Introduction
Gastric tumor is the type of tumor with the third
highest mortality rate worldwide and is the second most
frequently-diagnosed type of cancer in China (1,2).
Traditionally, gastric tumor was considered to be unrelated to
estrogen signaling. Epidemiological studies reported a male
predominance in gastric cancer (male/female ratio of 2-3:1), and it
has been proposed that there may be a protective function of
estrogen in gastric tumorigenesis (3,4).
However, studies into the expression patterns of estrogen receptor
(ER)-α in samples from gastric tumor patients were inconsistent
(5,6). ER-α expression was frequently low and
variable (0–62.5%) in gastric cancer specimens (5). ER-β was considered to be an
inhibitory factor in the invasiveness of gastric tumor. Therefore,
ER-β-positivity has been proposed as a prognostic marker (6). A previous study demonstrated that a
novel isoform, ER-α36, was expressed in specimens from patients
with gastric cancer (7).
Upregulated expression of ER-α36 was positively associated with
large size, increased nuclear fission, increased proliferation
marker protein Ki-67 expression and decreased E-cadherin expression
(8). However, the potential role
of ER-α36 in gastric carcinogenesis remains to be determined.
ER-α36 is predominantly expressed in the cytoplasm
and at the cell membrane, unlike ER-α which is primarily in the
cell nucleus (9–11). In breast cancer, ER-α36-mediated
signaling positively regulates ER-positive stem/progenitor cells
(12), and it serves an important
role in the malignant growth of ER-negative breast tumor cells via
the mitogen-activated protein kinase (MAPK)/extracellular
signal-regulated kinase (ERK) signaling pathway (13). In gastric tumor cells, ER-α36
conducts biphasic estrogen signaling (14). A decreased concentration of
estrogen (0.1 nM) has been demonstrated to promote cell growth,
while a high concentration (1 µM) inhibited cell growth (8,15).
However, the mechanism underlying estrogen signaling in cell growth
of the gastric tumor is still unclear.
The 78 kDa glucose-regulated protein (GRP78) is a
stress-inducible chaperone, and maybe induced under tumor
microenvironmental stress conditions (16). GRP78 has been implicated in cancer
cell growth, invasion, metastasis and angiogenesis (15,17,18).
In gastric carcinoma, GRP78 overexpression is positively-correlated
with larger tumor size, increased invasion and advanced stage
(19). Targeting GRP78 in gastric
cancer leads to a more effective therapeutic outcome (20). GRP78 expression suppressed
apoptosis induced by serine/threonine-protein kinase BIK in
estrogen-deprived breast cancer cells (21). GRP78 expression is induced by
treatment with estrogen in endometrial cancer cells (22). A previous study demonstrated that
elevated endoplasmin (GRP94) expression, another protein in the
heat shock protein family, was correlated with tumor malignancy and
upregulated expression of ER-α36 in gastric tumor cells (23,24).
In breast cancer, GRP94 was reported to positively-regulate ER-α36
expression, and enhance cell proliferation and invasion (25). However, the potential role and
mechanism through which GRP78 may regulate ER-α36 signaling remains
unclear.
In the present study, GRP78 and ER-α36 expression
patterns in samples from patients with gastric tumor, in addition
to the correlation between their expression levels and
clinicopathological features, were analyzed. GRP78, ER-α36 and
cyclin D1 expression in established gastric tumor cells with
overexpressed GRP78, and the cell growth of these cells following
treatment with estrogen, were additionally investigated.
Materials and methods
Reagents
17β-estradiol (E2) was obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). E2 was dissolved in absolute
alcohol (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) at a
concentration of 10 mM, and then stored at −20°C for cell
treatment. The rabbit polyclonal antibody recognizing GRP78 (cat.
no. ab21685) was from Abcam (Cambridge, UK). The mouse monoclonal
Cyclin D1 antibody was purchased from ProteinTech Group, Inc.
(Chicago, IL, USA; cat. no. 60186-1-Ig). The rabbit polyclonal
ER-α36 antibody was provided by D. Zhaoyi Wang, Shenogen Pharma
Group (Beijing, China). The antibody was generated using the custom
service provided by the Pacific Immunology (Ramona, CA, USA) using
the last 20 amino acids of ER-α36 encoded by exon 9 which are
unique to ER-α36 as an immunogen. The produced antibody was
purified using an affinity column consisting of immunogen peptides
(9–11). The monoclonal β-actin antibody
(cat. no. sc-47778) and the horseradish peroxidase-conjugated
antibodies (cat. nos. sc-2004 and sc-2005) were obtained from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). A lentiviral expression
vector (Lenti-HSPA5) and a lentiviral vector expressing GFP alone
(LV-control) were constructed and produced by Shanghai GeneChem
Co., Ltd. (Shanghai, China). The SuperPicture 3rd Gen
Immunohistochemistry kit was purchased from Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The Enhanced
Bicinchoninic Acid (BCA) Protein Assay kit and
radioimmunoprecipitation assay (RIPA) buffer were purchased from
Beyotime Institute of Biotechnology (Haimen, China).
