Introduction
Despite the development of medical technology,
cerebrovascular disease, approximately 80% of which is ischemic
brain damage, remains as the major cause of death and disability
worldwide (1). Based on its
multi-directional differentiation and proliferation ability, bone
mesenchymal stem cell (BMSC) transplantation has attracted
increasing attention (2). When
BMSCs were injected into mice with a cerebral infarction, the cells
were observed to survive in the ischemic brain, migrate to the
cerebral infarct and promote neurological recovery (3). In addition, BMSCs have been observed
to secrete various cytokines and neurotrophic factors, such as
brain-derived neurotrophic factor (BDNF) and vascular endothelial
growth factor (VEGF) (4). BDNF has
been demonstrated to promote functional recovery of the ischemia
brain (5), and VEGF induces
neuroprotection and neurogenesis following cerebral ischemia
(6).
MicroRNAs (miRNAs) are a class of single-strand
non-coding RNAs consisting of 18–24 nucleotides in length (7), which are involved in multiple
physiological and pathological processes (8) by negatively regulating gene
expression at the post-transcriptional level by either target mRNA
degradation or translational repression (9). Increasing evidence has demonstrated
that miRNAs are crucial for BMSC differentiation (10). microRNA (miR)-705 was first
reported to be associated with steatohepatitis (11). Then miR-705 was identified to be a
novel negative regulator of cell lineage commitment of BMSCs by
directly targeting the homeobox A10 (HOXA10) mRNA 3′ untranslated
region (12). Furthermore, the
transfection of miR-705 mimics or inhibitors in BMSCs affected the
expression of forkhead box O1 (FoxO1) (13), suggesting miR-705-transfected BMSCs
might be a good model for study of function. The present study
indicated that the expression of miR-705 was associated with
ischemic brain damage of mice. Although previous studies have
observed that BMSCs could rescue ischemic brain damage (2–4), the
role of miR-705 combined with BMSCs in ischemic brain injury
remained to be elucidated.
In the present study, a lentiviral expression vector
was designed for miR-705 and BMSCs were infected with lentiviral
particles. Subsequently, the BMSCs overexpressing miR-705 were
injected into mice with ischemic brain injuries induced by middle
cerebral artery occlusion (MCAO). The effect of BMSCs infected with
miR-705 on neurological deficit scores, neuronal morphology and
cell apoptosis was investigated in order to confirm the function of
miR-705 in ischemic brain damage therapy.
Materials and methods
Experimental animals
A total of 50 male C57BL/6J mice in a SPF grade,
aged 8 weeks, weight 200+10 g, were purchased from Beijing
Huafukang Biotechnology Company (Beijing, China). Mice were housed
in a colony room under controlled temperature (22+3°C), and a 12:12
light-dark cycle, with food and water available. All animal care
and use was approved by the ethics committee of Beijing Chao-yang
Hospital.
Preparation of BMSCs
The C57BL/6J male mice in SPF grade were
anesthetized with chloral hydrate (30 mg/100 g; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) via peritoneal injection. The
diaphyses of the bones were cut in sterile conditions in order to
open the marrow cavity. Bone marrow was pushed out of the bone with
a syringe with a no. 9 needle containing Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.). A total of 5 ml bone marrow was aspired
and then mixed with phosphate-buffered saline (PBS) at a volume
ratio of 1:1. The mixture was added to the lymphocyte separation
solution at a volume ratio of 2:1, and centrifuged at 3,500 × g for
20 min at 4°C. The cloudy layer (lymphocytes) was harvested and
washed in serum-free DMEM twice after centrifugation at 1,500 × g
for 10 min at 4°C. The cells were incubated in complete medium in a
humidified environment with 5% CO2 at 37°C. The medium
was first changed after 24 h, and changed again after 48 h to
remove non-adherent cells. Cells were diluted with 9 ml Hanks
buffer and mixed with 1 ml 0.4% trypan blue for detection of the
cell viability. The BMSC passage was completed at a ratio of 1:2.
The cells of passage 3 were used in the following experiments.
Vector construction of a lentiviral
expression vector for miR-705
The target sequence of miR-705 was amplified by
polymerase chain reaction (PCR) and was inserted into the
pCDH-CMV-MCS-EF1-cop-green fluorescent protein (GFP) plasmid
(System Biosciences, Mountain View, CA). The recombinant plasmid
was verified by restriction endonuclease analysis and DNA
sequencing. 293T cells were purchased from American Type Culture
Collection and cultured in 25 cm2 flask containing 4 ml
DMEM with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.). At a density of 70–90%, 293T cells were cotransfected with
lentiviral vector pCDH-CMV-MCS-EF1-copGFP-miR-705 (System
Biosciences) and lentivirus package plasmid mixture. After 8 h, the
old medium was replaced with fresh medium for 48 h incubation at
37°C. The supernatant was collected and filtered with a 0.45 µm
membrane. The control cells were transfected with the control
lentiviral vector. Virus titers were measured according to the
expression of GFP. Furthermore, the expression level of miR-705 was
measured by reverse transcription-quantitative PCR (RT-qPCR).
Lentiviral particles were collected and stored at −80°C for further
experiments.
BMSCs were seeded into the 10 cm plate. At a density
of 80%, cells were infected with lentiviral particles of miR-705
[BMSCs-adenovirus (Ad)-miR-705], and the control was incubated with
control lentiviral particles (BMSCs-Ad). The cell density was
adjusted to 1×106 cells/ml prior to transplantation.
