Introduction
Carbonic anhydrase (EC4.2.1.1; CAIII) is considered
a biological marker or therapeutic target for skeletal muscle
disorders in a variety of pathophysiological conditions. Changes in
the expression of CAIII, or the modification of CAIII by
carbonylation or oxidation occur in Duchenne's muscular dystrophy,
chronic widespread pain and skeletal muscle disuse (1–3). In
previous years, various epidemiological, pathological and molecular
investigations have demonstrated that CAIII is also involved in the
development of myasthenia gravis (MG) (4), a neuromuscular autoimmune disorder
caused by anti-acetylcholine receptor antibodies. Our previous
investigations (4) showed that the
protein and gene expression levels of CAIII were significantly
decreased in the skeletal muscles of patients with MG. As the
severity of MG increased, the decrease in the expression of CAIII
also increased. As CAIII is expressed mainly in slow-twitch fibers
(type I myofibers), exhibiting oxidative metabolism and having a
low contraction speed and high resistance to fatigue, it was
hypothesized that the changes in CAIII reflect abnormalities of
type I myofibers in patients with MG. This may be the molecular
mechanism underlying muscle fatigability. Compared with other
carbonic anhydrase family isozymes, CAIII shows unique
characteristics in activity and tissue distribution (5). Firstly, CAIII is a multifunctional
enzyme with unique phosphatase activity in addition to lower
specific hydrase activity (~1% of CAII). Secondly, CAIII is
expressed at high levels in tissues with in high metabolic
activity, including fat, liver tissues and skeletal muscles. CAIII
constitutes ~10% of the soluble protein in the cytoplasm of
slow-twitch fibers, but is largely absent from fast-twitch fibers.
In rodents, the levels of CAIII in skeletal muscles change with age
according to the development of innervation and contractile
activity (6). Specifically, the
level of CAIII increases sharply with age, reaching the peak adult
level at puberty. The levels of CAIII are then maintained for a
time, but eventually decline with aging (7). Comparative analysis has revealed a
correlation between changes in the levels of CAIII and the
development of skeletal muscle contractility (6).
CAIII may be involved in resistance to fatigue and
its levels are affected by the innervation state (8,9). For
example, neuromuscular dysfunction caused by anti-acetylcholine
receptor autoantibodies in MG, denervation, or cross innervation,
have an effect on the expression of CAIII. In previous studies, the
expression of CAIII following denervation was examined by analyzing
its overall protein and mRNA levels in skeletal muscles. However,
whether changes in the levels of CAIII resulted from changes in
expression in the original type I myofibers or from remodeling of
skeletal muscle were not clarified. Additionally, denervation
animal models are constructed predominantly by transecting the
sciatic nerve. This surgery leads to rapid and complete
denervation, but also causes dysfunction of almost the entire hind
limb and impairs the blood supply to the musculature. These effects
interfere with the examination of denervation-induced changes to
skeletal muscle (10).
In the present study, to investigate the association
between changes in the expression of CAIII and skeletal muscle
characteristics, a highly selective denervation technique was used,
which reduced the interference caused by changes in limb activity
and blood supply. This technique involves transection of the nerves
innervating the soleus (slow-twitch) and extensor digitorum longus
(EDL; fast-twitch) muscles. In this model, specific skeletal
muscles were denervated, but the movement and blood supply of the
affected limb were maintained. This enables the first
investigation, to the best of our knowledge, of the association
between skeletal muscle remodeling and the expression of CAIII in
serial sections.
Materials and methods
Animals and reagents
Male adult Sprague-Dawley rats, weighing 160–180 g,
were obtained from the Experimental Animal Center of Shanghai
Medical College, Fudan University (Shanghai, China). The rats had
free access to food and water, and were subjected to standard
conditions of humidity (50±10%), temperature (25±2°C) and a 12-h
light/dark cycle. All experiments were approved by the Ethics
Committee of Animal Treatment of Fudan University. All reagents
were purchased from Sinopharm Chemical Regents (Shanghai, China),
unless indicated otherwise. All procedures performed on the rats
were in accordance with the European Union protocols (86/609/EEC)
for the care and use of laboratory animals.
Selective denervation
The rats were anaesthetized with pentobarbital (50
mg/kg). The right hind limb was prepared for surgery and a 1-cm
incision was made in the skin along the axis of the femur. The
nerve branches supplying the soleus and EDL muscles were separated,
ligated and truncated distally to the muscles; this step was
performed by visualization using a dissecting microscope. The nerve
stumps were marked by a knot and buried in the muscles close by to
avoid reinnervation, as described previously with modifications
(11,12). Sham surgery, which involved the
same surgical steps without transection of the nerve, was performed
on the contralateral hind limbs of the animals simultaneously, and
the soleus and EDL muscles served as the control. The denervation
was verified by the location of the knots when the muscles were
dissected at 7, 14, 28 or 56 days. At the end of the assigned
period, the soleus and EDL muscles were carefully excised from the
hind limbs, divided into two sections (one for morphological
analysis and the other for analyses of protein expression and
enzyme activity), cleaned of tendons and connective tissues, and
immediately frozen in liquid nitrogen. The muscles were stored at
−80°C until processing.
