Introduction
Lung cancer is one of the commonest malignant
carcinomas in the world, and its incidence is increasing in a
number of countries. Worldwide, lung cancer is the leading cause of
death from malignant tumor, accounting for ~30% of all
cancer-associated mortality (1).
Lung adenocarcinoma is the principal subtype of lung cancer, and
metastasis is the leading cause of mortality in patients with lung
adenocarcinoma.
The protein kinase C (PKC) family regulates cell
growth, differentiation, metabolism and transcriptional activation.
PKCs may affect the invasion and metastasis of tumor cells. PKCζ is
a member of the PKC family that serves important roles in cell
growth, metabolism and other associated signal transduction
pathways (2,3). It has been established that PKCζ is a
tumor suppressor for numerous types of human cancer (4). However, studies have additionally
identified pro-oncogenic functions of PKCζ, although a complete
understanding of the detailed molecular mechanisms is lacking
(2–4). Additionally, it has been suggested
that PKCζ may be involved in inflammatory responses to diverse
stimuli in vitro and in vivo (5–8).
However, PKCζ expression in lung adenocarcinoma and the possible
outcomes of PKCζ signaling in the context of lung adenocarcinoma
remain to be completely elucidated.
Matrix metalloproteinases (MMPs) are able to degrade
the extracellular matrix and basement membrane, and serve important
roles in promoting tumor invasion and metastasis (9,10).
MMPs proteolytically activate or degrade a variety of non-matrix
substrates, including cytokines and chemokines, exerting a
regulatory function in inflammation and immunity (11). At present, the most
well-established roles for MMPs are in colorectal carcinogenesis,
wherein MMP-2 and MMP-9 have been implicated in colon cancer
progression and metastasis (12).
Studies into the role of metalloproteinases and their inhibitors in
lung adenocarcinoma are limited, and the results have been varied
(13,14).
Recently, studies have demonstrated that PKCs may
promote the metastasis of tumor cells in breast cancer, glioma and
other malignancies (15,16). PKCζ is able to activate the
mitogen-activated protein kinase (MAPK) signaling pathway, which
terminates with extracellular signal-regulated kinase (ERK)
phosphorylation and consequent promotion of MMP-2 and MMP-9
secretion, which may facilitate invasion and metastasis (17,18).
However, there have been few studies focusing on lung
adenocarcinoma, and whether PKCζ may mediate the invasion and
metastasis of lung adenocarcinoma by regulating MMP-2 and MMP-9
secretion remains unknown.
In the present study, PKCζ, MMP-2 and MMP-9
expression was assessed in lung adenocarcinoma and adjacent normal
lung tissues using immunohistochemistry, and associations between
their relative expression levels were analyzed. PKCζ was knocked
down in the lung adenocarcinoma cell line A549, and invasive
capacity, and MMP-2 and MMP-9 expression were observed, in order to
examine the effects of PKCζ on invasion and metastasis in lung
adenocarcinoma and to provide a novel method for the treatment of
lung adenocarcinoma.
Materials and methods
Specimen collection
The present study included 110 patients with
invasive lung adenocarcinoma (including all subtypes) who underwent
histological diagnosis at the Second People's Hospital of Weifang
(Weifang, China) between January 2012 and December 2014. Cases with
preoperative therapy or a history of other known malignancies were
excluded. Medical records were reviewed for clinicopathological
features, including sex, age, tumor size, smoking status, lymph
node metastasis, distant metastasis and pathological tumor, node,
metastasis (pTNM) stage. Patients were divided into two groups by
age (≤60 years and >60 years) and smoking status [smokers (>5
pack-year history) and non-smokers]. The pTNM stage was evaluated
in accordance with the 7th lung cancer TNM classification and
staging system (19). Adjacent
normal lung tissue (taken 5 cm from the edge of the cancerous
tissue) was used as the control.
Among the 110 lung adenocarcinoma patients: 59 were
male and 51 were female; 66 were ≤60 years old and 44 were >60;
and 44 were smokers and 66 were non-smokers. Regarding pTNM stage,
36 were stages I+II and 74 were stages III+IV. The present study
was approved by the Institutional Ethics Committee of Second
People's Hospital of Weifang, and written informed consent was
obtained from all participants.