Cell culture and treatments
SGC7901 cells were obtained from Tongji Medical
College (Wuhan, China). A stable cell line with overexpressed GRP78
(SGC-High78 cells) and a control cell line were generated by
Shanghai GeneChem Co., Ltd. SGC7901 and SGC-High78 cells were
maintained in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) with 10% fetal calf serum (FCS; Zhejiang Tianhang
Biotechnology Co., Ltd., Zhejiang, China) in a 5% CO2
atmosphere at 37°C. For E2 treatment, the cells were maintained in
phenol red-free RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) with 5% charcoal-stripped FCS (Biological Industries,
Beit-Heamek, Israel) for 6 h at 37°C, and then in 2%
charcoal-stripped FCS for 24 h at 37°C prior to experiments; the
same volume of alcohol was used as the control.
Cell proliferation assay
Cells (3×103/well) were seeded and then
treated with 0.1 nM E2 for 5, 7 and 9 days were assessed using the
Scepter™ 2.0 automated cell counter (Merck KGaA). All
experiments were repeated three times with three 6-well plates for
each point.
Gastric tumor samples
Tissue from 136 patients with gastric cancer between
January 2006 and December 2010 were obtained from the Jiangda
Pathology Institute (Wuhan, China) with Institutional Review Board
approval and written informed consent. The samples were obtained
from 100 men and 36 women aged between 34 and 82 years (mean age,
56.84 years), and all samples were fixed in 10% formalin at room
temperature for 1 day prior to paraffin-embedding. No patient had
received any anticancer therapy prior to surgery. Tumor size,
differentiation and staging were assessed according to the
classification system of the World Health Organization (2013).
Tissue microarray
Representative areas of the tumors were identified
by hematoxylin and eosin (H&E)-staining of the sections
obtained from patients. Briefly, a 0.6-mm in diameter tissue core
block (1 per donor) was punched out of each sample and transferred
to a recipient block (novel paraffin block containing a maximum of
130 patient core samples), using a tissue microarrayer MTA-1
(Beecher Instruments, Inc., Sun Prairie, WI, USA). Consecutive
4-µm-thick sections were cut from the recipient block and
transferred to polylysine-coated glass slides. H&E staining
(Mayer's hematoxylin for 2 min and 1% eosin for 30 sec at room
temperature) was performed on the tissue microarray to check the
quality of the sections prior to experiments.
Western blot analysis
Western blotting was performed as previously
described (26,27). Cells were harvested, washed and
lysed in RIPA buffer. Following determination of the protein
concentration using the BCA kit, the samples were separated using
SDS-PAGE on a 10% gel and then blotted to polyvinylidene fluoride
filters (EMD Millipore, Billerica, MA, USA). The filters were
blocked in buffer containing 5% nonfat milk for 1 h, and detected
with appropriate primary antibodies at 4°C overnight. The dilutions
of the antibodies were as follows: GRP78, 1:1,000; ER-α36, 1:1,000;
cyclin D1, 1:1,000; and β-actin, 1:5,000. The blots were
subsequently probed with secondary antibodies for 1 h at 37°C,
visualized using enhanced chemiluminescence, and quantitatively
analyzed using Totallab version TL120 analysis software (Nonlinear
Dynamics Ltd., Newcastle upon Tyne, UK).
Immunohistochemistry assay
Immunohistochemical analysis was performed as
previously described (7). The
slides were dewaxed in xylene and gradually rehydrated. Antigen
retrieval was performed in EDTA buffer (pH 8.0) and by boiling in a
water bath for 20 min. The samples were rinsed, incubated with
antibodies against GRP78 (1:400) or ER-α36 (1:400) overnight at
4°C, and with the secondary antibody (horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin; 1:100; cat.
no. A16096; Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for
30 min, prior to counterstaining with hematoxylin at room
temperature for 5 min. The slides were independently evaluated
using a light microscope (Olympus BX51; ×10 ocular magnification)
by two pathologists in a blinded manner.
