ml
penicillin and 100 µg/ml streptomycin, maintained at 37°C in
humidified atmosphere with 5% CO2.
Proliferation assay by MTT and
EdU
MTT assay was used to assess cell viability. The
cells were seeded in 96-well plates at a density of
1×104/well overnight and treated without or with
resveratrol (Sigma, St. Louis, MO, USA) dissolved in 0.1% (v/v)
DMSO at various concentrations for 24 h. Then cells were incubated
with MTT solution for 4 h. The formazan crystals dissolved by 150
µl DMSO, the solution was absorbed at 492 nm using enzyme-linked
immunosorbent assay reader (Awareness, Palm City, FL, USA).
Cell proliferation was tested by EdU
(5-ethynyl-2-deoxyuridine) incorporation assay kit (Ribobio,
Guangzhou, China). Briefly, cells cultured in 96-well plates
exposed to 50 µM EdU for 2 h at 37°C, and fixed in 4% formaldehyde.
After permeabilization with 0.5% Triton-X, the cells were reacted
with 1xApollo reaction cocktail for 30 min, the DNA contents were
stained with Hoechst 33342 and visualized under fluorescent
microscope. Cells were counted in five selected arbitrarily fields,
at least 300 cells were counted per well. EdU positive cells were
calculated with (EdU incorporated-in cells/Hoechst stained cells)
×100%.
Apoptosis detection by TUNEL
assay
TUNEL staining was performed using an EdUTP TUNEL
cell detection kit (Ribobio, Gangzhou, China) according to the
manufacturer's instructions. The cells cultured in 96-well plates
were treated without or with 100 µM resveratrol, fixed in 4%
paraformaldehyde, permeabilized with 0.1% Triton X-100, washed
twice, incubated with TUNEL detecting liquid for 1 h at 37°C and
observed by a fluorescent microscope (Olympus, Tokyo, Japan) at 488
nm excitation and 530 nm emission. TUNEL positive cells were
calculated as the number of apoptotic cells/DAPI stained cells
×100%.
Western blotting and
Co-immunoprecipitation analysis
The cells were lysed by RIPA (Beyotime, Shanghai,
China). The inhibitor of SIRT1, EX-527 (Selleck Chemicals, Houston,
TX, USA), was used to PI3K/AKT pathway. Proteins were separated by
SDS-PAGE and transferred on membranes were incubated in primary
antibodies against SIRT1, p-AKT, AKT, p-PI3K, PI3K, PARP,
Cleaved-PARP, p-FoxO3a, Caspase-3/-7, Bax, Bcl-2 and p53 (CST,
Danvers, MA, USA) and FoxO1, FoxO3a (Santa Cruz Biotechnology, San
Diego, CA, USA) overnight at 4°C, followed by incubation with
HRP-conjugated rabbit/mouse secondary antibodies (ZSGB-BIO,
Beijing, China). Protein expressions were visualized ECL detection
system (Beyotime, Shanghai, China).
Immunoprecipitation (IP) was carried out using
Pierce Classic Magnetic IP/Co-IP Kit (Thermo Scientific, Waltham,
MA, USA) according to the manufacturer's protocol. The protein
lysates were incubated with DLC1 antibody (BD Biosciences, San
Jose, CA, USA), and precipitated with Protein A/G Magnetic Agarose
at 4°C. The immunocomplex collected was washed, and the
immunoprecipitates were subjected to western blotting and
phosphorylation signals were determined using phospho-AKT substrate
(PAS) antibody (CST, Danvers, MA, USA).
Wound healing assay
Cells were seeded into 24-well plates
(1.0×105 cells/well). Sterile pipette tip was used to
produce a wound line between cells after the cells grew to 80–90%
confluence and allowed the cells migrated for 24 h. Images were
captured and the relative distance traveled by the leading edge
from 0 to 24 h was assessed using Image Pro Plus 6.0 software
(n=5).
SIRT1 activity assays
SIRT1 activity was quantified with a SIRT1
Fluorometric Assay Kit (Sigma, St. Louis, MO, USA) according to the
manufacturer's protocol. Fluorescence intensities were measured
with a microplate fluorometer (excitation wavelength=360 nm,
emission wavelength=450 nm). Experimental values are represented as
a percentage of control.
Statistical analysis
All of the assays were performed three times
independently at least. Value presented as the means ± standard
deviation (SD) by GraphPad Prism software (GraphPad Software, CA,
USA). Statistical analyses were performed using one-way ANOVA and
Student's t-test, *P<0.05, **P<0.01 were considered to
indicate a statistically significant difference.