Reagents
Anti-PKCζ (TA312044), anti-MMP-2 (TA806846) and
anti-MMP-9 (TA353338) antibodies were purchased from OriGene
Technologies, Inc. (Beijing, China). Cell culture plates, Matrigel
and Transwell chambers were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Lipofectamine 2000 transfection reagent
was purchased from Invitrogen (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and RIPA lysis buffer was purchased from Beyotime
Institute of Biotechnology (Haimen, China). The A549 cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA).
Immunohistochemistry
Immunohistochemical staining was performed on 4-µm,
formalin-fixed, paraffin-embedded sections. PKCζ primary antibody
was diluted 1:200 and manually applied to sections. All steps were
performed in accordance with the manufacturer's protocol. MMP-2 and
MMP-9 were not diluted for these experiments. PBS was used as the
negative control. Staining intensity and the percentage of positive
cells were evaluated under a microscope (BX53; Olympus Corporation,
Tokyo, Japan) in 5 high-magnification fields of vision, and 100
cells were counted in each field. The specific methods were
performed according to a previous study (18).
Cell culture
The lung adenocarcinoma cell line A549 was cultured
in F12K culture medium (21127–022; Invitrogen; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (Gibco, Thermo
Fisher Scientific, Inc.) at 37°C in 5% CO2. Experiments
were performed on cells in the logarithmic growth phase. The cells
were divided into 3 groups as follows: Control group, A549 cells
without any treatment; Scr/A549 group, A549 cells transiently
transfected with empty plasmid; and small interfering (si)PKCζ/A549
group, A549 cells transiently transfected with the PKCζ target
fragment 5′-GAGGAAGTGAGAGACATGTGT-3′. A total of 0.4 µg
plasmid/siRNA were transfected into the Scr/A549 group and the
(si)PKCζ/A549 group. All the vectors were synthesized by Shanghai
GeneChem Co., Ltd. (Shanghai, China). Transfections were performed
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The subsequent
experimentation commenced 48 h following transfection.
Western blotting
For Western blot analysis, cells or tissues were
directly lysed in RIPA lysis buffer. Aliquots of 50 µg protein were
separated by 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked in 5% skimmed milk for 1 h at room
temperature, and then immunoblotted using the appropriate primary
antibodies at 4°C overnight and the HRP conjugated secondary
antibodies at 37°C for 2 h. They were visualized by using enhanced
chemiluminescence reagents ECL (Pierce; Thermo Fisher Scientific,
Inc.). Western blot data in the present study are representative
from three independent experiments. The intensities of bands in
western blots were quantified by densitometry analysis using
AlphaImager HP (version 3.4.0; ProteinSimple, San Leandro, CA, USA)
and NIH ImageJ software (version 1.44; National Institutes of
Health, Bethesda, MD, USA). The following commercial antibodies
were used in this study: PKCζ (TA312044; 1:1,000), MMP-2 (TA806846;
1:1,000) and MMP-9 (TA353338; 1:1,000) (all from OriGene
Technologies, Inc.), β-actin (4970; 1:1,000) and HRP-linked
anti-rabbit IgG antibody (7074; 1:2,000) (both from Cell Signaling
Technology, Inc., Danvers, MA, USA).
Transwell invasion assay
Matrigel was added to the top chamber of a Transwell
system to form the matrix layer. To this matrix was added 100 µl
(1×105) Scr/A549 or siPKCζ/A549 cells; epidermal growth
factor was added into the lower chamber (500 µl/well). The
Transwell device was placed in an incubator (37°C; 5%
CO2) for 24 h. Following incubation, invaded cells were
fixed for 1 min in precooled methanol and Giemsa stained for 30 min
at room temperature. All experiments were repeated at least three
times. The number of invading cells was counted under a microscope
(IX71; Olympus Corporation) in five predetermined fields, total
magnification, ×200, using CellSens Standard (version 1.7; Olympus
Corporation).