ATPase staining
For the myosin ATPase staining, frozen serial cross
sections (thickness, 8 µm) were prepared and staining was performed
according to the method described by Brooke and Kaiser (13). ATPase stained sections were
observed using an Olympus BX60 microscope (Olympus Corporation,
Tokyo, Japan). Those fibers that stained dark following
pre-incubation in acid and light following pre-incubation in alkali
were classified as type I fibers.
Immunohistochemistry
Freshly obtained skeletal muscle slices (thickness,
8 µm) were fixed at −20°C in 4% formaldehyde. Prior to antibody
staining, the tissue sections were treated with 3%
H2O2 for 10 min and blocked with 10% bovine
serum for 1 h. The sections were incubated with an in-house
generated anti-CAIII antibody (1:1,000) (14) at 25°C for 2 h. Subsequently, the
sections were incubated with peroxidase-labeled goat anti-rabbit
IgG (1:2,000, ab6721, Abcam, Cambridge, UK) at 25°C for 1 h. CAIII
was visualized with diaminobenzidene solution (0.5 mg/ml; 0.03%
H2O2). Stained sections were observed using
an Olympus BX60 microscope (Olympus Corporation).
Western blot analysis
The skeletal muscles were homogenized in 10 mM
sodium phosphate buffer (pH 7.4) containing a mixture of protease
inhibitors. The homogenate was centrifuged at 10,000 × g at 4°C for
20 min. The supernatant was collected and the protein concentration
was determined using Bradford's method with bovine serum albumin as
the standard. For the western blot assays, crude protein extracts
(15 µg) were separated by 10% sodium dodecyl sulfate polyacrylamide
gel electrophoresis and transferred onto nitrocellulose membranes.
The nitrocellulose membranes were blocked with 2.5% bovine serum
albumin at 4°C overnight and then incubated with rabbit anti-rat
CAIII antibody (1:1,600) for 3 h at 25°C. This was followed by
incubation with a peroxidase-conjugated goat anti-rabbit IgG
(Chemicon; EMD Millipore, Billerica, MA, USA; 1:2,000) for 2 h at
25°C. GAPDH (1:1,200, AG019, Beyotime Institute of Biotechnology,
Shanghai, China) served as the internal reference.
Phosphatase staining
Staining for phosphatase activity was performed as
reported previously (14).
Briefly, following sodium dodecyl sulfate polyacrylamide gel
electrophoresis in an ice bath, the proteins were transferred onto
a nitrocellulose membrane on ice. The membrane was incubated in ABS
buffer containing 20 mM sodium acetate; 0.8% (w/v) sodium chloride
and 0.02% (w/v) potassium chloride (pH 5.5) at 25°C for 20 min to
permit removal of sodium dodecyl sulfate and refolding of the
proteins. Subsequently, 5% (w/v) polyvinylpyrrolidone (40,000 kDa;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) in ABS buffer
was applied at 4°C overnight to block the non-specific binding
sites. Following washing with ABS buffer for 5 min, the membrane
was dipped into a 20-ml staining system (pH 5.5) with final
concentrations of 50 mM sodium acetate, 20 mM magnesium chloride
and 5 mM nitrophenyl phosphate (Fluka; Merck Millipore), and 2 mM
Fast Garnet GBC (Sigma-Aldrich; Merck Millipore). The staining
reaction was terminated following incubation at 37°C for 45
min.