Results
Effect of resveratrol on cell
viability and proliferation
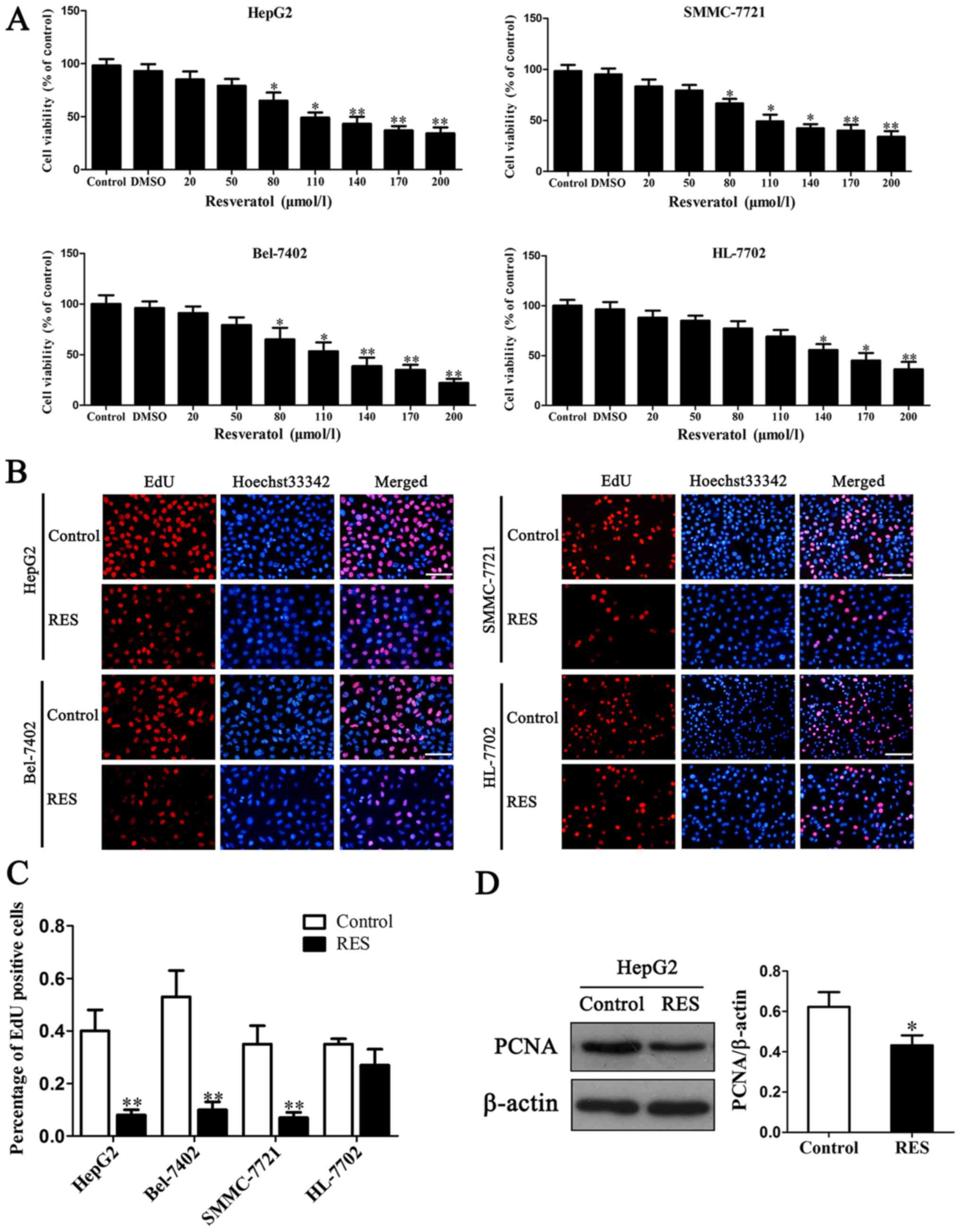
Three HCC cell viability was determined by MTT
assay. The results showed that resveratrol inhibited cell viability
when its concentrations were higher than 80 µM compared to normal
HL-7702 cell (Fig. 1A). From
80–200 µM, 100 µM resveratrol was selected as IC50 (half maximal
inhibitory concentration) in the subsequent experiments. It
implicated that resveratrol was able to reduce cancer cell
viability in a dose-dependent manner. The percentage of EdU
positive cells was markedly reduced in HCC cells with 100 µM
resveratrol treatment compared to the controls, while
non-tumorigenic cell line HL-7702 had a slight reduction (Fig. 1B and C).
To understand the molecular basis of proliferation
inhibition caused by resveratrol, proliferation regulation protein
PCNA (proliferating cell nuclear antigen) was evaluated in HepG2
cells. The level of PCNA reduced after 100 µM resveratrol treatment
(Fig. 1D). It was consistent with
above results of EdU assay. These findings demonstrate the
anti-proliferative effect of resveratrol on HCC cells.