Statistical analysis
All statistical analyses were performed using SPSS
version 19.0 (IBM Corp., Armonk, NY, USA). Data are presented as
the mean ± standard deviation. Statistical significance was
evaluated using Student's t-test or χ2 test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Immunohistochemical findings
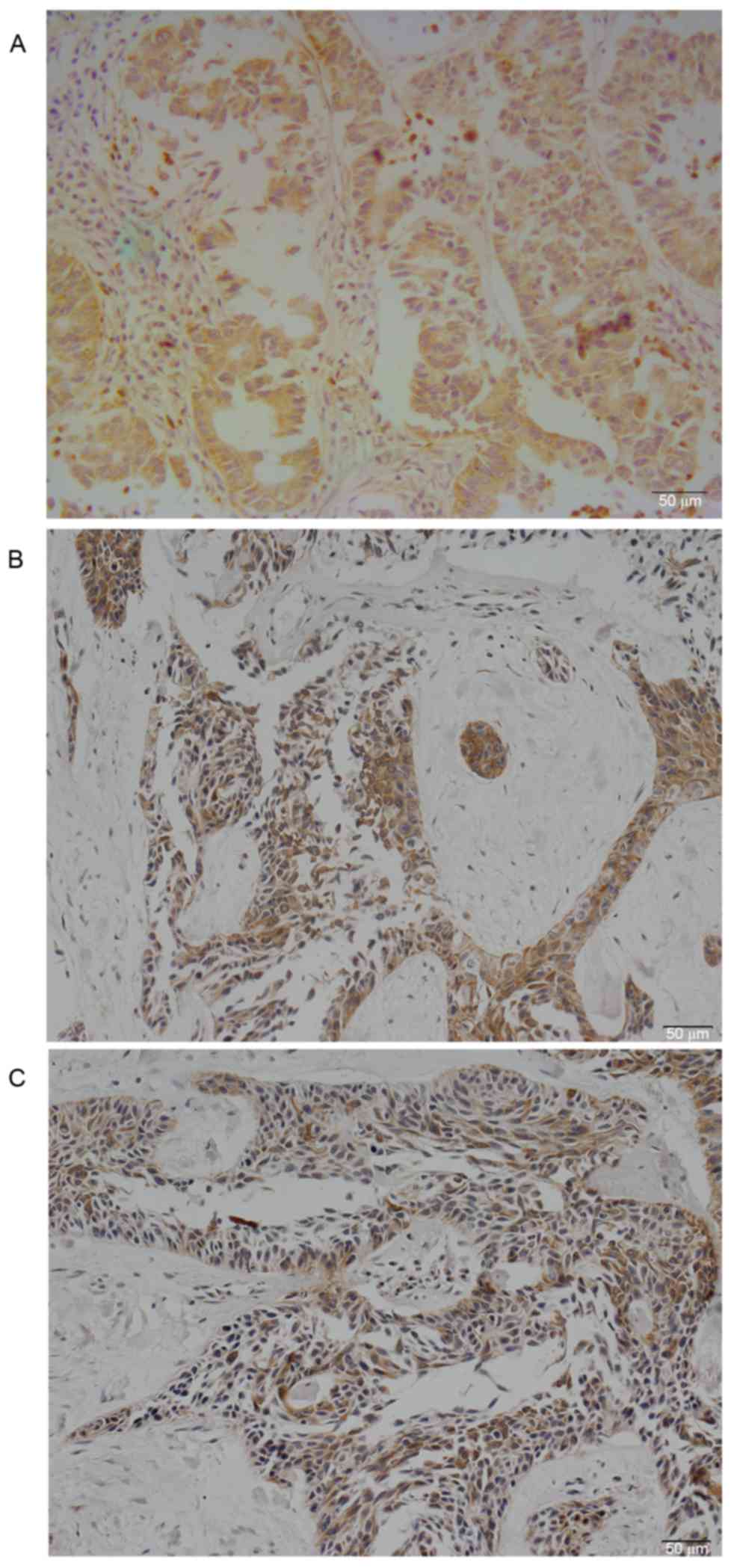
PKCζ was expressed in the cytoplasm of lung
adenocarcinoma cells (Fig. 1A).
