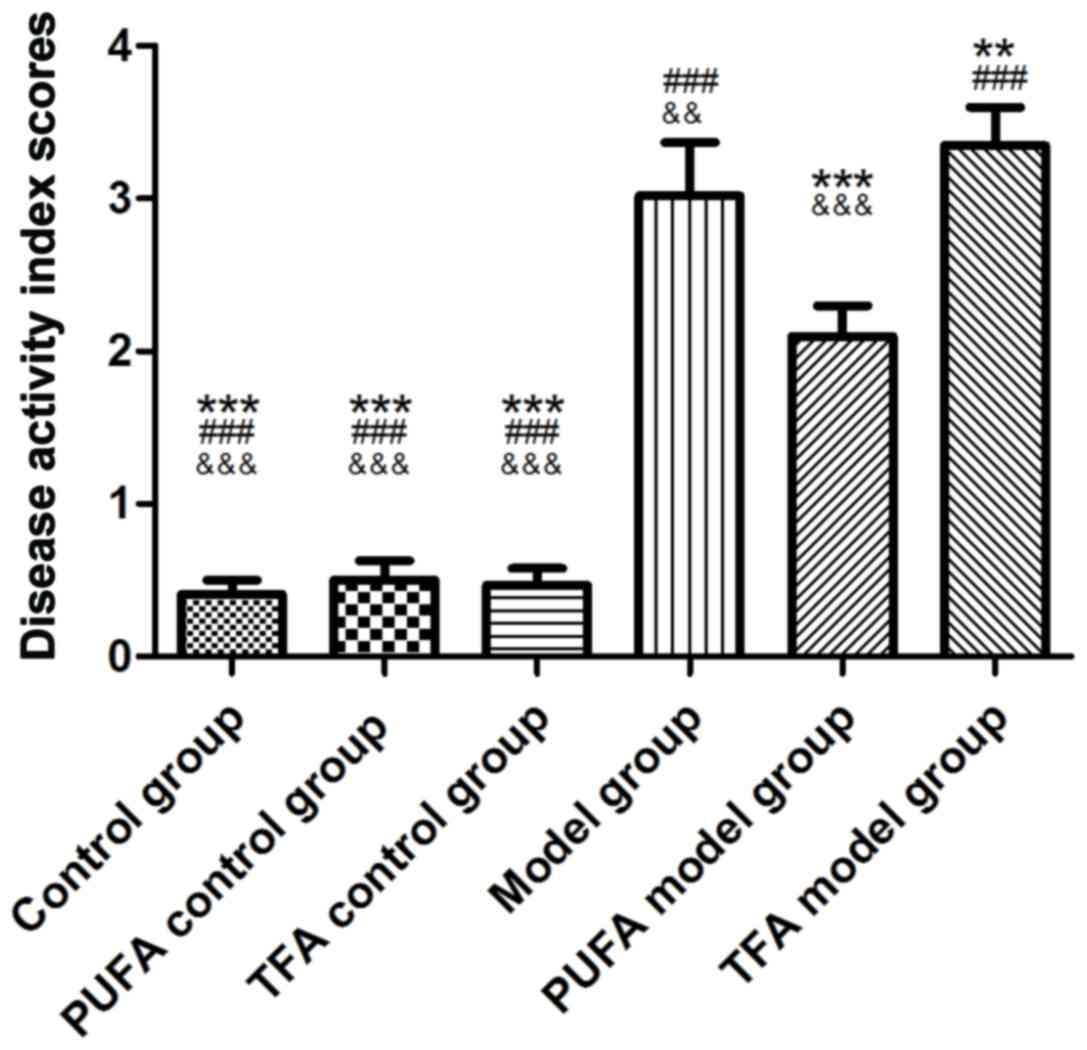
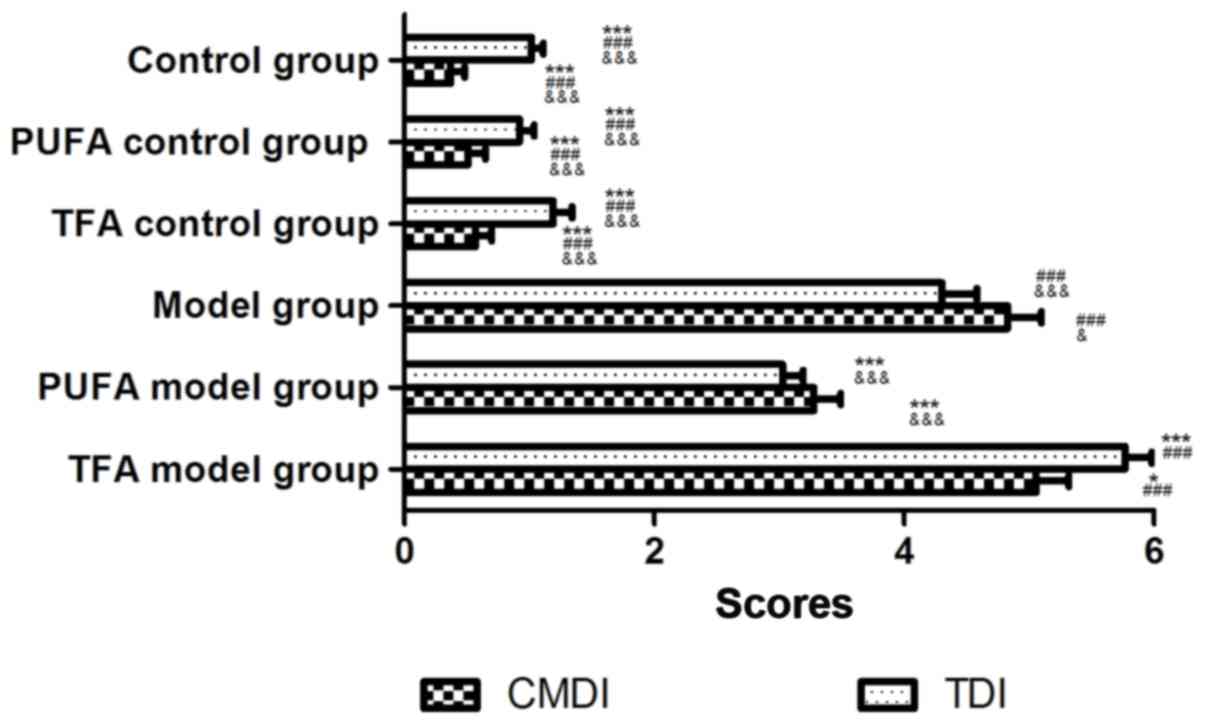
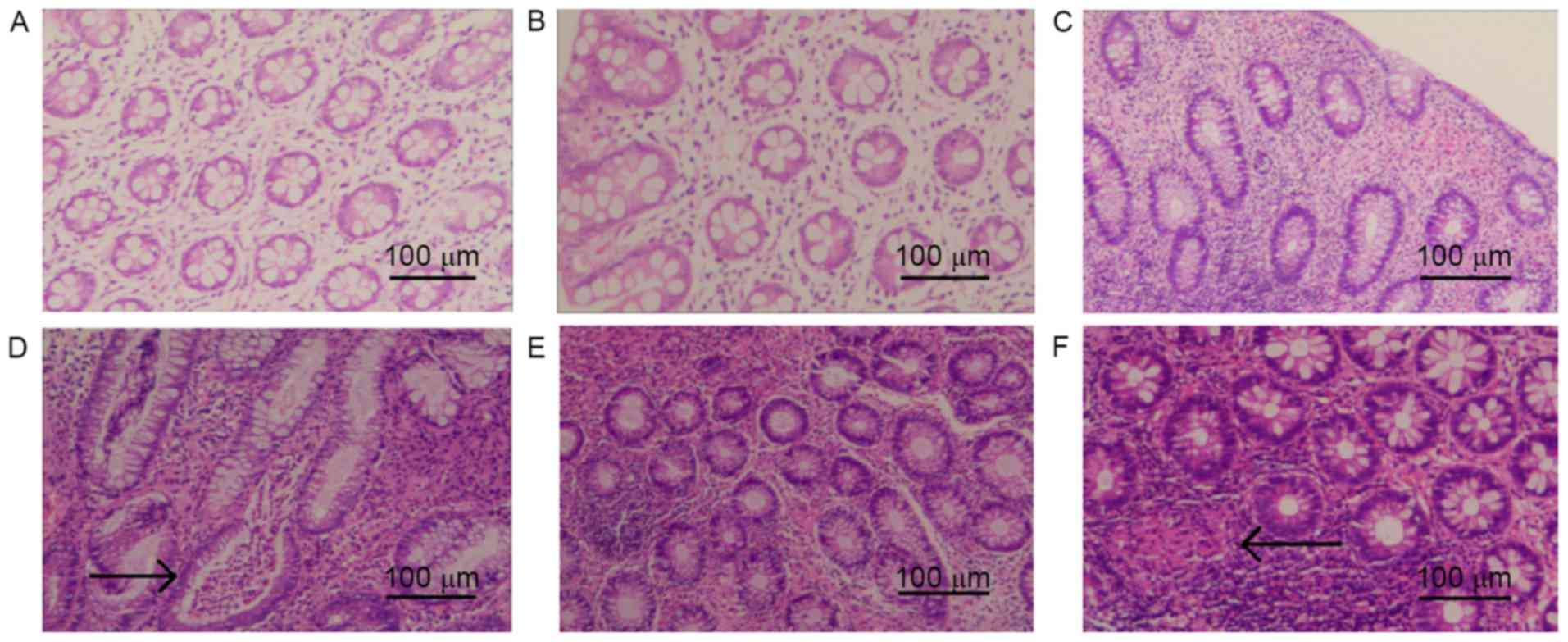
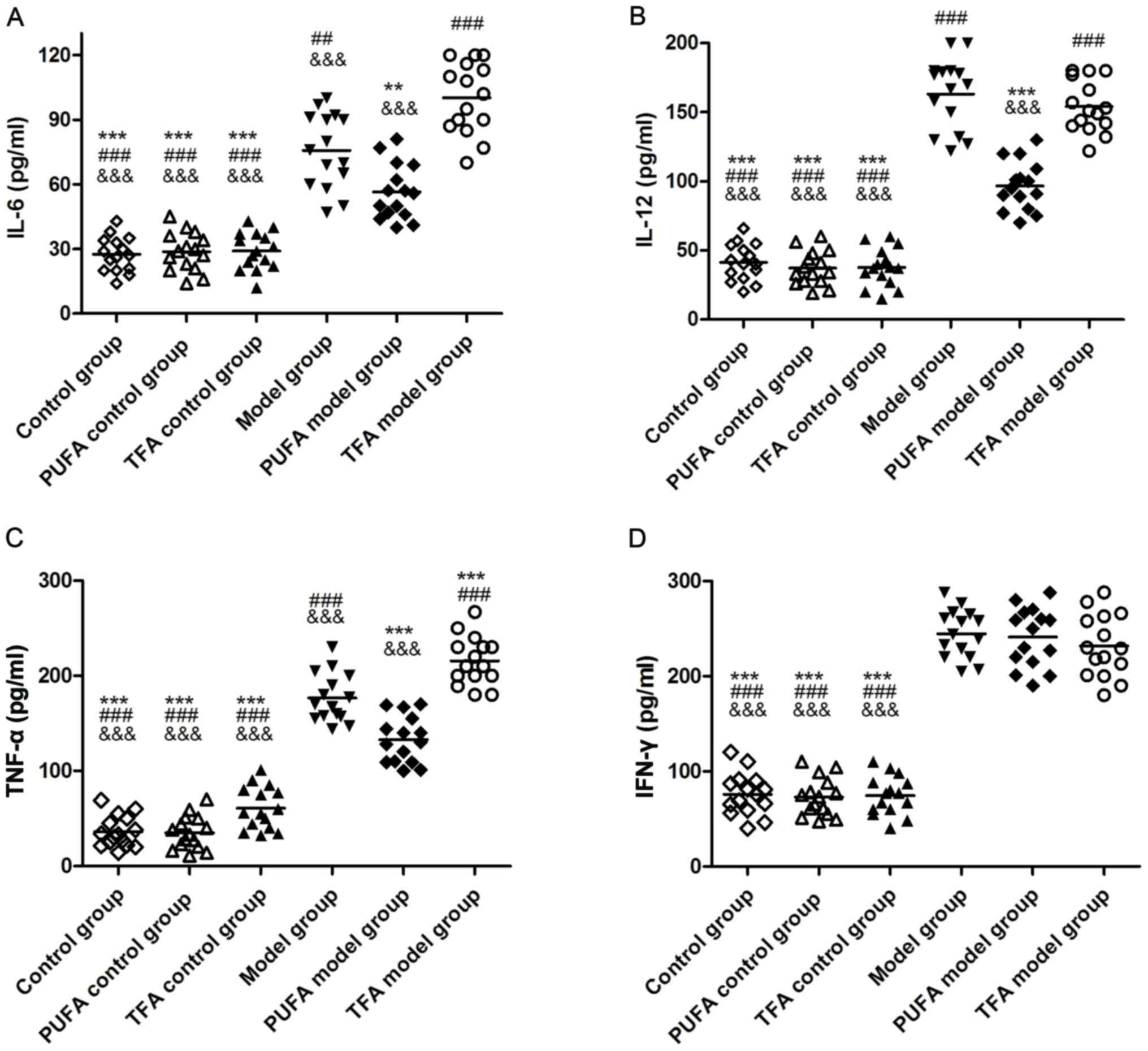
|
1
|
Abraham C and Cho JH: Inflammatory bowel
disease. N Engl J Med. 361:2066–2078. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khor B, Gardet A and Xavier RJ: Genetics
and pathogenesis of inflammatory bowel disease. Nature.
474:307–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asakura H, Suzuki K, Kitahora T and
Morizane T: Is there a link between food and intestinal microbes
and the occurrence of Crohn's disease and ulcerative colitis? J
Gastroenterol Hepatol. 23:1794–1801. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thia KT, Loftus EV Jr, Sandborn WJ and
Yang SK: An update on the epidemiology of inflammatory bowel
disease in Asia. Am J Gastroenterol. 103:3167–3182. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakanishi S, Nagano C, Miyahara M and