Statistical analysis
The results of the western blot analysis and
phosphatase staining were analyzed and quantified using Image
Pro-Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA). The expression of CAIII was normalized to that of GAPDH. Data
are expressed as the mean ± standard error of the mean. Differences
between denervated muscles and sham controls were analyzed using
Student's paired t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of denervation on skeletal
muscle structure and expression of CAIII
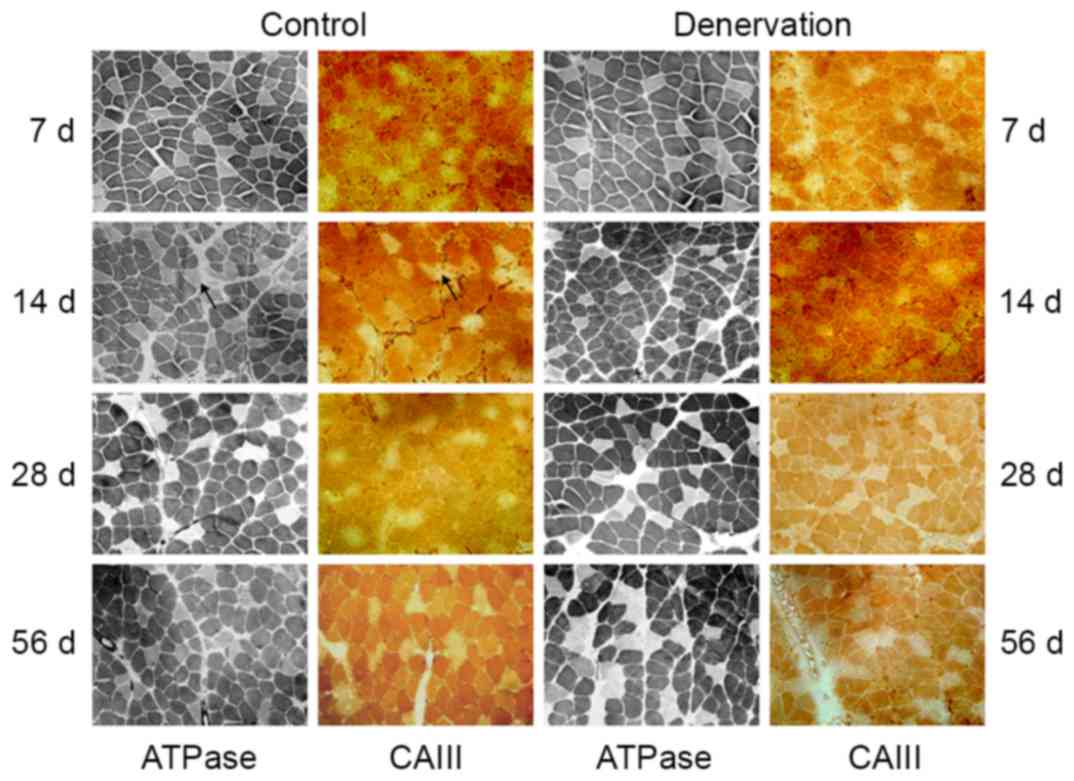
Comparative analyses of the staining for ATPase and
immunohistochemical staining of CAIII were performed on serial
sections of the soleus and EDL muscles. The soleus muscles were
composed mainly of type I fibers, as shown by the dark ATPase
staining at pH 4.6 (Fig. 1).
Throughout the 56-day observation period, there was a decline in
type I fibers, but no significant change in fiber morphology or the
ratio of slow-twitch fibers in the control or denervated soleus
muscle (~83% of type I fibers in the control muscles and ~80% of
type I fibers in the denervated muscles at day 56; P>0.05). In
addition, there was no visible atrophy, hypertrophy or hyperplasia
of connective tissues. Immunohistochemical staining of the soleus
muscle for CAIII showed that the location of the CAIII enzyme was
consistent with the type I fibers in the control group, and this
was maintained following denervation (Fig. 1).