Positive PKCζ staining was detected in 58 (52.73%) lung
adenocarcinoma samples, while only 5 (4.50%) normal lung tissues
exhibited weak positive staining. The difference was statistically
significant (χ2=62.479; P<0.01). The rate of positive
PKCζ staining in lung adenocarcinomas with lymph node metastases
(64.30%) was increased compared with non-metastatic samples
(45.60%) (P=0.017). The differences among other clinicopathological
parameters were not significant (Table
I).
 | Table I.Expression of PKCζ in lung
adenocarcinoma and association with clinical pathological
indices. |
Table I.
Expression of PKCζ in lung
adenocarcinoma and association with clinical pathological
indices.
|
|
| PKCζ |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinical pathological
index | Case no. | Positive | Negative | χ2 | P-value |
|---|
| Gender |
|
|
|
|
|
| Male | 59 | 32 | 27 | 0.116 | 0.848 |
|
Female | 51 | 26 | 25 |
|
|
| Age, years |
|
|
|
|
|
| ≤60 | 66 | 30 | 36 | 3.501 | 0.08 |
|
>60 | 44 | 28 | 16 |
|
|
| Diameter of tumor,
cm |
|
|
|
|
|
| ≤3 | 40 | 17 | 23 | 2.638 | 0.116 |
|
>3 | 70 | 41 | 29 |
|
|
| Smoker |
|
|
|
|
|
| Yes | 44 | 21 | 23 | 0.736 | 0.439 |
| No | 66 | 37 | 29 |
|
|
| Metastasis of LN |
|
|
|
|
|
| Yes | 39 | 26 | 13 | 4.710 | 0.045 |
| No | 71 | 32 | 39 |
|
|
| Distant
metastasis |
|
|
|
|
|
| Yes | 42 | 27 | 15 | 3.642 | 0.077 |
| No | 68 | 31 | 37 |
|
|
| TNM stage |
|
|
|
|
|
|
I+II | 36 | 16 | 20 | 1.473 | 0.309 |
|