Resveratrol induced apoptosis via
decreasing phosphorylation of FoxO3a with suppressing PI3K/AKT
pathway
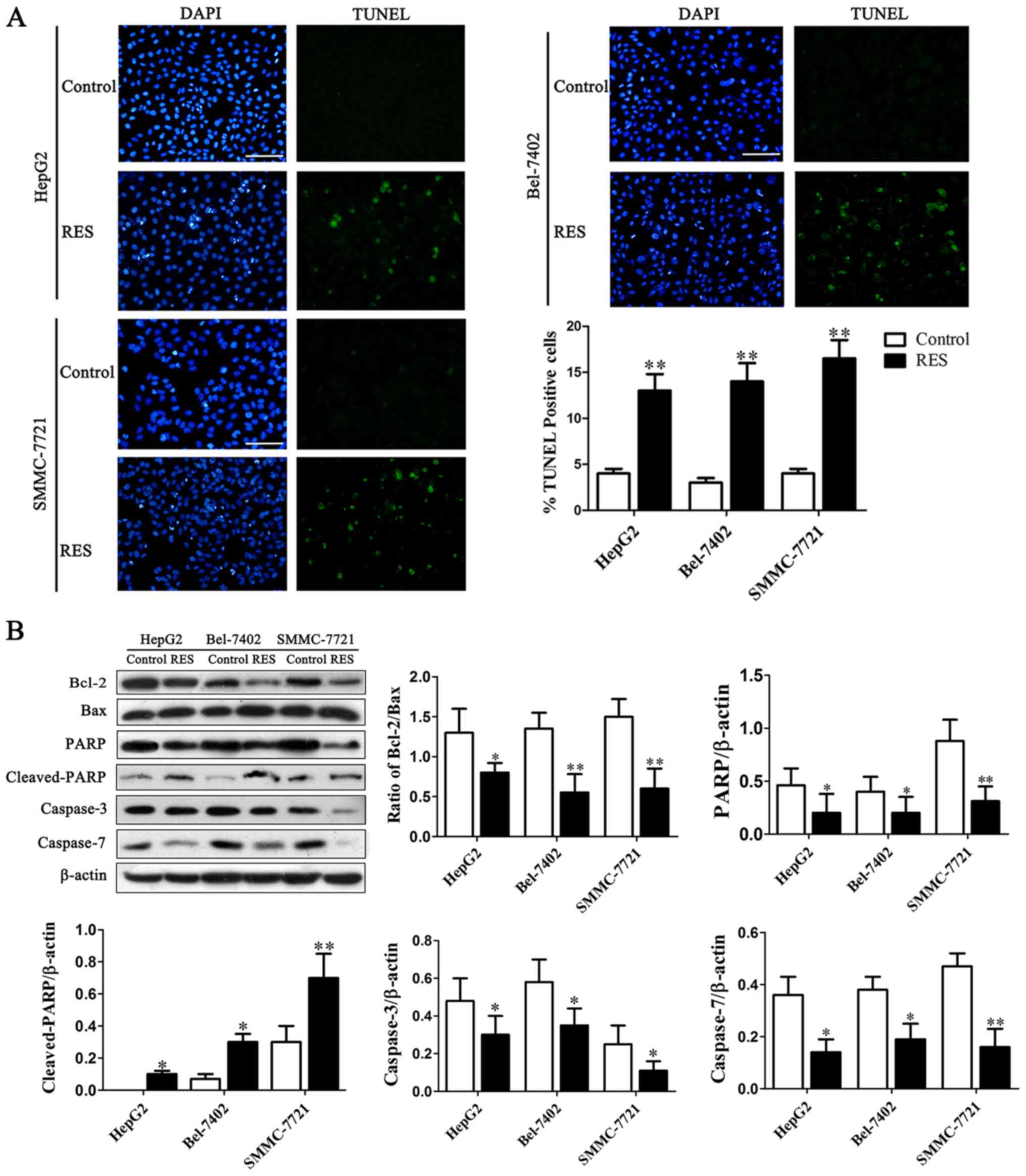
TUNEL assay was assessed whether anti-proliferative
effects of resveratrol against HCC cells are mediated via
apoptosis. Results showed that resveratrol increased apoptosis from
4±0.83% to 13±1.32%, 3±0.78% to 14±0.72%, 5±0.33% to 16±1.12% in
HepG2, Bel-7402 and SMMC-7721 cells (Fig. 2A).
Studies have reported that Bcl-2 could be a crucial
target gene of PI3K/AKT signaling, whereas AKT has been shown to
negatively regulate the activity of proapoptotic members of the
Bcl-2 family (8). Next effects of
resveratrol on apoptosis-related proteins were further detected. As
showed by Fig. 2B, resveratrol
inhibited Bcl-2 expression and concomitant up-regulated
proapoptotic protein Bax, causing a significant decrease in
Bcl-2/Bax ratio. The apoptosis regulators were further detected and
the precursor forms of caspase-3/7 induced by resveratrol were
down-regulated obviously in HCC cells (Fig. 2B). The activation of caspases were
also related to another marker of apoptosis, proteolysis of the DNA
repair enzyme PARP (18). The
results indicated that precursor form PARP decreased as active form
cleavage-PARP significantly enhanced (Fig. 2B).