Sawano F: Sex differences in the association between the
eicosapentaenoic acid/arachidonic acid ratio and the visceral fat
area among patients with type 2 diabetes. Intern Med. 55:1269–1274.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borgeraas H, Hertel JK, Seifert R, Berge
RK, Bohov P, Ueland PM, Nygård O and Hjelmesæth J: Serum trans
fatty acids, asymmetric dimethylarginine and risk of acute
myocardial infarction and mortality in patients with suspected
coronary heart disease: A prospective cohort study. Lipids Health
Dis. 15:382016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ananthakrishnan AN, Khalili H, Konijeti
GG, Higuchi LM, de Silva P, Fuchs CS, Willett WC, Richter JM and
Chan AT: Long-term intake of dietary fat and risk of ulcerative
colitis and Crohn's disease. Gut. 63:776–784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marion-Letellier R, Savoye G, Beck PL,
Panaccione R and Ghosh S: Polyunsaturated fatty acids in
inflammatory bowel diseases: A reappraisal of effects and
therapeutic approaches. Inflamm Bowel Dis. 19:650–661. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Y, Lu N, Chen D, Meng L, Zheng Y and
Hui R: Effects of n-3 PUFA supplementation on plasma soluble
adhesion molecules: A meta-analysis of randomized controlled
trials. Am J Clin Nutr. 95:972–980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calder PC: Mechanisms of action of (n-3)
fatty acids. J Nutr. 142 Suppl:592S–599S. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adachi M, Kurotani R, Morimura K, Shah Y,
Sanford M, Madison BB, Gumucio DL, Marin HE, Peters JM, Young HA
and Gonzalez FJ: Peroxisome proliferator activated receptor gamma
in colonic epithelial cells protects against experimental
inflammatory bowel disease. Gut. 55:1104–1113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Su CG, Wen X, Bailey ST, Jiang W, Rangwala
SM, Keilbaugh SA, Flanigan A, Murthy S, Lazar MA and Wu GD: A novel
therapy for colitis utilizing PPAR-gamma ligands to inhibit the
epithelial inflammatory response. J Clin Invest. 104:383–389. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bassaganya-Riera J, Viladomiu M, Pedragosa
M, De Simone C and Hontecillas R: Immunoregulatory mechanisms
underlying prevention of colitis-associated colorectal cancer by
probiotic bacteria. PLoS One. 7:e346762012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng C, Xiao JH, Chang MJ and Wang JL:
Beneficial effects of THSG on acetic acid-induced experimental
colitis: Involvement of upregulation of PPAR-γ and inhibition of
the Nf-Kb inflammatory pathway. Molecules. 16:8552–8568. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hontecillas R, Horne WT, Climent M, Guri
AJ, Evans C, Zhang Y, Sobral BW and Bassaganya-Riera J:
Immunoregulatory mechanisms of macrophage PPAR-γ in mice with
experimental inflammatory bowel disease. Mucosal Immunol.