Animal model and grouping
Mice were fixed on the table, anesthetized with an
intraperitoneal injection of chloral hydrate (30 mg/100 g), and
subjected to MCAO for ischemic brain damage. In brief, the skin of
the middle of the neck was opened, and the left common carotid
artery, external carotid artery (ECA) and internal carotid artery
(ICA) were exposed. The origin of MCA was occluded with a
monofilament suture with a distal cylinder from ECA to ICA. The
sham-operative mice received the same surgery, except that the
filament was inserted only 10 mm and withdrawn a minute later.
A total of 40mice underwent 24 h reperfusion after 2
h ischemic brain injury and were divided into four groups as
follows: i) Sham group, underwent sham operation and received PBS
(n=10); ii) PBS group, subjected to MCAO and received PBS (n=10);
iii) BMSCs-Ad group, subjected to MCAO and injected with BMSCs-Ad
(200 µl, 1×106 cells/ml) through ECA (n=10); iv)
BMSCs-Ad-miR-705 group, subjected to MCAO and injected with BMSCs
infected with Ad-miR-705 (200 µl, 1×106 cells/ml) via
ECA (n=10). After 7 days, brain tissues were isolated for further
experiments.
RT-qPCR
Total RNA was extracted from the brain tissues using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The purity of RNA was
determined by absorbance at 260 nm and 280 nm, and RNA integrity
was verified by agarose gel electrophoresis. A total of 5 mg of RNA
was converted to cDNA using the iScript cDNA Synthesis kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). RT-qPCR was performed using
Ultra SYBR mixture (CW Biotech, Beijing, China) containing SYBR
Green I staining on a CFX96™ Real-time system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The temperature protocol
for PCR was as follows: 95°C for 5 min, 95°C for 5 sec, 60°C for 45
sec and 72°C 45 sec, repeated for 40 cycles. The primers for
RT-qPCR were as follows: BDNF, forward primer
5′-AGGTCTGACGACGACATCACT-3′ and reverse primer,
5′-CTTCGTTGGGCCGAACCTT-3′; VEGF, forward primer
5′-GCACATAGAGAGAATGAGCTTCC-3′ and reverse primer
5′-CTCCGCTCTGAACAAGGCT-3′; miR-705, forward primer
5′-AGTAGTGGTGGGAGGTGGGGTGGGC-3′ and reverse primer
5′-AGGGAGGTAGGGAGGACTGC-3′. β-actin was used as the internal
control: Forward primer, 5′-CCTGTATGCCTCTGGTCG-3′; reverse primer,
5′-GGCGTAACCCTCGTAGAT-3′.
Western blotting
Brain tissues were isolated from different groups.
Subsequently, 1 ml lysis buffer was added to extract the proteins.
The quantity of proteins was determined by Bicinchoninic Acid
protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
The protein samples were boiled for 5 min. A total of 30 µg of
proteins were separated on 10% SDS polyacrylamide gels, then
transferred to polyvinylidene fluoride (PVDF) membrane (EMD
Millipore, Billerica, MA, USA). The PVDF membrane was blocked with
5% non-fat milk for 1 h at room temperature, and immunoblotted with
antibodies against BDNF (catalog no. sc-546, 1:500) or VEGF
(catalog no. sc-152, 1:500) (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), respectively overnight at 4°C. Subsequently, the
membranes were washed three times with Tris-buffered saline with
Tween-20 (20 mM Tris, pH 7.5; 150 mM NaCl; 0.1% Tween-20), and
incubated with the horseradish peroxidase-labeled secondary
antibody (1:5,000, catalog no. LK-GAR007, MultiSciences
Biotechnology Corporate Ltd., Hangzhou, Zhejiang, China) for 1 h at
room temperature. The bands were visualized by Enhanced
Chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.) and
analyzed by Quantity One software version 4.4 (Bio-Rad
Laboratories, Inc.). β-actin (1:3,000, catalog no. A5441;
Sigma-Aldrich) was used as the internal control.
Hematoxylin eosin and terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
staining
The brains were removed and postfixed in freshly
prepared ice 4% paraformaldehyde, dehydrated with graded alcohols
and embedded in paraffin. The paraffin-embedded sections with 3–4
µm were deparaffinized with xylene and rehydrated with graded
alcohol, then stained with hematoxylin and eosin (HE). The images
of slices at ×400 magnification were analyzed by a light
microscope. Three fields were randomly selected from the damaged
area.
TUNEL staining was performed to visualize apoptotic
cells according to the manufacturer's protocols (In Situ Cell Death
Detection kit; POD; Roche Diagnositics, Basel, Switzerland).
Briefly, subsequent to being deparaffinized, the sections were
incubated with proteinase K and permeabilized with 0.5% Triton
X-100. Then the sections were incubated with TUNEL reaction mixture
for 60 min. For each sample, five non-overlapping fields at ×400
magnification in sections were randomly captured using a
fluorescence inverted microscope. The apoptotic cells were stained
in a green color.
Statistical analysis
All data were expressed as the mean ± standard
deviation, and were analyzed using SPSS 20 software (IBM Corp.,
Armonk, NY, USA) with a one-way analysis of variance followed by
the Least Significant Difference post hoc test. A value of
P<0.05 was considered to indicate a statistically significant
difference. Each experiment was repeated for a minimum of three
times. For RT-qPCR and western blotting experiments, each mouse
sample was expressed as a ratio to its corresponding β-actin
value.