Resveratrol activated SIRT1 and
inhibited SIRT1-mediated post-translational modification of
PI3K/AKT signaling
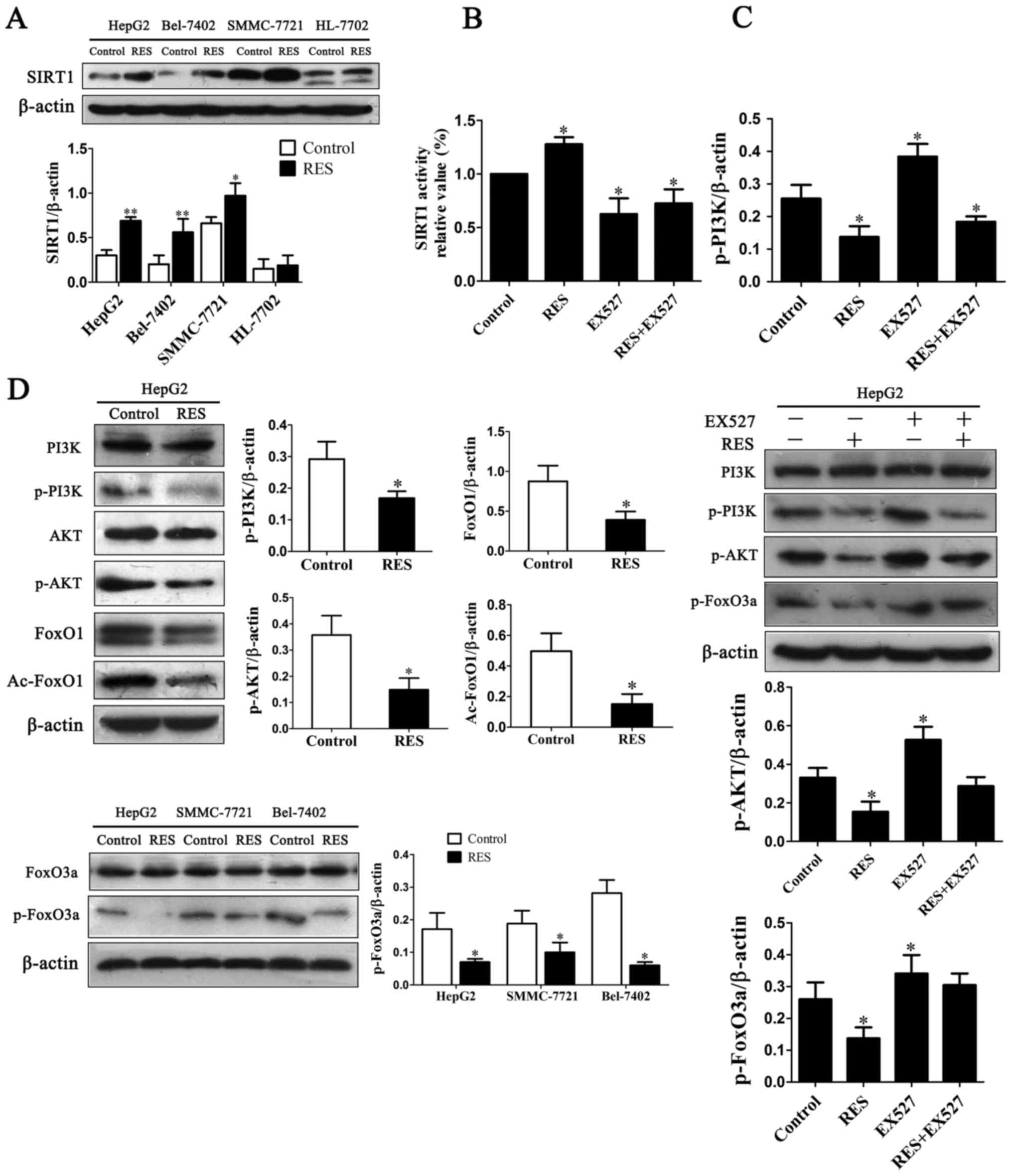
To investigate effects of resveratrol on the pathway
of PI3K/AKT/FoxO3a to induce cell apoptosis, western blotting was
performed for their phosphorylation levels. Resveratrol inhibited
phosphorylation of PI3K and AKT without effect on total levels of
PI3K and AKT in HepG2 cells (Fig.
3D), and inhibited FoxO3a phosphorylation in HCC cells with no
total FoxO3a change (Fig. 3D).
These data indicated that resveratrol down-regulated p-FoxO3a level
with reduction of phosphorylation level of PI3K/AKT.
We then examined whether resveratrol stimulates
expression of SIRT1. Resveratrol up-regulated protein expression of
SIRT1 in HCC cells but not in normal liver HL-7702 cells (Fig. 3A). Deacetylation of FoxO proteins
has been shown to result from the activity of SIRT1 (19). It has been shown that SIRT1
promotes transcription of FoxO target genes involved in stress
resistance, while decreasing transcription of genes involved in
apoptosis (20). Our result showed
that protein levels of FoxO1 and Ac-FoxO1 were significantly
decreased with resveratrol treatment compared with control
(Fig. 3D). Up-regulation of SIRT1
activated by resveratrol involved in deacetylation of FoxO1.
To determine the relationship between SIRT1 activity
and PI3K/AKT signaling pathway, the activity of intracellular SIRT1
was analyzed after SIRT1 inhibitor EX527 was used. Consistent with
its protein level, SIRT1 activity increased by resveratrol and
decreased after exposure to EX527 in HepG2 cells (Fig. 3B). Treatment of 1 µM EX527 enhanced
p-PI3K, p-AKT and p-FoxO3a levels while slight effect on total PI3K
(Fig. 3C). These results showed
that resveratrol suppressed post-translational modification of
SIRT1 mediated PI3K/AKT signaling.