III+IV | 74 | 42 | 32 |
|
|
MMP-2 and MMP-9 were primarily expressed in the
cytoplasm of lung adenocarcinomas (Fig. 1B and C); the rate of positive
staining was 55.45 and 61.82%, respectively. PKCζ expression was
associated with MMP-2 (P=0.012) and MMP-9 (P=0.006) expression in
lung adenocarcinoma (Table
II).
 | Table II.Association between PKCζ, MMP-2 and
MMP-9 expression in lung adenocarcinoma. |
Table II.
Association between PKCζ, MMP-2 and
MMP-9 expression in lung adenocarcinoma.
|
|
| PKCζ |
|
|
|---|
|
|
|
|
|
|
|---|
| Group | Case no. | Positive | Negative | χ2 | P-value |
|---|
| MMP-2 |
|
|
|
|
|
|
Positive | 61 | 39 | 22 | 6.900 | 0.012 |
|
Negative | 49 | 19 | 30 |
|
|
| MMP-9 |
|
|
|
|
|
|
Positive | 68 | 43 | 25 | 7.889 | 0.006 |
|
Negative | 42 | 15 | 27 |
|
|
Western blot analysis results
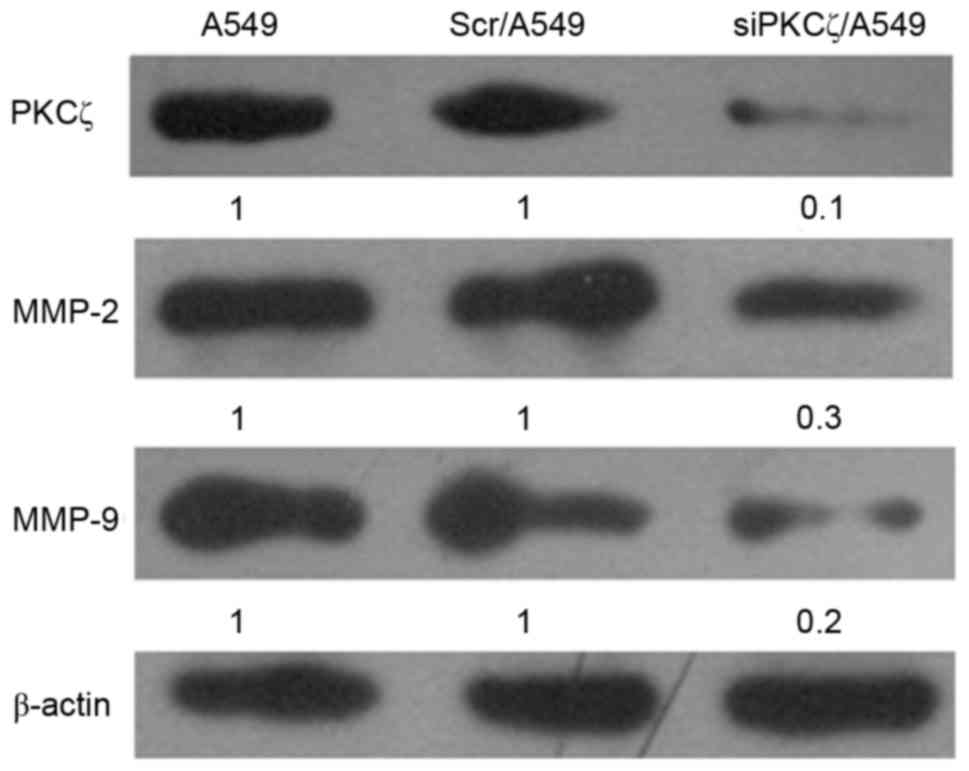
PKCζ expression siPKCζ/A549 cells was markedly
decreased compared with Scr/A549 cells, confirming that the reagent
successfully disrupted the expression of the target gene (Fig. 2). In addition, MMP-2 and MMP-9
protein expression in siPKCζ/A549 cells was markedly decreased
compared with Scr/A549 cells (Fig.
2).
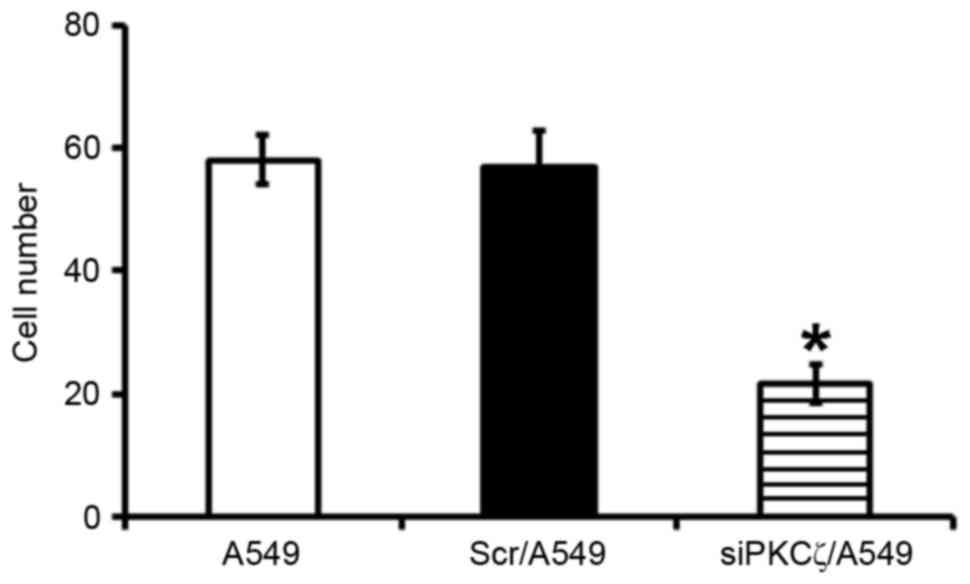
Transwell invasion assay findings
Fewer siPKCζ/A549 cells invaded through the membrane
and into the bottom chamber compared with Scr/A549 cells
(P<0.05), suggesting that PKCζ downregulation was able to
decreased the invasive ability of lung adenocarcinoma cells
(Fig. 3).