Statistical analysis
The association between GRP78 expression, clinical
pathological features and ER-α36 expression was examined using the
Pearson χ2 test. SPSS 12.0 software (SPSS Inc., Chicago,
IL, USA) was employed for statistical analysis. Data are presented
as the mean ± standard error of the mean. Statistical analysis was
performed using one-way analysis of variance, followed by
Bonferroni's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Association between GRP78, ER-α36
expression and clinicopathological properties of gastric tumor
samples
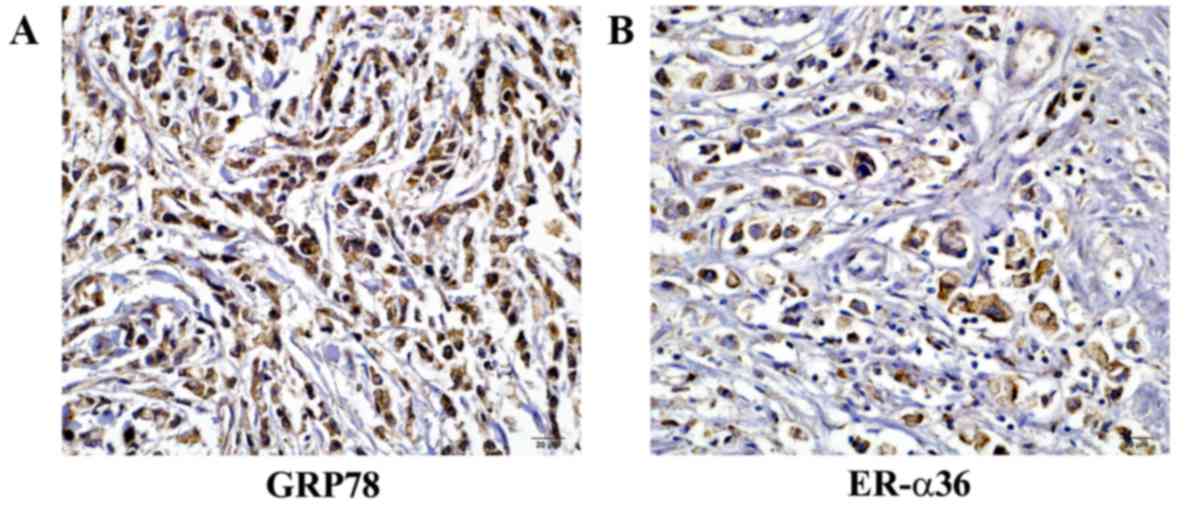
GPR78 expression was assessed in 136 specimens by
immunohistochemical analysis. GRP78 and ER-α36 were detected in the
cytoplasm of gastric cancer cells (Fig. 1). GRP78 expression (2+
or 3+) was observed in 95 of the cases of gastric
carcinoma (95/136; 69.85%). ER-α36 expression (2+ or
3+) was observed in 110 out of the 136 cases (80.88%)
(Table I).
 | Table I.Association between GRP78 expression,
clinicopathological features of gastric carcinoma, and ER-α36
expression. |
Table I.
Association between GRP78 expression,
clinicopathological features of gastric carcinoma, and ER-α36
expression.
|
| GRP78 expression |
|---|
|
|
|
|---|
| Factor | Positive | Negative | P-value |
|---|
| Age, years |
|
|
|
| ≤60 | 61 | 20 | 2.83 |
|
>60 | 34 | 21 |
|
| Sex |
|
|
|
| Male | 78 | 22 | 11.91 |
|
Female | 17 | 19 |
|
| Tumor size, cm |
|
|
|
| ≤5 | 50 | 15 | 2.96 |
|
>5 | 45 | 26 |
|
| Histological
differentiation |
|
|
|
| High
differentiation | 65 | 29 | 0.07 |
| Low
differentiation | 30 | 12 |
|
| T stage |
|
|
|
|
T2-3 | 67 | 29 | <0.01 |
| T4 | 28 | 12 |
|
| N stage |
|
|
|
| N0 | 20 | 8 | 0.04 |
|
N1-3 | 75 | 33 |
|
| ER-α36 |
|
|
|
|
Positive | 77 | 33 | 0.01 |
|
Negative | 18 | 8 |
|
Analysis of the association between GRP78 expression
and the clinical pathological characteristics of gastric cancer
specimens was performed. High GRP78 was positively-associated with
tumor stage (P<0.01) and an increased incidence of lymphatic
metastasis (P<0.05), although no association was observed with
age, gender, histological differentiation and tumor size
(P>0.05). Compared with female patients, GRP78 positivity was
detected in more male patients (male-to-female ratio, 2.78:1;
Table I).
A positive association between GPR78 and ER-α36
expression (P<0.05; Table I)
was observed, suggesting that GPR78 and ER-α36 may be involved in
gastric tumorigenesis.
Estrogen induces GRP78 and ER-α36
expression
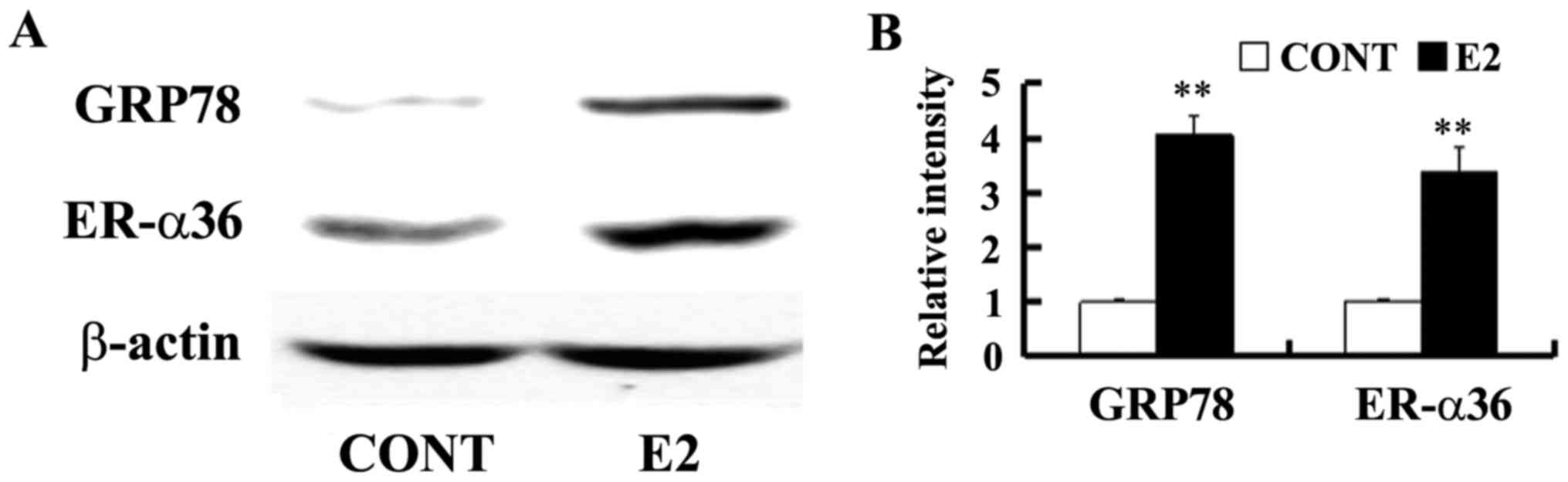
Estrogen-deprived SGC7901 cells were cultured in the
presence of E2 at a concentration of 0.1 nM for 24 h to determine
whether estrogen is able to regulate GRP78 expression. GRP78
expression was assessed by western blotting. It was demonstrated
that a low concentration of E2 upregulated GPR78 and ER-α36
expression in SGC7901 cells (Fig.