Results
A lentiviral expression vector for
miR-705 was successfully constructed
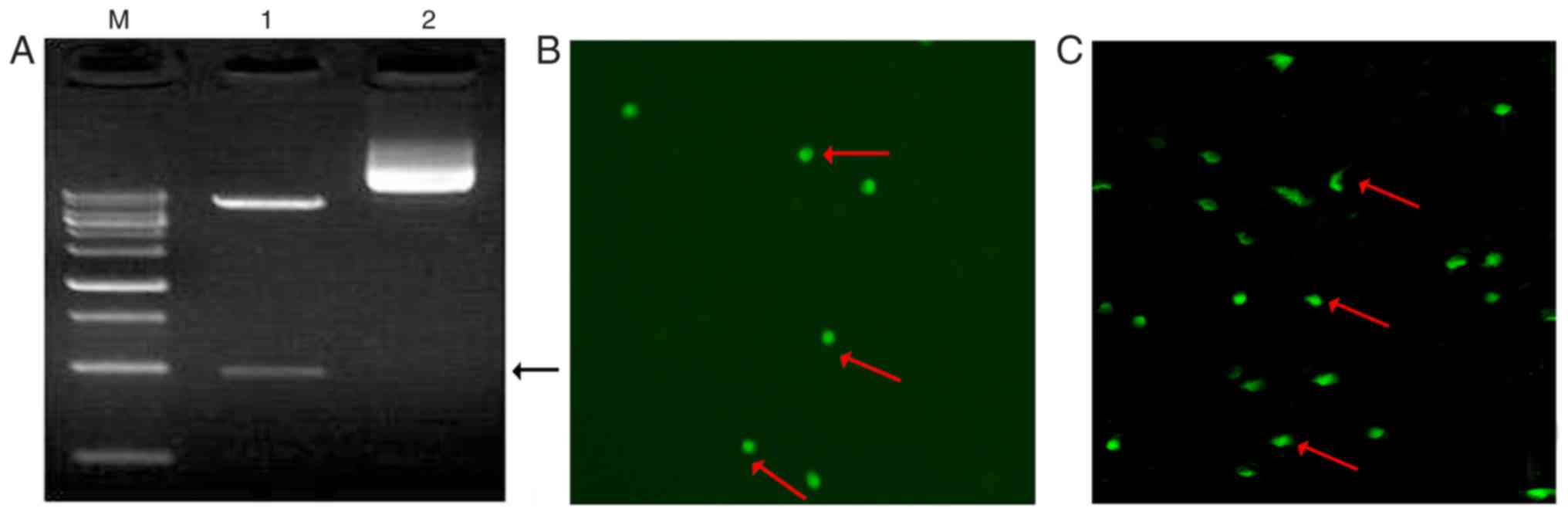
To examine the validity of the plasmid, the
recombinant plasmid (pCDH-CMV-MCS-EF1-copGFP-miR-705) was digested
with two restriction enzymes. Approximately 960 bp of the fragment
of interest was detected as presented in Fig. 1A. Combined with the DNA sequencing
results, it was concluded that the overexpression vector for
miR-705 was successfully constructed. To verify the transfection
efficiency, 293T cells were transfected with
pCDH-CMV-MCS-EF1-copGFP-miR-705. GFP was clearly expressed in 293T
cells (Fig. 1B) and BMSCs cells
(Fig. 1C). These results indicated
that a lentiviral expression vector for miR-705 had been
successfully constructed.
BMSCs-Ad-miR-705 mitigates the
neurological impairment in ischemic brain injury mice
The effect of different treatments on nerve function
were evaluated by neurological deficit scores (Nds), according to a
previous study (14). As presented
in Table I, the sham operation has
no impairment on nerve function. The NDs of PBS group were the
highest, suggesting that nerve function was severely damaged. The
NDs of BMSCs group was reduced, indicating that BMSCs
transplantation partly reduced the neurological impairment. In
addition, compared with PBS and BMSCs groups, the NDs of
BMSCs-Ad-miR-705 were the lowest with a significant difference
(P<0.05). These results indicated that BMSC transplantation
mitigated the neurological impairment, and that Ad-miR-705
infection improved the repair capacity of BMSCs.
 | Table I.Neurological deficit scores from
different groups. |
Table I.
Neurological deficit scores from
different groups.