Discussion
In the present study, it was observed that positive
PKCζ expression in lung adenocarcinoma was associated with lymph
node metastasis, and MMP-2 and MMP-9 expression. It was
additionally observed that MMP-2 and MMP-9 expression was decreased
in A549 cells following PKCζ knockdown by siRNA, which weakened the
invasive ability of the cells in vitro.
PKCs are lipid-dependent serine/threonine protein
kinases present in mammalian cells that serve important roles in
growth, metabolism, proliferation and cytoskeletal remodeling. PKCs
are also important intracellular signaling molecules that have been
demonstrated to act as oncogenes and tumor suppressors, depending
on the cellular context and upon which protein adaptors interact
with which PKC isoforms (20–23).
PKC isozymes comprise three classes: Conventional (cPKC, α, β and
γ), novel (nPKC, δ, ε, η and θ) and atypical (aPKC, ζ and ι).
Different PKC isotypes are known to serve distinct regulatory
roles. PKCζ is an important subtype of atypical PKCs that is
involved in numerous signal transduction pathways.
PKC family proteins have been intensively studied
due to their association with cancer. Previous studies have
demonstrated that PKCζ may promote tumor cell chemotaxis in glioma,
liver cancer and breast cancer, thus promoting cancer cell invasion
and metastasis (16,24). When PKCζ is activated, it may
phosphorylate Lim domain kinase 1 and cofilin, promoting F-actin
depolymerization and polymerization, respectively, which affects
the cytoskeleton structure and inhibits cancer cell chemotaxis and
migration. Moreover, PKCζ is able to activate integrin-β1, which
enhances adhesion between cells, activates the MAPK pathway, and
promotes vascular endothelial growth factor (VEGF) expression and
angiogenesis, which may consequently promote tumor invasion and
metastasis (17,18).
Ma et al (25) observed that PKCζ is involved in
lung cancer cell adhesion and chemotaxis, and thus may affect the
invasion and metastasis of lung cancer. In the present study, it
was demonstrated that the rate of positive PKCζ staining in
patient-derived lung adenocarcinoma paraffin sections assayed by
immunohistochemistry was significantly increased compared with
adjacent tissues, and that PKCζ expression was associated with
lymph node metastasis. This result also suggested that PKCζ
affected the invasion and metastasis of lung adenocarcinoma. In
vitro Transwell invasion experiments using A549 lung
adenocarcinoma cells further confirmed that reducing PKCζ inhibited
the invasive capacity of tumor cells. Therefore, the results of the
present study demonstrated that PKCζ was able to promote the
invasion and metastasis of lung adenocarcinoma through in
vitro and in vivo methods.
MMPs are a family of Zn2+-dependent
endopeptidases that are able to degrade the extracellular matrix
and basement membrane, and serve an important role in physiological
and pathological processes. They have been regarded as critical
factors that promote tumor cell invasion. MMP-2 and MMP-9 are the
most important enzymes for type IV collagen degradation, and serve
important roles in tumor angiogenesis, invasion and metastasis
(26,27). The mechanism underlying this effect
involves the increase of VEGF secretion from tumor cells induced by
MMP-2 and MMP-9, promoting invasion and metastasis, which is
dependent on MAPK activation and ERK phosphorylation. In addition,
MMP-9 expression is known to cause emphysema in chronic obstructive
pulmonary disorder and angiogenesis/metastasis in lung cancer
(28).
Studies have demonstrated that MMP-2 and MMP-9
expression in non-small cell lung cancer is significantly increased
compared with normal tissue adjacent to the cancer, and that their
expression levels are associated with pathological grading and
staging, invasion and metastasis (20,29).
PKCζ was able to activate MAPK and the MAPK signaling pathway, and
promote VEGF overexpression, angiogenesis, tumor invasion and
metastasis (17). In the present
study, it was observed that PKCζ expression was associated with the
expression of MMP-2 and MMP-9 in lung adenocarcinoma, using
immunohistochemical detection. By decreasing the expression of PKCζ
in A549 cells, the invasiveness of siPKCζ/A549 cells decreased
significantly; decreased PKCζ expression coincided with reduced
secretion of MMP-2 and MMP-9. The above results suggested that the
PKCζ may promote lung cancer invasion and metastasis by affecting
MMP-2 and MMP-9 secretion in lung adenocarcinoma cells.