2).
Increased ER-α36 and cyclin D1
expression, and enhanced growth in GRP78 expressing cells
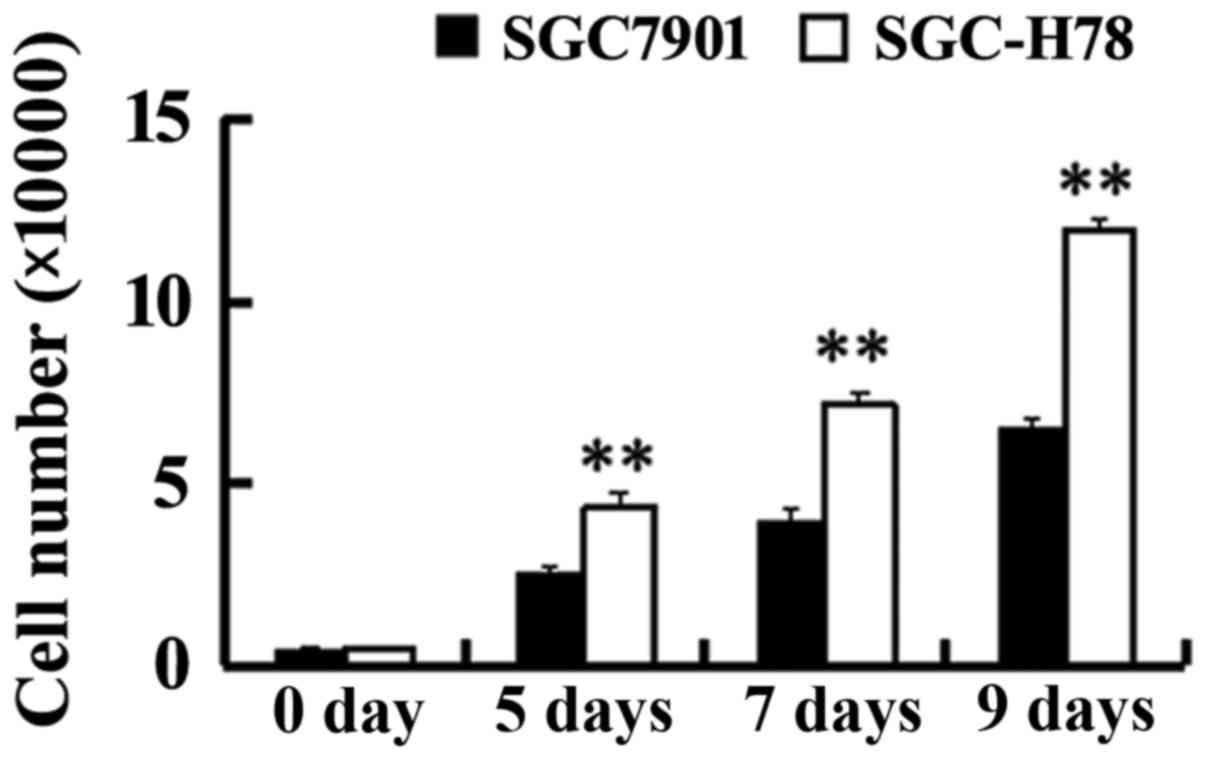
In order to study the role and potential mechanism
of GRP78 in the growth of gastric tumor cells, SGC-High78 cells
that overexpressed recombinant GRP78 and SGC-Control cells were
examined for cell growth. It was observed that SGC-High 78 cells
exhibited a higher growth rate compared with SGC7901-Control cells
(Fig. 3). A significant increase
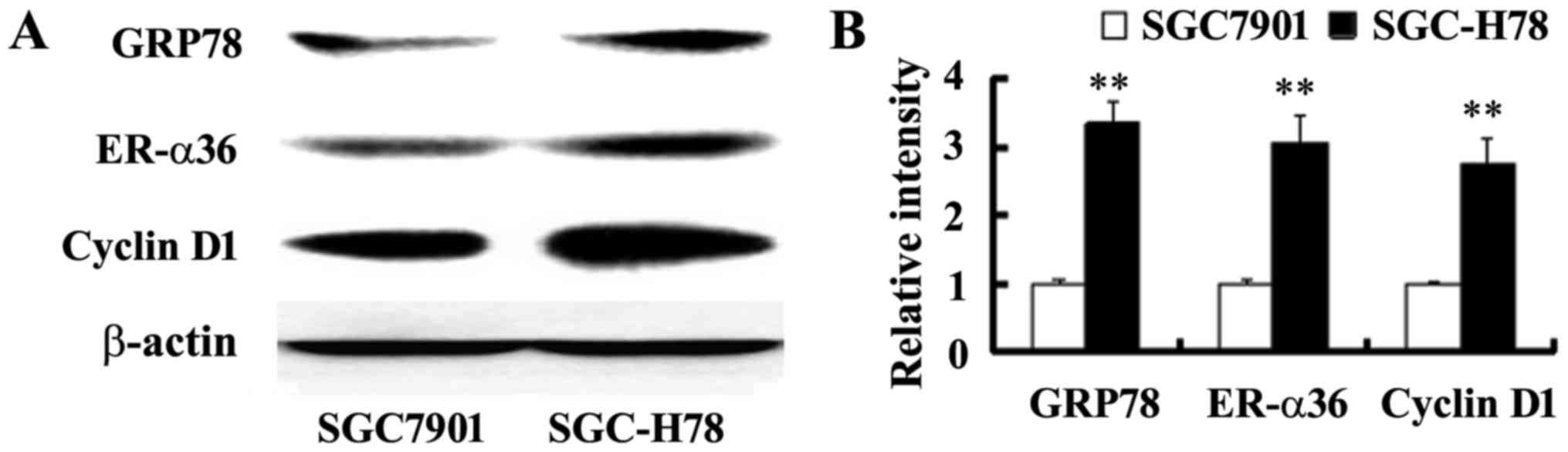
in ER-α36 and cyclin D1 expression was noted in the cells with
overexpressed GRP78, compared with SGC-Control cells (Fig. 4), indicating that upregulated
ER-α36 expression in GPR78-expressing cells may be important for
the increased cell growth of GPR78-expressing cells.
GRP78 induces ER-α36 and cyclin D1
expression via estrogen in gastric tumor cells
In order to confirm the function of GRP78 in the
responsiveness of gastric tumor cells to estrogen SGC-High78 cells