Resveratrol enhanced phosphorylation
of DLC1 by AKT and inhibited cell migration
As PI3K/AKT pathway is an important cell survival
cascade, DLC1 might be a substrate of AKT. In order to explore
whether AKT involved in regulation of tumor suppressor DLC1,
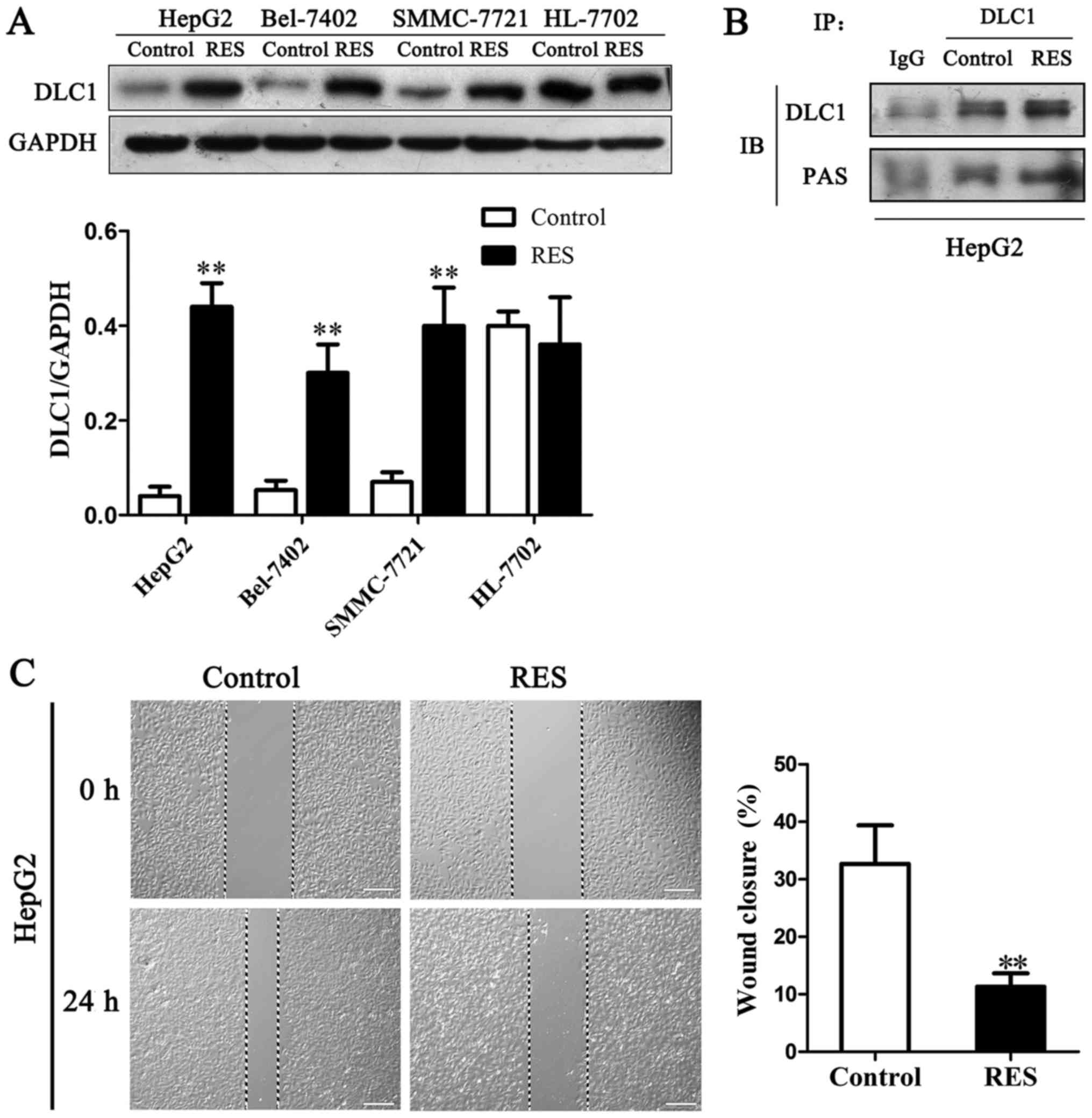
phosphorylation on the biological activities of DLC1 was
demonstrated in HCC cells. DLC1 protein expression elevated in
three kinds of HCC cells and no effect on HL-7702 cells by
resveratrol (Fig. 4A). An antibody
against PAS (phospho-AKT substrate) was employed to detect
phosphorylation of DLC1. Immunoprecipitation result demonstrated
that DLC1 phosphorylation level was enhanced by resveratrol in
HepG2 cells (Fig. 4B). The
relevance and functional effect of AKT mediated phosphorylation of
DLC1 remain unclear and await further investigation.
Wound healing assay measures the ability of cells to
migrate into an area of a cell culture plate denuded of cells
(wound). Our result showed that resveratrol inhibited the wound
closures from 32.5 to 11.5% in HepG2 cells by 24 h treatment
(Fig. 4C). The findings revealed
that up-regulation of DLC1 and its phosphorylation level via
resveratrol treatment might cause motility inhibition in cancer
cells.
Discussion
Although the capacity of resveratrol to prevent
cancer development has been studied for many years, its mechanism
underlying remains to be fully elucidated. Proliferating cell
nuclear antigen (PCNA) is a critical event in growth regulation of
cancer cells (21). The
anti-metastatic effect of resveratrol was associated with
restriction of invasion, mobility, adhesion, and MMP expression in
colon carcinoma (22). Here, we
found that resveratrol inhibited the viability and proliferation by
MTT and EdU assays and suppressed expression of PCNA accompanying
proliferation inhibition in HCC cells.