| Groups | Neurological deficit
scores |
|---|
| Sham group | 0 |
| Phosphate-buffered
saline group | 9.34±1.21 |
| BMSCs-Ad | 6.32±1.33 |
|
BMSCs-Ad-microRNA-705 | 2.13±1.14 |
BMSCs-Ad-miR-705 suppresses neuronal
cell apoptosis in MCAO-induced cerebral infarction
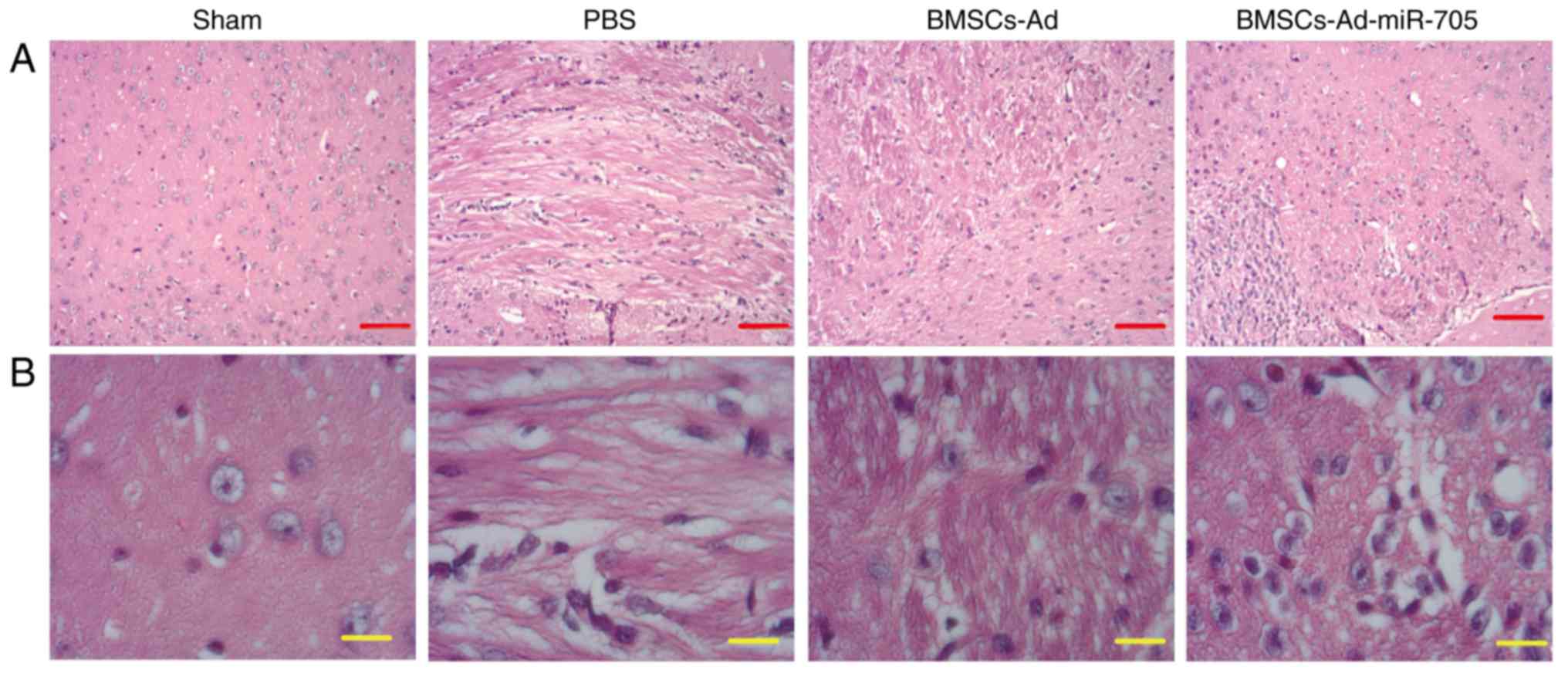
To examine the effect of BMSC transplantation on
neuronal morphology, brain tissues were isolated from different
groups and HE staining was conducted. In Fig. 2, normal neuronal structure and
morphology were observed in the sham group. In the PBS group, MCAO
resulted in marked neuronal injury with loose nerve fibers, and
certain neocortical neurons were clearly damaged with
characteristic vacuolation. Transplantation with BMSCs or
BMSCs-Ad-miR-705 markedly improved neurological recovery, for
example the number of neurons was increased and the neuronal
morphology became normal. In addition, BMSCs-Ad-miR-705 enhanced
the effect of BMSCs on neuronal morphology recovery.
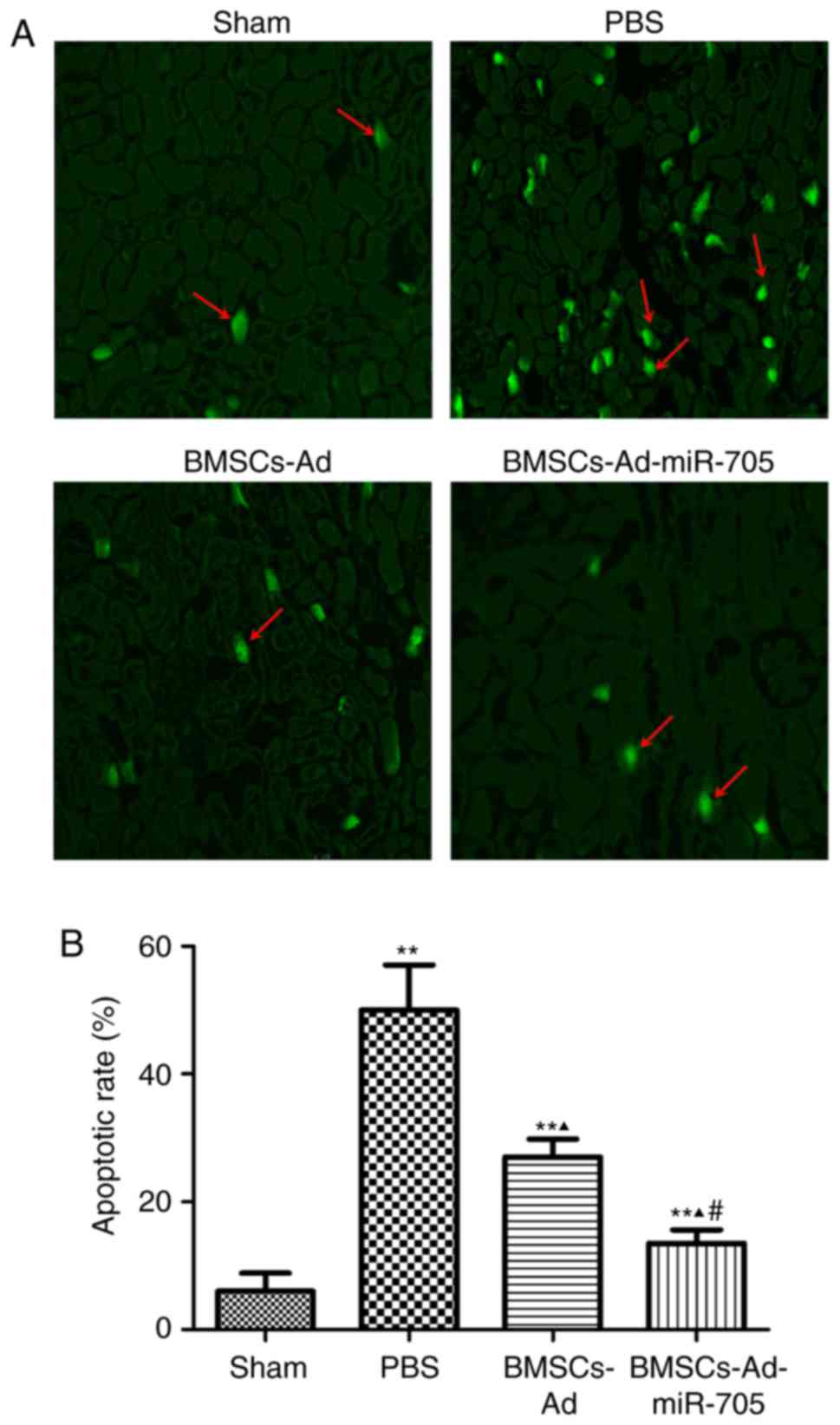
The TUNEL assay was used to further detect the