overexpressing recombinant GRP78 and SGC7901-Control cells were
treated with E2 at a concentration of 0.1 nM for different time
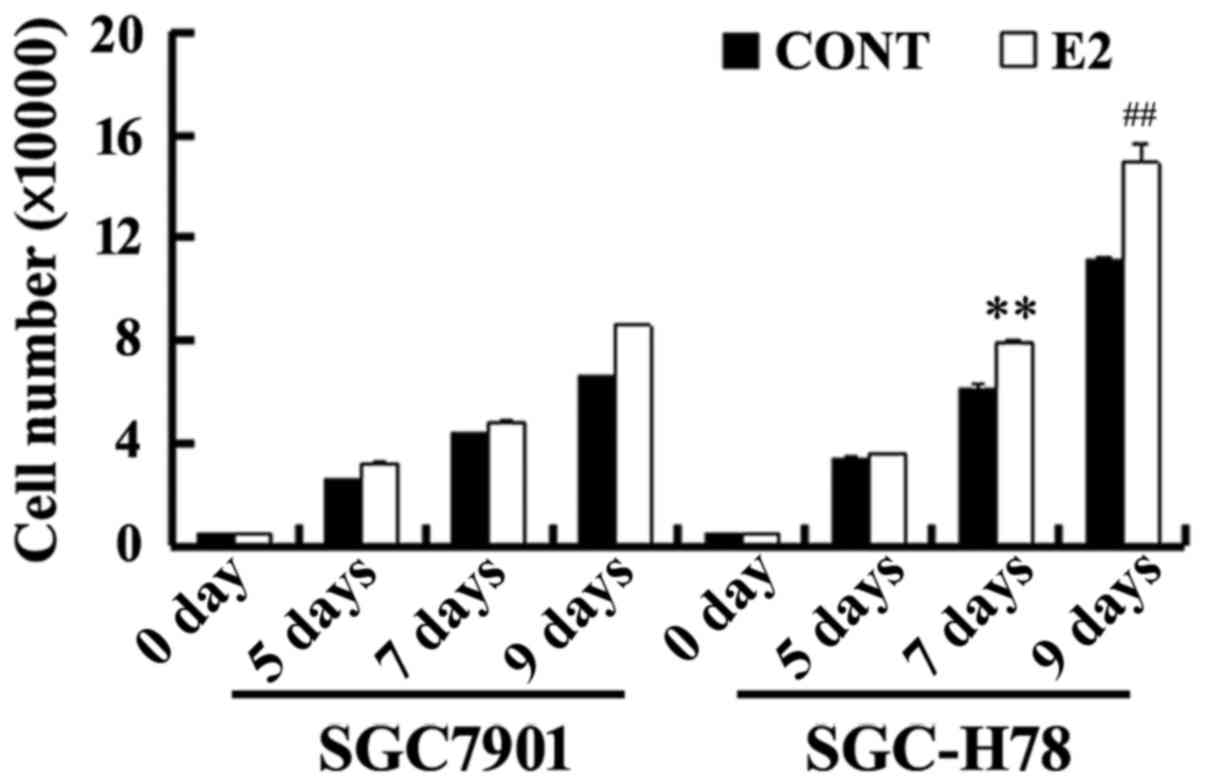
periods, and cell growth was examined. As presented in Fig. 5, SGC-High78 cells exhibited an
increased growth rate with treatment with estrogen compared with
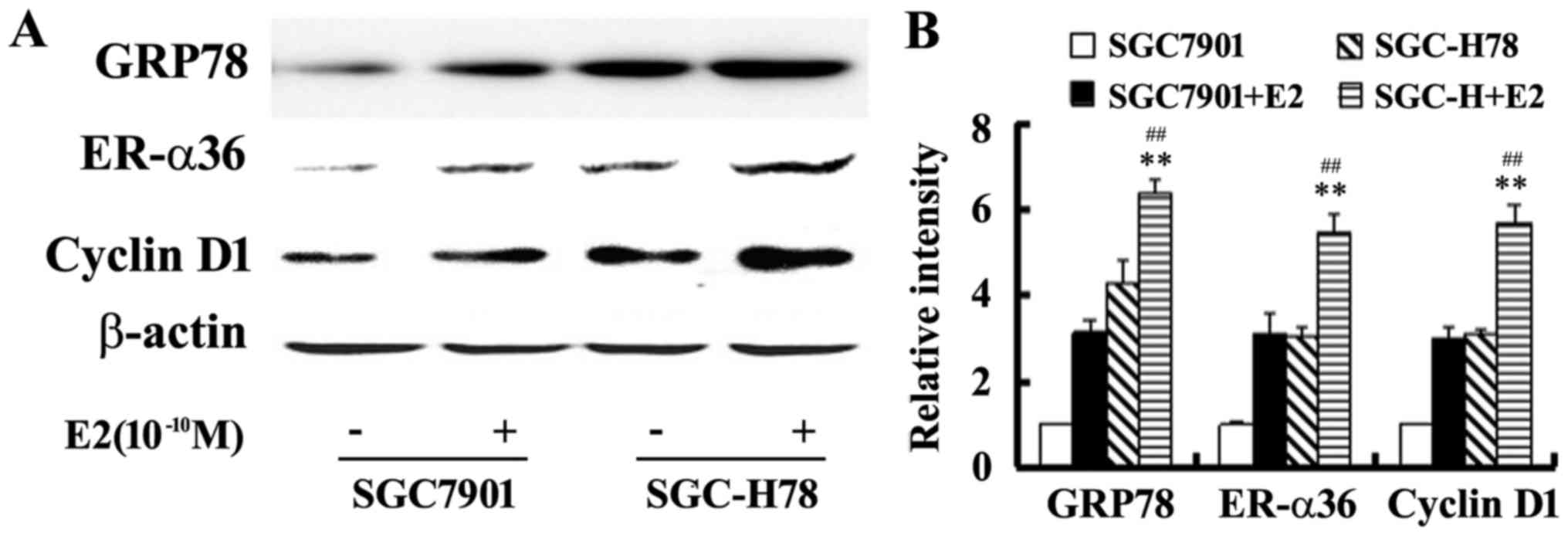
SGC7901-Control cells. Western blot analysis illustrated that E2
upregulated the levels of GRP78, ER-α36 and cyclin D1 expression,
and these increases were more marked in SGC-High78 cells compared
with those in SGC-Control cells (Figs.
5 and 6). The results of the
present study suggested that overexpressed GRP78 promoted the
growth of gastric tumor cells via upregulation of ER-α36
signaling.
Discussion
ER-α36 expression has been reported in gastric,
breast, lung and endometrial cancer, and its function is associated
with the carcinogenesis and progression of these tumors (7,11,28,29).
In gastric tumor, increased ER-α36 expression was associated with
more advanced lymphatic metastasis (7). ER-α36 enhanced the growth of gastric
tumor cells by augmenting proto-oncogene tyrosine-protein kinase
Src (Src) signaling and upregulating cyclin D1 expression (14). In the present study, GRP78 and
ER-α36 were expressed in gastric tumor specimens. Estrogen promoted
gastric cancer cell growth and upregulated GRP78 and ER-α36 in
SGC7901 cells. The result of the present study suggested an
involvement of GRP78 in the estrogen-enhanced growth of gastric
tumor cells via the ER-α36 signaling pathway.
ER-α36 is primarily expressed in the cytoplasm and
at the plasma membrane. ER-α36 mediates the membrane-initiated
rapid estrogen pathway and inhibits genomic estrogen signaling
mediated by ER-α66 and ER-β, and it functions as an important
factor in the increased cell growth and tumorigenesis of breast
cancer stimulated by estrogen (9,30).