The PI3K/AKT signaling is a critical pathway in cell
proliferation, survival, neovascularization and tumor growth
(23). AKT is an important
downstream target kinase of PI3K signaling pathway. Activated AKT
can inhibit release of cytochrome c and apoptosis factor, thereby
inhibiting apoptosis and promote the growth of cancer cells
(8). Resveratrol has been shown to
inhibit constitutive activation of PI3K/AKT pathway to induce
apoptosis in several types of cancer cells (12,24).
FoxO3a is the downstream targets of AKT and AKT can promote FoxO3a
phosphorylation, leading to FoxO3a translocation from the nucleus
to the cytoplasm, which de-activates FoxO3a; conversely, inhibition
of AKT promotes de-phosphorylation of FoxO3a, resulting in nuclear
translocation of FoxO3a (25). Our
results showed that resveratrol resulted in significant inhibition
in constitutively elevated levels of phosphorylated PI3K/AKT and
reduced phosphorylated FoxO3a significantly in HepG2 cells. AKT
inhibits apoptosis through multiple targets, including Bcl-2 family
and caspase proteases (8). Bcl-2
members are well characterized as regulators of apoptosis, such as
Bax and Bim. The ratio of Bcl-2/Bax protein regarded as a driving
force for apoptosis in cancer cells (26). Caspases are a family of cell death
proteases triggered in response to proapoptotic signals and play an
essential role in the execution phase of apoptosis (27). TUNEL assays are used to detect DNA
fragmentation from apoptosis. In the present study, resveratrol
resulted in an increase of green fluorescence signal which was
indicative of apoptosis. Also, resveratrol caused a significant
down-modulation of Bcl-2/Bax ratio and activated caspase-3,
caspase-7, PARP and induced the cleavage-PARP in HCC cells. It
suggested that the apoptosis of HCC cells induced by resveratrol
might act through the mitochondrial pathways.
SIRT1 plays a key role in both cell death and
survival with p53 family members, FoxOs and the nuclear factor-κB
family (28). Furthermore,
resveratrol suppresses the proliferation of gastric cancer cells in
a SIRT1-dependent manner in vitro and in vivo
(29). We showed that resveratrol
significantly increased SIRT1 expression in HCC cells. As a
nicotinamide adenine dinucleotide-dependent protein deactylase,
SIRT1 is known to be directly involved in the acetylation of FoxOs
and expression of proapoptotic protein Bim (19). FoxO1 has emerged as an important
protein that modulates the expression of apoptosis-related genes in
cancer cells (7). SIRT1 knockdown
enhanced Ac-FoxO1 expression to block reactive oxygen
species-induced apoptosis in mouse embryonic stem cells (30). Our results showed that resveratrol
significantly decreased expressions of FoxO1 and Ac-FoxO1 with
activation of SIRT1 by resveratrol.
SIRT1 has been also implicated as a negative
regulator for the PI3K/AKT pathway by deacetylating the tumor
suppressor PTEN (31) and by
down-regulation of both AKT and phosphorylation levels to inhibit
the PI3K/AKT pathway in glioblastoma cell (32). The regulation of PI3K/AKT pathway
by SIRT1 may provide a potential mechanism in tumorigenesis, and
SIRT1 inhibitor EX527 was used to evaluate the underlying
mechanism. We found that resveratrol up-regulated SIRT1 level to
decrease PI3K and AKT phosphorylation and the phosphorylation of
PI3K and AKT became significantly higher when SIRT1 was inhibited
in HepG2 cells. It indicated that the inhibition of PI3K/AKT
pathway by resveratrol is mediated by up-regulation of SIRT1.
DLC1 is a Rho GTPase-activating protein (RhoGAP) and
frequently deleted and underexpressed in cancers (14). Restoration of DLC1 gene expression
induces apoptosis and inhibits both cell growth and tumorigenicity
in HCC cells (33). Our previous
results has been shown that DLC1 is a multifunctional protein which
interacts with tensin, talin, FAK in focal adhesion (34,35).
DLC1 expression could significantly suppress Rho-dependent actin
stress fiber formation in hepatocellular carcinoma and fibroblast
cell lines (16). Cell migration
is tightly regulated by the activity of Rho proteins through actin
cytoskeletal rearrangements (36).
In addition, DLC1 overexpression inhibited cell migration by
induced disassembly of stress fibers and extensive membrane
protrusions around cells on laminin-1 in HCC (37). Our result showed that resveratrol
significantly up-regulated expression of DLC1 protein and inhibited
the migration ratio from 32.5 to 11.5% in HCC cells, indicating
that induced DLC1 level was associated with tumor suppression
effect. The post-translational modification of DLC1 has garnered
much attention as the important regulatory mechanism of DLC1
activity, and kinases such as AKT, PKC and PKD have been shown to
phosphorylate DLC1 at different residues and regulate its
biological activities via RhoGAP-dependent as well as
RhoGAP-independent pathways (15,38).