effect of BMSCs on neuronal cell apoptosis. Few apoptotic cells
were identified in the sham group, and different apoptotic rates
were observed in the three other groups in Fig. 3. The apoptotic rate of the PBS
group was the greatest at ~50%. BMSC transplantation partly reduced
the apoptotic rate (27%). And the apoptotic rate of
BMSCs-Ad-miR-705 was the lowest at ~13%. These results indicated
that BMSCs-Ad-miR-705 significantly inhibited cell apoptosis caused
by MCAO.
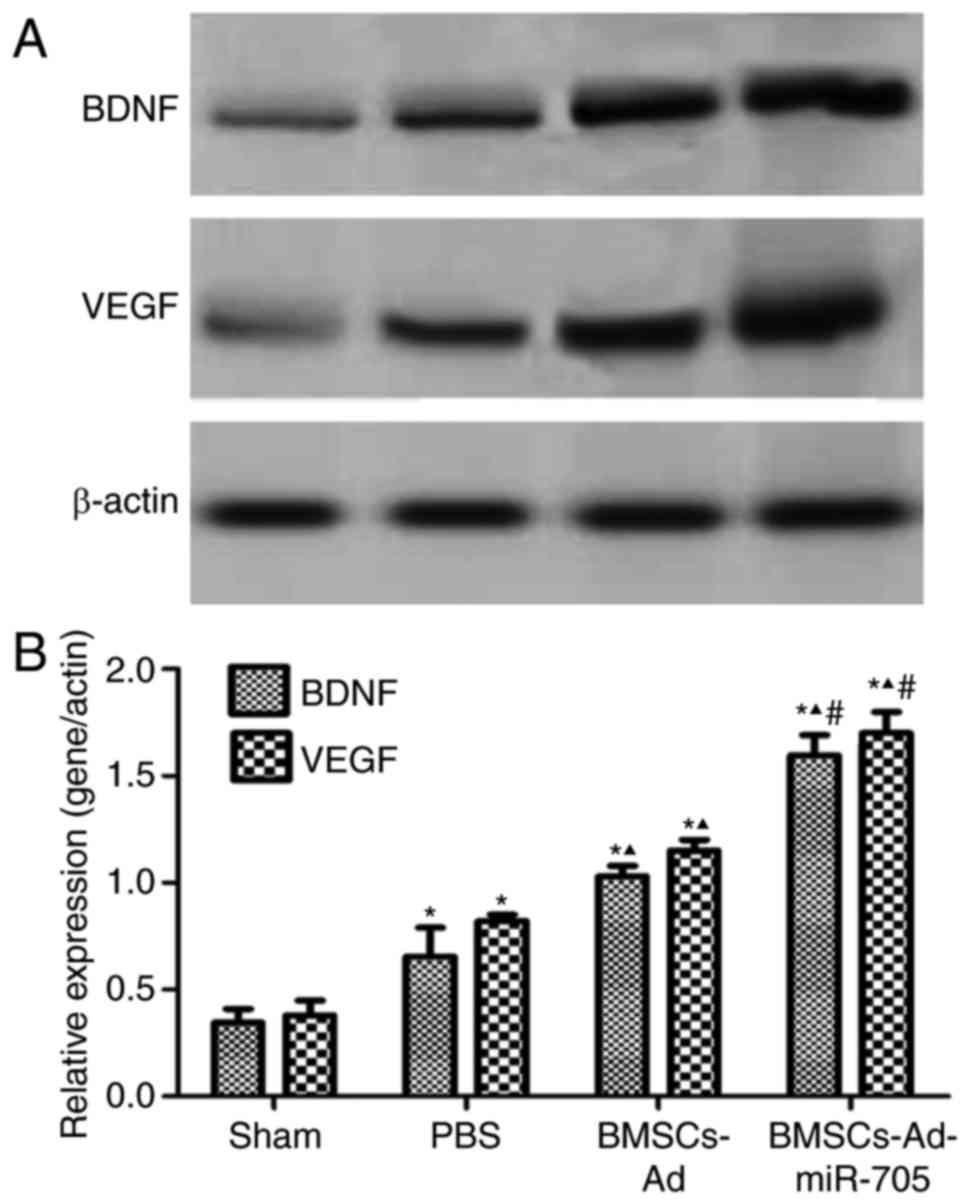
BMSCs-Ad-miR-705 improves BDNF and
VEGF expression on mRNA and protein levels
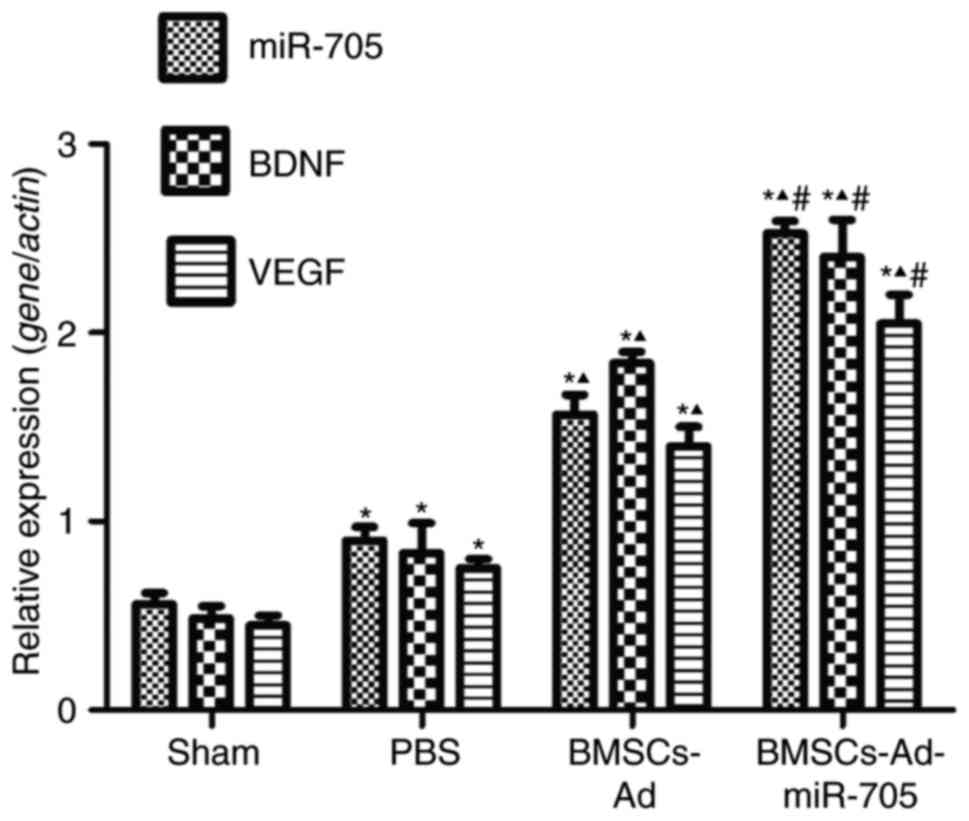
To investigate the mechanism of BMSCs-Ad-miR-705
stimulating neurological recovery, the expression of two factors,
BDNF and VEGF, were examined in brain tissues. Compared with the
sham, PBS and BMSCs groups, the BMSCs-Ad-miR-705 group
significantly increased the expression of miR-705, reconfirming the
successful construction of Ad-miR-705. In Fig. 4, low and stable levels of BDNF,
VEGF and miR-705 were detected in the sham group. Compared with the
sham group, the expression levels of BDNF, VEGF and miR-705 were
upregulated with significance (P<0.05). However, the levels of
BDNF, VEGF and miR-705 in BMSCs-Ad and BMSCs-Ad-miR-705 groups were
increased compared with those in the PBS group. The levels of BDNF,
VEGF and miR-705 in BMSCs-Ad-miR-705 group were the greatest. In
agreement, similar results were detected when protein expression
levels of BDNF and VEGF were measured using western blotting
(Fig. 5). This suggested that BMSC
transplantation stimulated the expression of BDNF, VEGF and
miR-705, and Ad-miR-705 infection more significantly enhanced the
mRNA and protein levels of BDNF and VEGF.
Discussion
The present study demonstrated that administration
of BMSCs can effectively improve functional recovery in animal
models of cerebral ischemia, which is in agreement with that of a
previous study (15). The current
study identified that BMSCs-Ad-miR-705 significantly ameliorated
neurological deficit, suppressed neuronal apoptosis and improved
the expression of BDNF and VEGF in mice with ischemic brain injury.