Estrogen has been demonstrated to stimulate the growth of gastric
cancer cells (14,15). It has been reported that cells with
high levels of ER-α36 require lower concentrations of estrogen (in
the pM range) to enhance cell growth, compared with cells
expressing low levels of the receptor (13). In the present study, a low
concentration of estrogen (equivalent to the level observed in
postmenopausal women) was demonstrated to promote gastric tumor
cell growth and to increase GRP78 and ER-α36 expression, which
provided a potential explanation for the observed male predominance
in gastric tumor and a possible mechanism underlying postmenopausal
ER-α36-mediated rapid estrogen signaling in gastric
tumorigenesis.
It was additionally demonstrated in the present
study that GRP78 expression was positively associated with tumor
stage, increased lymphatic metastasis and ER-α36 expression in
gastric carcinoma specimens. In addition, a higher growth rate, and
increased levels of ER-α36 and cyclin D1, were detected in cells
with GRP78 overexpression. Cells with overexpressed GRP78 were more
sensitive to treatment with estrogen and the growth rate of these
cells was higher, with increased ER-α36 and cyclin D1 expressions
compared with SGC-Control cells. The present findings suggested
that ER-α36 may be positively regulated by GRP78, and may be
involved in the cell growth of gastric tumors. A recent report
indicated that GRP94, a scaffold protein, stabilized cell membrane
ER-α36 and upregulated its levels in breast cancer (25). Targeting GRP94 with a specific
small interfering RNA or a specific monoclonal inhibited
ER-α36-driven cell growth in vitro and in vivo
(25). A previous study reported
that the GRP94 expression level was upregulated by ER-α36 in
gastric cancer cells (23,24). In established gastric cancer cells
with knockdown of ER-α36 expression, GRP94 was markedly reduced
(23). ER-α36 was reported to be
involved in the testosterone-stimulated activation of the MAPK/ERK
and phosphatidylinositol 3-kinase/RAC-α serine/threonine protein
kinasesignaling pathways in endometrial cancer Hec1A cells
(29). E2 induced MAPK/ERK
activation via a mechanism involving ER-α36 and the epidermal
growth factor receptor/Src/SHC transforming protein 1complex
(31). Therefore, it is possible
that there exists a positive regulatory loop between GRPs and
ER-α36 expression, although the mechanism underlying their
association with tumorigenesis requires further investigation.
In conclusion, GRP78 expression was positively
correlated with advanced tumor stage, increased lymphatic
metastasis and increased ER-α36 expression in specimens from
patients with gastric tumors. ER-α36-mediated signaling positively
regulated by GRP78 enhanced cell growth in gastric tumors. The
results of the present study thereby provided evidence that GRP78
may function as an important regulator in the estrogen-enhanced
growth of gastric tumor through ER-α36 signaling.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402315).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Camargo MC, Goto Y, Zabaleta J, Morgan DR,
Correa P and Rabkin CS: Sex hormones, hormonal interventions, and
gastric cancer risk: A meta-analysis. Cancer Epidemiol Biomarkers
Prev. 21:20–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lindblad M, Ye W, Rubio C and Lagergren J:
Estrogen and risk of gastric cancer: A protective effect in a
nationwide cohort study of patients with prostate cancer in Sweden.
Cancer Epidemiol Biomarkers Prev. 13:2203–2207. 2004.PubMed/NCBI
|
|
5
|
Wang M, Pan JY, Song GR, Chen HB, An LJ
and Qu SX: Altered expression of estrogen receptor alpha and beta
in advanced gastric adenocarcinoma: Correlation with prothymosin
alpha and clinicopathological parameters. Eur J Surg Oncol.
33:195–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ryu WS, Kim JH, Jang YJ, Park SS, Um JW,
Park SH, Kim SJ, Mok YJ and Kim CS: Expression of estrogen
receptors in gastric cancer and their clinical significance. J Surg
Oncol. 106:456–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deng H, Huang X, Fan J, Wang L, Xia Q,
Yang X, Wang Z and Liu L: A variant of estrogen receptor-alpha,
ER-alpha36 is expressed in human gastric cancer and is highly
correlated with lymph node metastasis. Oncol Rep. 24:171–176.
2010.PubMed/NCBI
|
|
8
|
Wang XM, Liu JJ, Deng H, Chen Y and Liu
LJ: ER-α36 promotes the growth of SGC-7901 cells in nude mice.
World Chin J Digestol. 19:2919–2924. 2011.(In Chinese). View Article : Google Scholar
|
|
9
|
Wang ZY and Yin L: Estrogen receptor
alpha-36 (ER-α36): A new player in human breast cancer. Mol Cell
Endocrinol 418 Pt. 3:193–206. 2015. View Article : Google Scholar
|
|
10
|
Wang Z, Zhang X, Shen P, Loggie BW, Chang
Y and Deuel TF: Identification, cloning, and expression of human
estrogen receptor-alpha36, a novel variant of human estrogen
receptor-alpha66. Biochem Biophys Res Commun. 336:1023–1027. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Z, Zhang X, Shen P, Loggie BW, Chang
Y and Deuel TF: A variant of estrogen receptor-{alpha},
hER-{alpha}36: Transduction of estrogen- and antiestrogen-dependent
membrane-initiated mitogenic signaling. Proc Natl Acad Sci USA.