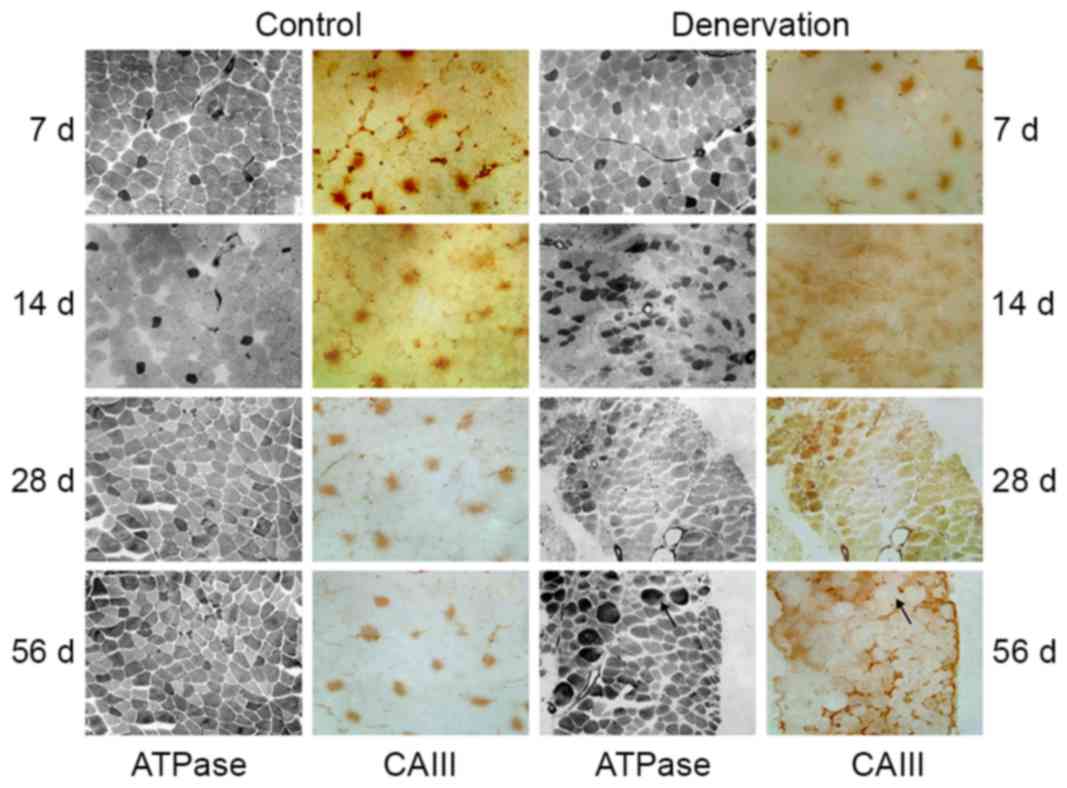
The EDL muscle was composed mainly of fast-twitch
fibers and a small number of type I fibers (Fig. 2). Throughout the observation
period, no significant change was observed in the ratio of the two
myofiber types, the consistency of type I myofibers or the location
of CAIII in the control EDL tissues. Following denervation, the
morphology of the EDL muscle changed significantly. Firstly, all
denervated muscle fibers (both type I and other fibers) gradually
lost their polygonal appearance and became rounded, changing from
uniform shapes to different sizes. Secondly, the composition ratio
of the muscle fibers changed following denervation. In the first 2
weeks, the number of type I fibers gradually increased, indicating
the conversion from fast- to slow-twitch fibers. Further
reconstruction was demonstrated by non-selective ATPase staining,
which indicated the appearance of transitional type fibers. During
this time course, the expression of CAIII changed accordingly. In
the first 2 weeks, the number of CAIII-immunoreactive fibers
increased, and the location of the fibers was consistent with the
fast-to-slow skeletal muscle remodeling. During the subsequent 2
weeks, in addition to the dedifferentiation of EDL muscle, with the
exception of typical type I fibers, CAIII immunostaining became
more diffuse over time. At 56 days post-denervation, a mismatch
between ATPase staining and CAIII immunohistochemical staining was
observed.