Taken together, these results indicate that injection of
BMSCs-Ad-miR-705 is a potential and effective therapy for
neurological recovery following cerebral ischemic injury.
In the present study, a lentiviral recombined
expression vector was constructed for miR-705 overexpression. GFP
was detected in plasmid-transfected 293T cells and lentiviral
particle-infected BMSCs, indicating successful construction of
Ad-miR-705. In Fig. 4, the highest
expression level of miR-705 was observed in
BMSCs-Ad-miR-705-injected mice, suggesting that miR-705 was
successfully overexpressed directed by Ad-miR-705. Chen et
al (16) identified that
Ad-VEGF could infect BMSCs and direct the expression of VEGF in
BMSCs. Combined with the results of the present study, it is
suggested that this method of lentivirus-mediated overexpression of
target genes in BMSCs provides novel insight for cellular
therapy.
miR-705 was first identified to be significantly
expressed in BMSCs from osteoporosis bone marrow. TNFα and ROS have
been observed to mediate the overexpression of miR-705 to regulate
the cell lineage commitment of BMSCs through NF-κB signaling
(12). The present study focused
on investigating the function of miR-705 in cerebral ischemic
damage. In mice with cerebral ischemia, intravenous BMSC therapy
reduced cell apoptosis and promoted endogenous cell proliferation
(17), and stimulated functional
recovery for brain repair (18).
In the current study, compared with the PBS group, the NDs of the
BMSCs-Ad group were decreased, the neurological morphology was
improved and the apoptosis rate of neurons were declined. These
results were in agreement with the abovementioned previous
research. At present, two targets of miR-705 have been reported:
HOXA10 and FoxO1. HOXA10 is a member of the family of
homeodomain-containing transcription factors, and its
overexpression inhibits adipocyte differentiation of BMSCs
(12). However, the decrease of
FoxO1 leads to oxidative damage and inhibition of BMSC
differentiation (13). According
to the results of the current study, it was suggested that HOXA10
is the main target gene of miR-705 in improving BMSC therapy.
However, further experiments are required.
Previous studies have indicated that administration
of BMSCs facilitates the release of neurovascular trophic factors
from activated astrocytes, including BDNF, nerve growth factor,
VEGF and basic fibroblast growth factor through activating
astrocytic phosphatidylinositol 3-kinase (PI3K) and extracellular
signal-regulated kinase pathways (19,20).
During the process of neurological recovery, these factors reduced
neuronal apoptosis and enhanced neuronal regeneration and cellular
differentiation (21). It was
observed that BMSC transplantation increased the expression of BDNF
and VEGF. Mature BDNF is crucial in the protection of the neonatal
or developing brain from ischemia injury (5). Administration of BMSCs-BDNF into mice
significantly promoted functional recovery and reduced the numbers
of TUNEL-positive apoptotic cells (22). VEGF promotes the formation of new
cerebral blood vessels, and injection of VEGF improves neurological
function and protects the brain against ischemia (23). VEGF-BMSCs combination therapy
ameliorates ischemic damage by the activation of the PI3K/protein
kinase B/glycogen synthase kinase 3β signaling pathway (16). VEGF, in addition to BDNF, has been
implicated in the regulation of brain neurogenesis by promoting the
proliferation and differentiation of neuronal precursors (24). The current on the effect of BMSCs
on the expression of BDNF and VEGF was in agreement with the
previous data and it was observed that BMSCs-Ad-miR-705 enhanced
the outcome of BMSCs transplantation on NDs improvement, neuronal
apoptosis and the expression VEGF and BDNF. Thus, these results
provided novel insight into that the role of miR-705 in function
neuronal recovery via induction of the secretion of growth factors,
and indicated miR-705 as a potential therapeutic target for stem
cell-mediated regenerative medicine for cerebral ischemia.
In summary, BMSCs-Ad-miR-705 promoted the secretion
of VEGF and BDNF, suppressed the neuronal apoptosis and stimulated
the neuronal regeneration, in turn functioning in the impairment of
ischemic brain damage. However, the mechanism by which miR-705
functioned in the BMSCs-mediated signaling pathway requires further
investigation.
Glossary
Abbreviations
Abbreviations:
|
BDNF
|
brain-derived neurotrophic factor
|
|
ECA
|
external carotid artery
|
|
HE
|
hematoxylin eosin
|
|
ICA
|
internal carotid artery
|
|
MCAO
|
middle cerebral artery occlusion
|
|
miR-705
|
miRNA-705
|
|
NDs
|
neurological deficit scores
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Wang Hy, Wang Gl, Yu Yh and Wang Y: The
role of phosphoinositide-3-kinase/Akt pathway in propofol-induced
postconditioning against focal cerebral ischemia-reperfusion injury
in rats. Brain Res. 1297:177–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schouten JW, Fulp CT, Royo NC, Saatman KE,
Watson DJ, Snyder EY, Trojanowski JQ, Prockop DJ, Maas AI and
McIntosh TK: A review and rationale for the use of cellular
transplantation as a therapeutic strategy for traumatic brain
injury. J Neurotrauma. 21:1501–1538. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Z, Cai X, Xu A, Xu F and Liang Q:
Bone marrow stromal cell transplantation through tail vein
injection promotes angiogenesis and vascular endothelial growth
factor expression in cerebral infarct area in rats. Cytotherapy.