Phosphorylation of DLC1 by PKA contributes to enhance RhoGAP
activity and promotes activation of DLC1, which suppresses hepatoma
cell growth, motility and metastasis both in vitro and in
vivo models (39). To
elucidate whether AKT could phosphorylate DLC1, an antibody against
PAS (phospho-AKT substrate) was employed to detect phosphorylation
of DLC1. Our findings showed that DLC1 was directly phosphorylated
by AKT in HepG2 cells. These results suggested that DLC1 as a tumor
suppressor was up-regulated by resveratrol and its
post-translational modification was mediated by PI3K/AKT signaling.
Although previous studies have characterized functional effects of
the identified phosphorylated residues of DLC1 (40), the physiological stimuli of these
phosphorylations remain unclear. Future work are warrant to clarify
how DLC1 regulated by its domains and phosphorylation as well as
precise downstream mechanisms through post-translational
modification of DLC1 acts as a tumor suppressor.
Taken together, our findings suggested that
resveratrol activated SIRT1 to induce liver cancer cell apoptosis
and to inhibit migration through SIRT1 mediated post-translational
regulation of PI3K/AKT signaling and phosphorylation level of
FoxO3a and DLC1 and deacetylation of FoxO1 leading to tumor
suppression in HCC cells.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (Grant No. 31672377), the Major Key
Science and Technology Project of Shandong Province
(2015ZDJS04003), the Key Program of Shandong Provincial Natural
Science Foundation of China (ZR2013CZ002), Science and Technology
Program of Jinan (201202033).
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsieh TC and Wu JM: Resveratrol:
Biological and pharmaceutical properties as anticancer molecule.
Biofactors. 36:360–369. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frazzi R, Valli R, Tamagnini I, Casali B,
Latruffe N and Merli F: Resveratrol-mediated apoptosis of hodgkin
lymphoma cells involves SIRT1 inhibition and FOXO3a
hyperacetylation. Int J Cancer. 132:1013–1021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Zhu W, Li J, Liu M and Wei M:
Resveratrol suppresses the STAT3 signaling pathway and inhibits
proliferation of high glucose-exposed HepG2 cells partly through
SIRT1. Oncol Rep. 30:2820–2828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang Y and Nicholl MB: Sirtuin 1 in
malignant transformation: Friend or foe? Cancer Lett. 306:10–14.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buhrmann C, Shayan P, Popper B, Goel A and
Shakibaei M: Sirt1 is required for resveratrol-mediated
chemopreventive effects in colorectal cancer cells. Nutrients.
8:1452016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu Z and Tindall DJ: FOXOs, cancer and
regulation of apoptosis. Oncogene. 27:2312–2319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu MH, Lin XL, Li J, He J, Tan TP, Wu SJ,
Yu S, Chen L, Liu J, Tian W, et al: Resveratrol induces apoptosis
through modulation of the Akt/FoxO3a/Bim pathway in HepG2 cells.
Mol Med Rep. 13:1689–1694. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shankar S, Chen Q and Srivastava RK:
Inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to
enhance antiangiogenic effects of EGCG through activation of FOXO
transcription factor. J Mol Signal. 3:72008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tzivion G, Dobson M and Ramakrishnan G:
FoxO transcription factors; Regulation by AKT and 14-3-3 proteins.
Biochim Biophys Acta. 1813:1938–1945. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hussain AR, Uddin S, Bu R, Khan OS, Ahmed
SO, Ahmed M and Al-Kuraya KS: Resveratrol suppresses constitutive
activation of AKT via generation of ROS and induces apoptosis in
diffuse large B cell lymphoma cell lines. PLoS One. 6:e247032011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan BZ, Miller MJ, Keck CL, Zimonjic DB,
Thorgeirsson SS and Popescu NC: Cloning, characterization, and
chromosomal localization of a gene frequently deleted in human
liver cancer (DLC-1) homologous to rat RhoGAP. Cancer Res.
58:2196–2199. 1998.PubMed/NCBI
|
|
14
|
Lahoz A and Hall A: DLC1: A significant
GAP in the cancer genome. Genes Dev. 22:1724–1730. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scholz RP, Regner J, Theil A, Erlmann P,
Holeiter G, Jähne R, Schmid S, Hausser A and Olayioye MA: DLC1
interacts with 14-3-3 proteins to inhibit RhoGAP activity and block
nucleocytoplasmic shuttling. J Cell Sci. 122:92–102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wong CM, Yam JW, Ching YP, Yau TO, Leung
TH, Jin DY and Ng IO: Rho GTPase-activating protein deleted in
liver cancer suppresses cell proliferation and invasion in
hepatocellular carcinoma. Cancer Res. 65:8861–8868. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan BZ, Jefferson AM, Baldwin KT,
Thorgeirsson SS, Popescu NC and Reynolds SH: DLC-1 operates as a
tumor suppressor gene in human non-small cell lung carcinomas.
Oncogene. 23:1405–1411. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Communal C, Sumandea M, de Tombe P, Narula
J, Solaro RJ and Hajjar RJ: Functional consequences of caspase
activation in cardiac myocytes. Proc Natl Acad Sci USA. 99:pp.