103:pp. 9063–9068. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng H, Zhang XT, Wang ML, Zheng HY, Liu
LJ and Wang ZY: ER-α36-mediated rapid estrogen signaling positively
regulates ER-positive breast cancer stem/progenitor cells. PLoS
One. 9:e880342014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi L, Dong B, Li Z, Lu Y, Ouyang T, Li J,
Wang T, Fan Z, Fan T, Lin B, et al: Expression of ER-{alpha}36, a
novel variant of estrogen receptor {alpha} and resistance to
tamoxifen treatment in breast cancer. J Clin Oncol. 27:3423–3429.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Huang X, Fu Z, Zou F, Li Y, Wang Z
and Liu L: Biphasic ER-α36-mediated estrogen signaling regulates
growth of gastric cancer cells. Int J Oncol. 45:2325–2330. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Deng H, Zou F, Fu Z, Chen Y, Wang
Z and Liu L: ER-α36-mediated gastric cancer cell proliferation via
the c-Src pathway. Oncol Lett. 6:329–335. 2013.PubMed/NCBI
|
|
16
|
Li Z and Li Z: Glucose regulated protein
78: A critical link between tumor microenvironment and cancer
hallmarks. Biochim Biophys Acta. 1826:13–22. 2012.PubMed/NCBI
|
|
17
|
Lee AS: GRP78 induction in cancer:
Therapeutic and prognostic implications. Cancer Res. 67:3496–3499.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee AS: Glucose-regulated proteins in
cancer: Molecular mechanisms and therapeutic potential. Nat Rev
Cancer. 14:263–276. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng HC, Takahashi H, Li XH, Hara T,
Masuda S, Guan YF and Takano Y: Overexpression of GRP78 and GRP94
are markers for aggressive behavior and poor prognosis in gastric
carcinomas. Hum Pathol. 39:1042–1049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng CC, Lu N, Peng CL, Chang CC, Mai FD,
Chen LY, Liao MH, Wang WM and Chang J: Targeting to overexpressed
glucose-regulated protein 78 in gastric cancer discovered by 2D
DIGE improves the diagnostic and therapeutic efficacy of
micelles-mediated system. Proteomics. 12:2584–2597. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu Y, Li J and Lee AS: GRP78/BiP inhibits
endoplasmic reticulum BIK and protects human breast cancer cells
against estrogen starvation-induced apoptosis. Cancer Res.
67:3734–3740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luvsandagva B, Nakamura K, Kitahara Y,
Aoki H, Murata T, Ikeda S and Minegishi T: GRP78 induced by
estrogen plays a role in the chemosensitivity of endometrial
cancer. Gynecol Oncol. 126:132–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu Z, Deng H, Wang X, Yang X, Wang Z and
Liu L: Involvement of ER-α36 in the malignant growth of gastric
carcinoma cells is associated with GRP94 overexpression.
Histopathology. 63:325–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu Z, Zhen H, Zou F, Wang X, Chen Y and
Liu L: Involvement of the Akt signaling pathway in
ER-α36/GRP94-mediated signaling in gastric cancer. Oncol Lett.
8:2077–2080. 2014.PubMed/NCBI
|
|
25
|
Hou J, Deng M, Li X, Liu W, Chu X, Wang J,
Chen F and Meng S: Chaperone gp96 mediates ER-α36 cell membrane
expression. Oncotarget. 6:31857–31867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu ZQ, Yang Y, Song J, Jiang Q, Lin ZC,
Wang Q, Zhu LQ, Wang JZ and Tian Q: LiCl attenuates
thapsigargin-induced tau hyperphosphorylation by inhibiting GSK-3β
in vivo and in vitro. J Alzheimers Dis. 21:1107–1117. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu Z, Zou F, Deng H, Zhou H and Liu L:
Estrogen protects SGC7901 cells from endoplasmic reticulum
stress-induced apoptosis by the Akt pathway. Oncol Lett. 7:560–564.
2014.PubMed/NCBI
|
|
28
|
Zhang S, Qiu C, Wang L, Liu Q and Du J:
The elevated level of ERα36 is correlated with nodal metastasis and
poor prognosis in lung adenocarcinoma. Steroids. 87:39–45. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin SL, Yan LY, Liang XW, Wang ZB, Wang
ZY, Qiao J, Schatten H and Sun QY: A novel variant of ER-alpha,
ER-alpha36 mediates testosterone-stimulated ERK and Akt activation
in endometrial cancer Hec1A cells. Reprod Biol Endocrinol.
7:1022009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Zheng N, Dong J, Wang X, Liu L and
Huang J: Estrogen receptor-α36 is involved in icaritin induced
growth inhibition of triple-negative breast cancer cells. J Steroid
Biochem Mol Biol. 171:318–327. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang XT, Kang LG, Ding L, Vranic S,
Gatalica Z and Wang ZY: A positive feedback loop of ER-α36/EGFR
promotes malignant growth of ER-negative breast cancer cells.
Oncogene. 30:770–780. 2011. View Article : Google Scholar : PubMed/NCBI
|