17:1200–1212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shichinohe H, Ishihara T, Takahashi K,
Tanaka Y, Miyamoto M, Yamauchi T, Saito H, Takemoto H, Houkin K and
Kuroda S: Bone marrow stromal cells rescue ischemic brain by
trophic effects and phenotypic change toward neural cells.
Neurorehab Neural Repair. 29:80–89. 2015. View Article : Google Scholar
|
|
5
|
Chen A, Xiong LJ, Tong Y and Mao M: The
neuroprotective roles of BDNF in hypoxic ischemic brain injury
(Review). Biomed Rep. 1:167–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun Y, Jin K, Xie L, Childs J, Mao XO,
Logvinova A and Greenberg DA: VEGF-induced neuroprotection,
neurogenesis, and angiogenesis after focal cerebral ischemia. J
Clin Invest. 111:1843–1851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshino H, Seki N, Itesako T, Chiyomaru T,
Nakagawa M and Enokida H: Aberrant expression of microRNAs in
bladder cancer. Nat Rev Urol. 10:396–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lian JB, Stein GS, Van Wijnen AJ, Stein
JL, Hassan MQ, Gaur T and Zhang Y: MicroRNA control of bone
formation and homeostasis. Nat Rev Endocrinol. 8:212–227. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dolganiuc A, Petrasek J, Kodys K, Catalano
D, Mandrekar P, Velayudham A and Szabo G: MicroRNA expression
profile in Lieber-DeCarli diet-induced alcoholic and methionine
choline deficient diet-induced nonalcoholic steatohepatitis models
in mice. Alcohol Clin Exp Res. 33:1704–1710. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liao L, Yang X, Su X, Hu C, Zhu X, Yang N,
Chen X, Shi S, Shi S and Jin Y: Redundant miR-3077-5p and miR-705
mediate the shift of mesenchymal stem cell lineage commitment to
adipocyte in osteoporosis bone marrow. Cell Death Dis. 4:e6002013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liao L, Su X, Yang X, Hu C, Li B, Lv Y,
Shuai Y, Jing H, Deng Z and Jin Y: TNF-α Inhibits FoxO1 by
Upregulating miR-705 to aggravate oxidative damage in bone
marrow-derived mesenchymal stem cells during osteoporosis. Stem
Cells. 34:1054–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schallert T, Kozlowski DA, Humm JL and
Cocke RR: Use-dependent structural events in recovery of function.
Adv Neurol. 73:229–238. 1997.PubMed/NCBI
|
|
15
|
Jahromi G Pirzad, Seidi S, Sadr SS,
Shabanzadeh AP, Keshavarz M, Kaka GR, Hosseini SK, Sohanaki H and
Charish J: Therapeutic effects of a combinatorial treatment of
simvastatin and bone marrow stromal cells on experimental embolic
stroke. Basic Clin Pharmacol Toxicol. 110:487–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen B, Zhang F, Li QY, Gong A and Lan Q:
Protective effect of Ad-VEGF-bone mesenchymal stem cells on
cerebral infarction. Turk Neurosurg. 26:8–15. 2016.PubMed/NCBI
|
|
17
|
Gutiérrez-Fernández M, Rodríguez-Frutos B,
Ramos-Cejudo J, Vallejo-Cremades M Teresa, Fuentes B, Cerdán S and
Díez-Tejedor E: Effects of intravenous administration of allogenic
bone marrow-and adipose tissue-derived mesenchymal stem cells on
functional recovery and brain repair markers in experimental
ischemic stroke. Stem Cell Res Ther. 4:112013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Lin Z, Shao B, Zhuge Q and Jin K:
Therapeutic applications of bone marrow-derived stem cells in
ischemic stroke. Neurol Res. 35:470–478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu J, Sun Z, Sun HS, Wu J, Weisel RD,
Keating A, Li ZH, Feng ZP and Li RK: Intravenously administered
bone marrow cells migrate to damaged brain tissue and improve
neural function in ischemic rats. Cell Transplant. 16:993–1005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Q, Chen ZW, Zhao YH, Liu BW, Liu NW,
Ke CC and Tan HM: Bone marrow stromal cells combined with sodium
ferulate and n-butylidenephthalide promote the effect of
therapeutic angiogenesis via advancing astrocyte-derived trophic
factors after ischemic stroke. Cell Transplant. 26:229–242. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wan H, Li F, Zhu L, Wang J, Yang Z and Pan
Y: Update on therapeutic mechanism for bone marrow stromal cells in
ischemic stroke. J Mol Neurosci. 52:177–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yao RQ, Qi DS, Yu HL, Liu J, Yang LH and
Wu XX: Quercetin attenuates cell apoptosis in focal cerebral
ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling
pathway. Neurochem Res. 37:2777–2786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li N, Wang P, Ma XL, Wang J, Zhao LJ, Du
L, Wang LY, Wang XR and Liu KD: Effect of bone marrow stromal cell
transplantation on neurologic function and expression of VEGF in
rats with focal cerebral ischemia. Mol Med Rep. 10:2299–2305. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deindl E: Mechanistic insights into the
functional role of vascular endothelial growth factor and its
signalling partner brain-derived neurotrophic factor in angiogenic
tube formation. Acta Physiol (Oxf). 211:268–270. 2014. View Article : Google Scholar : PubMed/NCBI
|