Effect of denervation on the protein
expression of CAIII in skeletal muscles
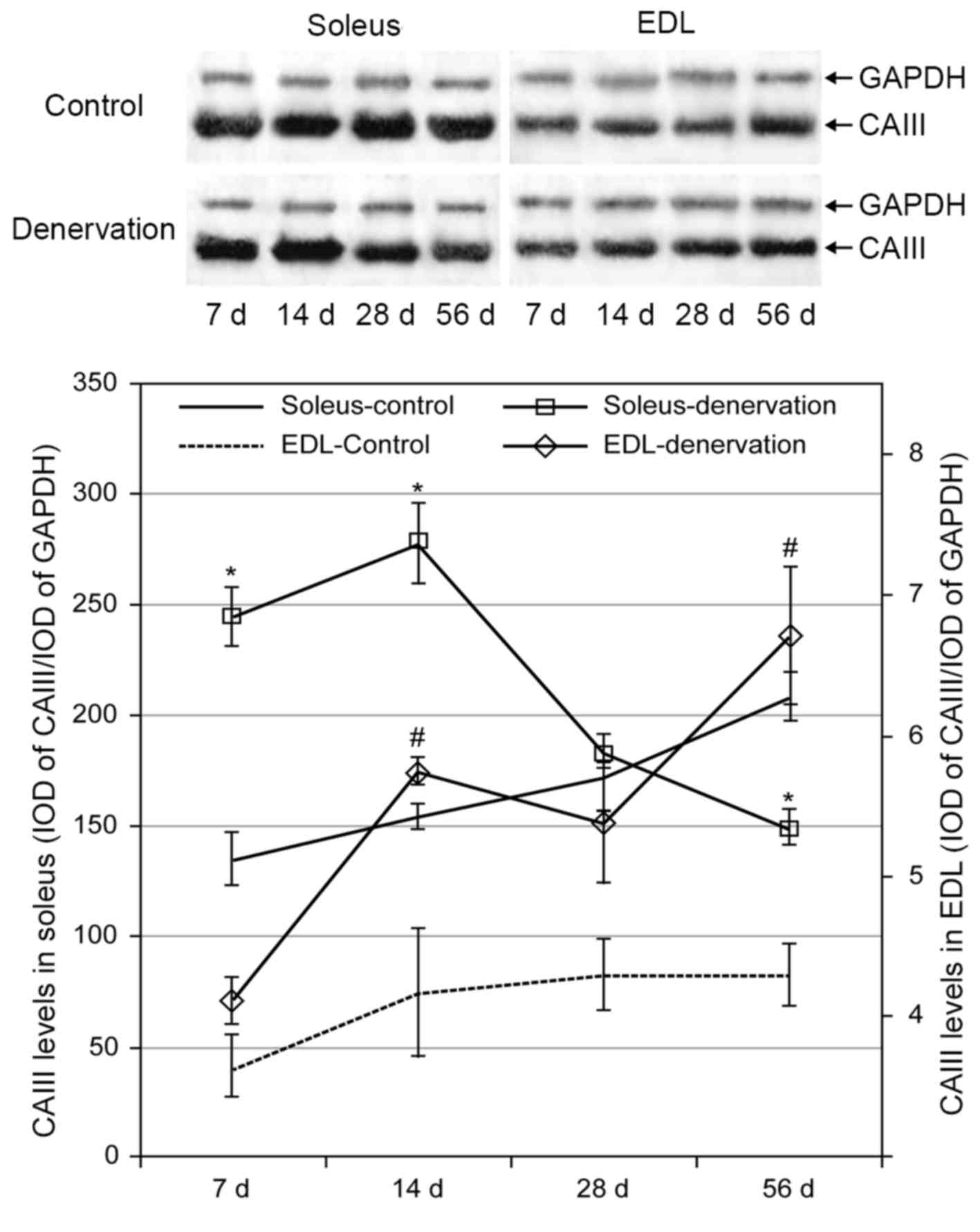
In the control group, the protein levels of CAIII in
the soleus and EDL muscles increased gradually over time. This
trend was more marked in the soleus than in the EDL muscle, and the
level of CAIII in the soleus was markedly higher than that in the
EDL muscle. Following denervation, the levels of CAIII in the
soleus and EDL muscles were higher, compared with the levels in the
control groups (Fig. 3). Compared
with the increased protein level of CAIII in the EDL muscle over
time following denervation, the level in the soleus muscle was
significantly increased at 2 weeks but then decreased gradually,
reaching control levels at 28 days and dropping below the control
level at 56 days (P<0.05).
Effect of denervation on the
phosphatase activity of CAIII in skeletal muscle
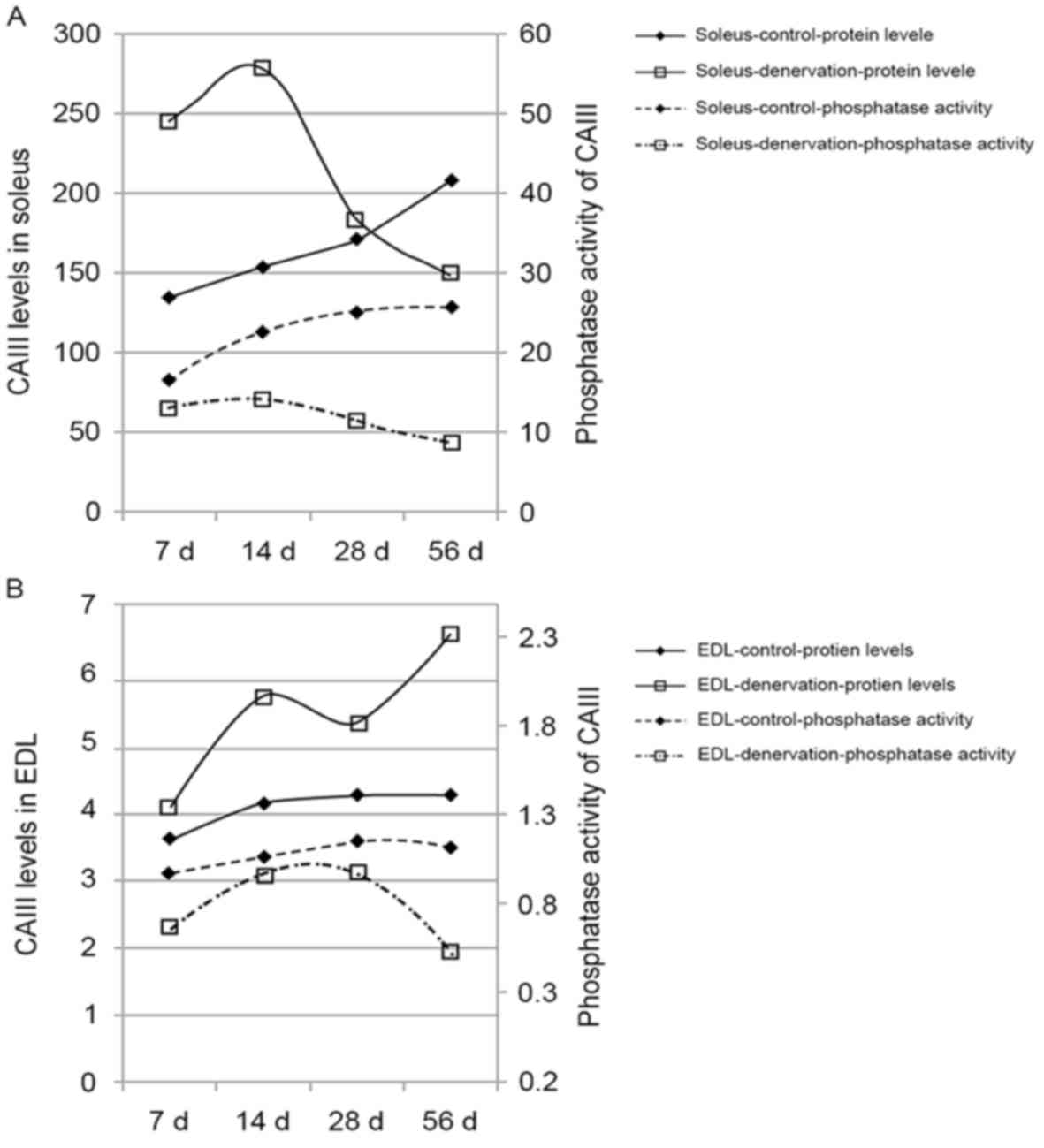
The phosphatase activity of CAIII in the control
group increased with time in the soleus and EDL muscles,
maintaining a steady enzyme activity-protein ratio (Table I). Following denervation, the
phosphatase activity of CAIII in the soleus and EDL muscles
decreased sharply, compared with the previously observed increase
in CAIII. This decrease was more marked with time, particularly in
the soleus muscle (P<0.05). These data indicated a deviation
between the phosphatase activity and protein expression of CAIII in
denervated muscle (Fig. 4A and
B).