4:304–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakajima A, Wada K, Miki H, Kubota N,
Nakajima N, Terauchi Y, Ohnishi S, Saubermann LJ, Kadowaki T,
Blumberg RS, et al: Endogenous PPAR gamma mediates
anti-inflammatory activity in murine ischemia-reperfusion injury.
Gastroenterology. 120:460–469. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raman P, Kaplan BL, Thompson JT, Heuvel JP
Vanden and Kaminski NE: 15-Deoxy-delta12,14-prostaglandin
J2-glycerol ester, a putative metabolite of 2-arachidonyl glycerol,
activates peroxisome proliferator activated receptor gamma. Mol
Pharmacol. 80:201–209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chung SW, Kang BY and Kim TS: Inhibition
of interleukin-4 production in CD4+ T cells by
peroxisome proliferator-activated receptor-gamma (PPAR-gamma)
ligands: Involvement of physical association between PPAR-gamma and
the nuclear factor of activated T cells transcription factor. Mol
Pharmacol. 64:1169–1179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Research Council, . Guide For The
Care And Use Of Laboratory Animals. No. 85-23. National Academies
Press; Washington, DC: 1996
|
|
20
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. PLoS Biol.
8:e10004122010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mizoguchi A: Animal models of inflammatory
bowel disease. Prog Mol Biol Transl Sci. 105:263–320. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei W: Pharmacokinetics and
bioavailability of drug metabolismExperimental Methodology of
Pharmacology. 6th edition. People's Medical Publishing Company;
Guangzhou: 2010, View Article : Google Scholar
|
|
23
|
Ito R, Shin-Ya M, Kishida T, Urano A,
Takada R, Sakagami J, Imanishi J, Kita M, Ueda Y, Iwakura Y, et al:
Interferon-gamma is causatively involved in experimental
inflammatory bowel disease in mice. Clin Exp Immunol. 146:330–338.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao JY, Lu Y, Zhi M, Li CJ, Hu PJ and Gao
X: Inhibition of the interleukin-23/interleukin-17 pathway by
anti-interleukin-23p19 monoclonal antibody attenuates
2,4,6-trinitrobenzene sulfonic acid-induced Crohn's disease in
rats. Mol Med Rep. 10:2105–2110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding H, Gan HZ, Fan WJ, Cao LY, Xu JM and
Mei Q: Homocysteine promotes intestinal fibrosis in rats with
trinitrobenzene sulfonic acid-induced colitis. Dig Dis Sci.
60:375–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zingarelli B, Hake PW, Burroughs TJ,
Piraino G, O'Connor M and Denenberg A: Activator protein-1
signalling pathway and apoptosis are modulated by poly(ADP-ribose)
polymerase-1 in experimental colitis. Immunology. 113:509–517.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deepak P and Loftus EV Jr: Ustekinumab in
treatment of Crohn's disease: Design, development, and potential
place in therapy. Drug Des Devel Ther. 10:3685–3698. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bassaganya-Riera J, Reynolds K,
Martino-Catt S, Cui Y, Hennighausen L, Gonzalez F, Rohrer J,
Benninghoff AU and Hontecillas R: Activation of PPAR gamma and
delta by conjugated linoleic acid mediates protection from
experimental inflammatory bowel disease. Gastroenterology.