6252–6256. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brunet A, Sweeney LB, Sturgill JF, Chua
KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et
al: Stress-dependent regulation of FOXO transcription factors by
the SIRT1 deacetylase. Science. 303:2011–2015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Greer EL and Brunet A: FOXO transcription
factors at the interface between longevity and tumor suppression.
Oncogene. 24:7410–7425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao H, Ho PC, Lo YH, Espejo A, Bedford
MT, Hung MC and Wang SC: Interaction of proliferation cell nuclear
antigen (PCNA) with c-Abl in cell proliferation and response to DNA
damages in breast cancer. PLoS One. 7:e294162012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu H, Liang X, Fang Y, Qin X, Zhang Y and
Liu J: Resveratrol inhibits hypoxia-induced metastasis potential
enhancement by restricting hypoxia-induced factor-1 alpha
expression in colon carcinoma cells. Biomed Pharmacother.
62:613–621. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aziz MH, Nihal M, Fu VX, Jarrard DF and
Ahmad N: Resveratrol-caused apoptosis of human prostate carcinoma
LNCaP cells is mediated via modulation of phosphatidylinositol
3′-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther.
5:1335–1341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu MH, Yuan C, He J, Tan TP, Wu SJ, Fu
HY, Liu J, Yu S, Chen YD, Le QF, et al: Resveratrol protects PC12
cells from high glucose-induced neurotoxicity via PI3K/Akt/FoxO3a
pathway. Cell Mol Neurobiol. 35:513–522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niquet J and Wasterlain CG: Bim, Bad and
Bax: A deadly combination in epileptic seizures. J Clin Invest.
113:960–962. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaushal GP, Kaushal V, Hong X and Shah SV:
Role and regulation of activation of caspases in cisplatin-induced
injury to renal tubular epithelial cells. Kidney Int. 60:1726–1736.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blander G and Guarente L: The Sir2 family
of protein deacetylases. Annu Rev Biochem. 73:417–435. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Q, Wang B, Zang W, Wang X, Liu Z, Li
W and Jia J: Resveratrol inhibits the growth of gastric cancer by
inducing G1 phase arrest and senescence in a Sirt1-dependent
manner. PLoS One. 8:e706272013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chae HD and Broxmeyer HE: SIRT1 deficiency
downregulates PTEN/JNK/FOXO1 pathway to block reactive oxygen
species-induced apoptosis in mouse Embryonic stem cells. Stem Cells
Dev. 20:1277–1285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ikenoue T, Inoki K, Zhao B and Guan KL:
PTEN acetylation modulates its interaction with PDZ domain. Cancer
Res. 68:6908–6912. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang G, Wang JJ, To TS, Zhao HF and Wang
J: Role of SIRT1-mediated mitochondrial and Akt pathways in
glioblastoma cell death induced by Cotinus coggygria flavonoid
nanoliposomes. Int J Nanomedicine. 10:5005–5023. 2015.PubMed/NCBI
|
|
33
|
Zhou X, Thorgeirsson SS and Popescu NC:
Restoration of DLC-1 gene expression induces apoptosis and inhibits
both cell growth and tumorigenicity in human hepatocellular
carcinoma cells. Oncogene. 23:1308–1313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li G, Du X, Vass WC, Papageorge AG, Lowy
DR and Qian X: Full activity of the deleted in liver cancer 1
(DLC1) tumor suppressor depends on an LD-like motif that binds
talin and focal adhesion kinase (FAK). Proc Natl Acad Sci USA.
108:pp. 17129–17134. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qian X, Li G, Asmussen HK, Asnaghi L, Vass
WC, Braverman R, Yamada KM, Popescu NC, Papageorge AG and Lowy DR:
Oncogenic inhibition by a deleted in liver cancer gene requires
cooperation between tensin binding and Rho-specific
GTPase-activating protein activities. Proc Natl Acad Sci USA.
104:pp. 9012–9017. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maddox AS and Burridge K: RhoA is required
for cortical retraction and rigidity during mitotic cell rounding.
J Cell Biol. 160:255–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim TY, Lee JW, Kim HP, Jong HS, Kim TY,
Jung M and Bang YJ: DLC-1, a GTPase-activating protein for Rho, is
associated with cell proliferation, morphology, and migration in
human hepatocellular carcinoma. Biochem Biophys Res Commun.
355:72–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Scholz RP, Gustafsson JO, Hoffmann P,
Jaiswal M, Ahmadian MR, Eisler SA, Erlmann P, Schmid S, Hausser A
and Olayioye MA: The tumor suppressor protein DLC1 is regulated by
PKD-mediated GAP domain phosphorylation. Exp Cell Res. 317:496–503.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ko FC, Chan LK, Sze KM, Yeung YS, Tse EY,
Lu P, Yu MH, Ng IO and Yam JW: PKA-induced dimerization of the
RhoGAP DLC1 promotes its inhibition of tumorigenesis and
metastasis. Nat Commun. 4:16182013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hers I, Wherlock M, Homma Y, Yagisawa H
and Tavaré JM: Identification of p122RhoGAP (deleted in liver
cancer-1) Serine 322 as a substrate for protein kinase B and
ribosomal S6 kinase in insulin-stimulated cells. J Biol Chem.
281:4762–4770. 2006. View Article : Google Scholar : PubMed/NCBI
|