 | Table I.Effect of denervation on the
phosphatase activity of carbonic anhydrase III in skeletal
muscles. |
Table I.
Effect of denervation on the
phosphatase activity of carbonic anhydrase III in skeletal
muscles.
| Group | n | Day 7 | Day 14 | Day 28 | Day 56 |
|---|
| Soleus-control | 6 | 16.75±1.25 | 22.75±1.80 | 25.26±3.15 | 25.82±2.97 |
|
Soleus-denervation | 6 | 13.29±0.94 |
14.39±1.93a |
11.48±1.46a |
9.04±1.46a |
| EDL-control | 6 | 0.98±0.19 | 1.06±0.21 | 1.15±0.16 | 1.12±0.16 |
| EDL-denervation | 6 | 0.67±0.09 | 0.96±0.21 | 0.98±0.19 |
0.52±0.09b |
Discussion
The present study adopted a selective denervation
technique to assess the impact of nerve conduction on CAIII.
Comparative analyses to examine relative changes in muscle
structure and CAIII location, and to evaluate the expression of
CAIII and its phosphatase activity following denervation, revealed
that: i) CAIII was distributed selectively in type I myofibers; ii)
following denervation, skeletal muscle fibers of the EDL muscle
exhibited significant type transformation (remodeling). This was
followed by a change in the localization of CAIII, which was more
pronounced in the EDL muscle than in the soleus muscle.
Specifically, there was a fast-to-slow conversion of myofibers
staining positively for active CAIII; iii) Protein levels of CAIII
increased to varying extents in denervated muscles, whereas its
phosphatase activity decreased significantly. Innervation is
critical for myofiber differentiation, maturation and maintenance.
Denervation in animals is a common approach to assess the impact of
innervation on skeletal muscle structure and functions.
Traditionally, sciatic denervation causes near paralysis of the
entire limb and leads to fiber atrophy, including denervation and
disuse atrophy. Myofiber remodeling from fast-to-slow or
slow-to-fast type is also observed as a result of the conversion of
original myofibers and/or newly-formed fibers from satellite cells
(15,16). This change is more markedin the
soleus than in the EDL muscle (17).
In certain studies, selective denervation of a
specific muscle has been performed, which differs from traditional
surgery (12,18). In the present study, following
selective denervation, no significant structural change was
observed in the soleus muscle. By contrast, prominent muscle
remodeling occurred shortly following denervation of the EDL
muscle, demonstrating that fast fibers were more susceptible to
atrophy than slow fibers (19).
Pathologically, the denervated EDL fibers lost their polygonal
shape and, over time, the fibers began to exhibit dedifferentiation
(17). These different effects of
selective denervation maybe associated with the absence of
disruption from mechanical forces and blood supply, which exist in
traditional animal denervation models (10,20,21).
It was also apparent that the necessity of innervation for
maintaining muscle morphology is more important in the EDL muscle
than in the soleus muscle. Considering the differences between the
different denervation animal models, even within the same study
(12,21), it is reasonable and important to
consider and evaluate the differing effects between selective and
non-selective denervation in a selected animal model.