127:777–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Viladomiu M, Hontecillas R, Yuan L, Lu P
and Bassaganya-Riera J: Nutritional protective mechanisms against
gut inflammation. J Nutr Biochem. 24:929–939. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Borniquel S, Jädert C and Lundberg JO:
Dietary conjugated linoleic acid activates PPARγ and the intestinal
trefoil factor in SW480 cells and mice with dextran sulfate
sodium-induced colitis. J Nutr. 142:2135–2140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bassaganya-Riera J, DiGuardo M, Viladomiu
M, de Horna A, Sanchez S, Einerhand AW, Sanders L and Hontecillas
R: Soluble fibers and resistant starch ameliorate disease activity
in interleukin-10-deficient mice with inflammatory bowel disease. J
Nutr. 141:1318–1325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lian M, Luo W, Sui Y, Li Z and Hua J:
Dietary n-3 PUFA protects mice from con A induced liver injury by
modulating regulatory T cells and PPAR-γ expression. PLoS One.
10:e01327412015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu J, Zhou F, Chen Q, Kang A, Lu M, Liu
W, Zang X, Wang G and Zhang J: Chronic inflammation up-regulates
P-gp in peripheral mononuclear blood cells via the STAT3/Nf-κb
pathway in 2,4,6-trinitrobenzene sulfonic acid-induced colitis
mice. Sci Rep. 5:135582015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lytle C, Tod TJ, Vo KT, Lee JW, Atkinson
RD and Straus DS: The peroxisome proliferator-activated receptor
gamma ligand rosiglitazone delays the onset of inflammatory bowel
disease in mice with interleukin 10 deficiency. Inflamm Bowel Dis.
11:231–243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Speca S, Rousseaux C, Dubuquoy C, Rieder
F, Vetuschi A, Sferra R, Giusti I, Bertin B, Dubuquoy L, Gaudio E,
et al: Novel PPARγ modulator GED-0507-34 levo ameliorates
inflammation-driven intestinal fibrosis. Inflamm Bowel Dis.
22:279–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kundu P, Ling TW, Korecka A, Li Y,
D'Arienzo R, Bunte RM, Berger T, Arulampalam V, Chambon P, Mak TW,
et al: Absence of intestinal PPARγ aggravates acute infectious
colitis in mice through a lipocalin-2-dependent pathway. PLoS
Pathog. 10:e10038872014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hwang D: Fatty acids and immune
responses-a new perspective in searching for clues to mechanism.
Annu Rev Nutr. 20:431–456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim HB, Kumar A, Wang L, Liu GH, Keller
SR, Lawrence JC Jr, Finck BN and Harris TE: Lipin 1 represses
NFATc4 transcriptional activity in adipocytes to inhibit secretion
of inflammatory factors. Mol Cell Biol. 30:3126–3139. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mozaffarian D, Katan MB, Ascherio A,
Stampfer MJ and Willett WC: Trans fatty acids and cardiovascular
disease. N Engl J Med. 354:1601–1613. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Micha R and Mozaffarian D: Trans fatty
acids: Effects on cardiometabolic health and implications for
policy. Prostaglandins Leukot Essent Fatty Acids. 79:147–152. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ganguly R and Pierce GN: Trans fat
involvement in cardiovascular disease. Mol Nutr Food Res.
56:1090–1096. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Okada Y, Tsuzuki Y, Ueda T, Hozumi H, Sato
S, Hokari R, Kurihara C, Watanabe C, Tomita K, Komoto S, et al:
Trans fatty acids in diets act as a precipitating factor for gut
inflammation? J Gastroenterol Hepatol. 28(Suppl 4): S29–S32. 2013.
View Article : Google Scholar
|