In conclusion, PKCζ expression was associated with
the invasion and metastasis of lung adenocarcinoma, making PKCζ a
potential target for gene therapy in lung cancer and providing a
theoretical basis for enhancing the survival rate of patients with
lung adenocarcinoma. PKCζ, MMP-2 and MMP-9 synergistically promoted
lung cancer invasion and metastasis, although the specific
mechanism remains unclear and requires further research.
Acknowledgements
The present study was supported by the Program of
Weifang Health Bureau in China (grant no. 2012012) and the Program
of Bureau of Science and Technology in Weifang Kuiwen District in
China (grant no. 201620).
References
|
1
|
Lu J, Wang W, Xu M, Li Y, Chen C and Wang
X: A global view of regulatory networks in lung cancer: An approach
to understand homogeneity and heterogeneity. Semin Cancer Biol.
42:31–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu J, Zhang B, Wu M, Li H, Niu R, Ying G
and Zhang N: Screening of a PKC zeta-specific kinase inhibitor
PKCzI257.3 which inhibits EGF-induced breast cancer cell
chemotaxis. Invest New Drugs. 28:268–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Butler AM, Scotti BML, Li S, Smith KE,
Fields AP and Murray NR: Protein kinase C zeta regulates human
pancreatic cancer cell transformed growth and invasion through a
STAT3-dependent mechanism. PLoS One. 8:e720612013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Queisser MA, Dada LA, Deiss-Yehiely N,
Angulo M, Zhou G, Kouri FM, Knab LM, Liu J, Stegh AH, DeCamp MM, et
al: HOIL-1L functions as the PKCζ ubiquitin ligase to promote lung
tumor growth. Am J Respir Crit Care Med. 190:688–698. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
LaVallie ER, Chockalingam PS,
Collins-Racie LA, Freeman BA, Keohan CC, Leitges M, Dorner AJ,
Morris EA, Majumdar MK and Arai M: Protein kinase Czeta is
up-regulated in osteoarthritic cartilage and is required for
activation of NF-kappaB by tumor necrosis factor and interleukin-1
in articular chondrocytes. J Biol Chem. 281:24124–24137. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdel-Halim M, Darwish SS, ElHady AK,
Hoppstädter J, Abadi AH, Hartmann RW, Kiemer AK and Engel M:
Pharmacological inhibition of protein kinase C (PKC)ζ downregulates
the expression of cytokines involved in the pathogenesis of chronic
obstructive pulmonary disease (COPD). Eur J Pharm Sci. 93:405–409.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diaz-Meco MT and Moscat J: The atypical
PKCs in inflammation: NF-κB and beyond. Immunol Rev. 246:154–167.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morin C, Fortin S and Rousseau E:
Bronchial inflammation induced PKCζ over-expression: Involvement in
mechanical properties of airway smooth muscle. Can J Physiol
Pharmacol. 90:261–269. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu M, Wang HF and Zhang HZ: Expression of
RECK and MMPs in hepatoblastoma and neuroblastoma and comparative
analysis on the tumor metastasis. Asian Pac J Cancer Prev.
16:4007–4011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Merdad A, Karim S, Schulten HJ, Dallol A,
Buhmeida A, Al-Thubaity F, Gari MA, Chaudhary AG, Abuzenadah AM and
Al-Qahtani MH: Expression of matrix metalloproteinases (MMPs) in
primary human breast cancer: MMP-9 as a potential biomarker for
cancer invasion and metastasis. Anticancer Res. 34:1355–1366.