As reported previously, CAIII is expressed
selectively in type I myofibers (5). Following denervation, the
localization of CAIII changed in response to myofiber remodeling,
and this effect was significantly different between the soleus and
EDL muscles. No significant change was observed in the structure of
the soleus muscle following denervation. This was consistent with
the absence of changes in the distribution of type I myofibers and
the localization of CAIII during the observation period. However,
the tissue structure and immunostaining for CAIII altered
significantly in the denervated EDL muscle. In the first 2 weeks
(early stage), the localization of CAIII-positive fibers was
consistent with the localization of type I fibers. At later time
points, the ATPase staining showed no complete or clear
distinguished skeletal muscle fiber types, indicating
dedifferentiation (17,18). In addition, there was a lack of
complete overlap between CAIII immunostaining and ATPase staining,
indicating the existence of transitional myofibers (22) in which the cells may or may not
express CAIII. Although immunostaining for CAIII and myosin heavy
chain isoforms was not applied to the serial sections to describe
the relative changes of these two indices, the present study did
show consistent changes in the location of CAIII and type I
myofiber (slow-twitch fiber) distribution.
The remodeling of skeletal muscle is an adaptive
change in response to physiological and pathological signals
(23). Fast-slow and slow-fast
transformations occur through intracellular signal transduction,
resulting in a change in the gene expression of specific myosin
heavy chain isoforms (15,24). In the present study, CAIII was
localized in type I myofibers, and the change of its location was
consistent with the change in type I myofibers following
denervation. Therefore, it is possible that CAIII is regulated by
the signaling pathway, which regulates slow fiber programming
(25).
The transformation of muscle structure (CAIII and
myofibers) was confirmed in the present study by analyzing the
protein levels of CAIII. Following denervation, the protein levels
of CAIII increased in the slow- and fast-twitch fibers at an early
stage, but long-term changes appeared to differ. Specifically, at
day 14, the protein levels of CAIII began to decline in the
denervated soleus muscle, whereas the levels in the denervated EDL
muscle continued to increase gradually. This difference may have
been caused by the increased release of CAIII from reserves (i.e.,
the transcriptional pool) in the early stage following denervation,
followed by the de novo synthesis of CAIII through gene
transcription and translation. The levels of CAIII are determined
by the number of type I myofibers (CAIII-immunostained myofibers).
The number of type I myofibers in the EDL muscle increased
gradually, leading to a concomitant increase in the level of
CAIII.
In contrast to the differential changes in protein
levels, the phosphatase activity of CAIII decreased significantly
in the two types of denervated muscles. Changes in neurotrophic and
contraction activity occur in denervated muscles, resulting in
decreases in muscle metabolism associated with changes in the
levels of enzymes and contraction-associated proteins (26,27).
The selective expression of CAIII in skeletal muscles, liver
tissues and adipose tissues suggests that it is directly or
indirectly involved in energy metabolism (5). Therefore, the significant reduction
inCAШ phosphatase activity following denervation may be an adaptive
response to reduced skeletal muscle contraction and cell
metabolism. Intracellular redox reactions and
dephosphorylation/phosphorylation in skeletal muscles are also
affected by denervation (28), and
the change in CAIII phosphatase activity maybe a reflection of
these changes (29).
In conclusion, the present study demonstrated that
the expression and activity of CAIII, a contraction-associated
enzyme, were regulated by innervation. Following denervation, the
expression and activity of CAIII were altered with myofiber
remodeling. The differences in manifestations observed in the
present study from those in previous studies on muscle structure
following denervation maybe the result of the maintenance of
passive movements of the denervated muscles in the present study.
Therefore, further investigations are required to assess the
effects of movement inhibition, including tenotomy or suspension,
on CAIII. Although the mechanism requires further investigation,
the changes in the expression of CAIII observed in the present
study reflect changes in type I myofibers, indicating that CAIII is
important for muscle contraction, particularly in resistance to
fatigue.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant no. 30170329).
References
|
1
|
Ayoglu B, Chaouch A, Lochmüller H,
Politano L, Bertini E, Spitali P, Hiller M, Niks EH, Gualandi F,
Pontén F, et al: Affinity proteomics within rare diseases: A
BIO-NMD study for blood biomarkers of muscular dystrophies. EMBO
Mol Med. 6:918–936. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olausson P, Gerdle B, Ghafouri N, Sjöström
D, Blixt E and Ghafouri B: Protein alterations in women with
chronic widespread pain-An explorative proteomic study of the
trapezius muscle. Sci Rep. 5:118942015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen CN, Ferrington DA and Thompson LV:
Carbonic anhydrase III and four-and-a-half LIM protein 1 are
preferentially oxidized with muscle unloading. J Appl Physiol
(1985). 105:1554–1561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du AL, Ren HM, Lu CZ, Tu JL, Xu CF and Sun
YA: Carbonic anhydrase III is insufficient in muscles of myasthenia
gravis patients. Autoimmunity. 42:209–215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harju AK, Bootorabi F, Kuuslahti M,
Supuran CT and Parkkila S: Carbonic anhydrase III: A neglected
isozyme is stepping into the limelight. J Enzyme Inhib Med Chem.
28:231–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Côté CH, Ambrosio F and Perreault G:
Metabolic and contractile influence of carbonic anhydrase III in
skeletal muscle is age dependent. Am J Physiol. 276:R559–R565.
1999.PubMed/NCBI
|
|
7
|
Cabiscol E and Levine RL: Carbonic
anhydrase III. Oxidative modification in vivo and loss of
phosphatase activity during aging. J Biol Chem. 270:14742–14747.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shang X, Chen S, Ren H, Li Y and Huang H:
Carbonic anhydrase III: The new hope for the elimination of
exercise-induced muscle fatigue. Med Hypotheses. 72:427–429. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Milot J, Frémont P, Côté C and Tremblay
RR: Differential modulation of carbonic anhydrase (CA III) in slow-
and fast-twitch skeletal muscles of rat following denervation and
reinnervation. Biochem Cell Biol. 69:702–710. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Argadine HM, Hellyer NJ, Mantilla CB, Zhan
WZ and Sieck GC: The effect of denervation on protein synthesis and
degradation in adult rat diaphragm muscle. J Appl Physiol (1985).
107:438–444. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu DX, Huang SK and Carlson BM: Electron
microscopic study of long-term denervated rat skeletal muscle. Anat
Rec. 248:355–365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ashley Z, Sutherland H, Lanmüller H,
Russold MF, Unger E, Bijak M, Mayr W, Boncompagni S, Protasi F,
Salmons S and Jarvis JC: Atrophy, but not necrosis, in rabbit
skeletal muscle denervated for periods up to one year. Am J Physiol
Cell Physiol. 292:C440–C451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brooke MH and Kaiser KK: Muscle fiber
types: How many and what kind? Arch Neurol. 23:369–379. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang H, Ren HM, Shang XL and Liu XY:
Detection of the phosphatase activity of carbonic anhydrase III on
a nitrocellulose membrane following 2D gel electrophoresis. Mol Med
Rep. 10:1887–1892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bassel-Duby R and Olson EN: Signaling
pathways in skeletal muscle remodeling. Annu Rev Biochem. 75:19–37.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dumont NA, Bentzinger CF, Sincennes MC and
Rudnicki MA: Satellite cells and skeletal muscle regeneration.
Compr Physiol. 5:1027–1059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Midrio M: The denervated muscle: Facts and
hypotheses. A historical review. Eur J Appl Physiol. 98:1–21. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szabó A, Wuytack F and Zádor E: The effect
of passive movement on denervated soleus highlights a differential
nerve control on SERCA and MyHC isoforms. J Histochem Cytochem.
56:1013–1022. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Viguie CA, Lu DX, Huang SK, Rengen H and
Carlson BM: Quantitative study of the effects of long-term
denervation on the extensor digitorum longus muscle of the rat.
Anat Rec. 248:346–354. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eisenberg HA and Hood DA: Blood flow,
mitochondria, and performance in skeletal muscle after denervation
and reinnervation. J Appl Physiol (1985). 76:859–866.
1994.PubMed/NCBI
|
|
21
|
Adhihetty PJ, O'Leary MF, Chabi B, Wicks
KL and Hood DA: Effect of denervation on mitochondrially mediated
apoptosis in skeletal muscle. J Appl Physiol (1985). 102:1143–1151.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Patterson MF, Stephenson GM and Stephenson
DG: Denervation produces different single fiber phenotypes in fast-
and slow-twitch hindlimb muscles of the rat. Am J Physiol Cell
Physiol. 291:C518–C528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blaauw B, Schiaffino S and Reggiani C:
Mechanisms modulating skeletal muscle phenotype. Compr Physiol.
3:1645–1687. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cohen TJ, Choi MC, Kapur M, Lira VA, Yan Z
and Yao TP: HDAC4 regulates muscle fiber type-specific gene
expression programs. Mol Cells. 38:343–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mitterberger MC, Kim G, Rostek U, Levine
RL and Zwerschke W: Carbonic anhydrase III regulates peroxisome
proliferator-activated receptor-γ2. Exp Cell Res. 318:877–886.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang H, Cheung WM, Ip FC and Ip NY:
Identification and characterization of differentially expressed
genes in denervated muscle. Mol Cell Neurosci. 16:127–140. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sakuma K, Watanabe K, Sano M, Kitajima S,
Sakamoto K, Uramoto I and Totsuka T: The adaptive response of
transforming growth factor-beta 2 and -beta RII in the overloaded,
regenerating and denervated muscles of rats. Acta Neuropathol.
99:177–185. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abruzzo PM, di Tullio S, Marchionni C,
Belia S, Fanó G, Zampieri S, Carraro U, Kern H, Sgarbi G, Lenaz G
and Marini M: Oxidative stress in the denervated muscle. Free Radic
Res. 44:563–576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Räisänen SR, Lehenkari P, Tasanen M,
Rahkila P, Härkönen PL and Väänänen HK: Carbonic anhydrase III
protects cells from hydrogen peroxide-induced apoptosis. FASEB J.
13:513–522. 1999.PubMed/NCBI
|