2014.PubMed/NCBI
|
|
11
|
Parks WC, Wilson CL and López-Boado YS:
Matrix metalloproteinases as modulators of inflammation and innate
immunity. Nat Rev Immunol. 4:617–629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Babykutty S, Suboj P, Srinivas P, Nair AS,
Chandramohan K and Gopala S: Insidious role of nitric oxide in
migration/invasion of colon cancer cells by upregulating MMP-2/9
via activation of cGMP-PKG-ERK signaling pathways. Clin Exp
Metastasis. 29:471–492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim HIe, Lee HS, Kim TH, Lee JS, Lee ST
and Lee SJ: Growth-stimulatory activity of TIMP-2 is mediated
through c-Src activation followed by activation of FAK
PI3-kinase/AKT, and ERK1/2 independent of MMP inhibition in lung
adenocarcinoma cells. Oncotarget. 6:42905–42922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamura T, Nakanishi K, Hiroi S, Kumaki
F, Sato H, Aida S and Kawai T: Expression of membrane-type-1-matrix
metalloproteinase and metalloproteinase-2 in nonsmall cell lung
carcinomas. Lung Cancer. 35:249–255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang F, Dong W, Zeng W, Zhang L, Zhang C,
Qiu Y, Wang L, Yin X, Zhang C and Liang W: Naringenin prevents
TGF-β1 secretion from breast cancer and suppresses pulmonary
metastasis by inhibiting PKC activation. Breast Cancer Res.
18:382016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paul A, Danley M, Saha B, Tawfik O and
Paul S: PKCζ Promotes Breast Cancer Invasion by Regulating
Expression of E-cadherin and Zonula Occludens-1 (ZO-1) via
NFκB-p65. Sci Rep. 5:125202015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
S LH Yang, Li W YL, Ni M ZL and Yin CaZB:
Expression and correlation of PKCζ, MMP-2 and MMP-9 in breast
cancer. Chin J Clin Exp Pathol. 30:958–962. 2014.
|
|
19
|
Mirsadraee S, Oswal D, Alizadeh Y, Caulo A
and van Beek E Jr: The 7th lung cancer TNM classification and
staging system: Review of the changes and implications. World J
Radiol. 4:128–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rajagopalan S, Moyle MW, Joosten I and
Long EO: DNA-PKcs controls an endosomal signaling pathway for a
proinflammatory response by natural killer cells. Sci Signal.
3:ra142010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lim PS, Sutton CR and Rao S: Protein
kinase C in the immune system: From signalling to chromatin
regulation. Immunology. 146:508–522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fields AP and Regala RP: Protein kinase C
iota: Human oncogene, prognostic marker and therapeutic target.
Pharmacol Res. 55:487–497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Antal CE, Hudson AM, Kang E, Zanca C,
Wirth C, Stephenson NL, Trotter EW, Gallegos LL, Miller CJ, Furnari
FB, et al: Cancer-associated protein kinase C mutations reveal
kinase's role as tumor suppressor. Cell. 160:489–502. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun R, Gao P, Chen L, Ma D, Wang J,
Oppenheim JJ and Zhang N: Protein kinase C zeta is required for
epidermal growth factor-induced chemotaxis of human breast cancer
cells. Cancer Res. 65:1433–1441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma NZF, Guo HZX, Cao SNR and Zhang N:
Correlation between PKCζ expression and invasion, metastasis and
prognosis of adenoearcinoma of the lung. Chin J Clin Oncol.
37:557–560. 2010.
|
|
26
|
Kuo HY, Huang YS, Tseng CH, Chen YC, Chang
YW, Shih HM and Wu CW: PML represses lung cancer metastasis by
suppressing the nuclear EGFR-mediated transcriptional activation of
MMP2. Cell Cycle. 13:3132–3142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu Y, Ding Z, Jian H, Shen L, Zhu L and Lu
S: Prognostic value of MMP9 activity level in resected stage I B
lung adenocarcinoma. Cancer Med. 5:2323–2331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
King PT: Inflammation in chronic
obstructive pulmonary disease and its role in cardiovascular
disease and lung cancer. Clin Transl Med. 4:682015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
González-Arriaga P, Pascual T,
García-Alvarez A, Fernández-Somoano A, López-Cima MF and Tardón A:
Genetic polymorphisms in MMP 2, 9 and 3 genes modify lung cancer
risk and survival. BMC Cancer. 12:1212012. View Article : Google Scholar : PubMed/NCBI
|